Abstract
External beam radiotherapy with photon beams is a highly accurate treatment modality, but requires extensive quality assurance programs to confirm that radiation therapy will be or was administered appropriately. In vivo dosimetry (IVD) is an essential element of modern radiation therapy because it provides the ability to catch treatment delivery errors, assist in treatment adaptation, and record the actual dose delivered to the patient. However, for various reasons, its clinical implementation has been slow and limited. The purpose of this report is to stimulate the wider use of IVD for external beam radiotherapy, and in particular of systems using electronic portal imaging devices (EPIDs). After documenting the current IVD methods, this report provides detailed software, hardware and system requirements for in vivo EPID dosimetry systems in order to help in bridging the current vendor-user gap. The report also outlines directions for further development and research. In vivo EPID dosimetry vendors, in collaboration with users across multiple institutions, are requested to improve the understanding and reduce the uncertainties of the system and to help in the determination of optimal action limits for error detection. Finally, the report recommends that automation of all aspects of IVD is needed to help facilitate clinical adoption, including automation of image acquisition, analysis, result interpretation, and reporting/documentation. With the guidance of this report, it is hoped that widespread clinical use of IVD will be significantly accelerated.
Keywords: In vivo dosimetry, External beam radiotherapy, Electronic portal imaging device, Task group report, Review
1. Introduction
External beam radiotherapy (EBRT) with photon beams has seen major progress in recent decades in the form of treatment planning, beam delivery, and image guidance. Nonetheless, despite best efforts, the actual delivered dose to the patient can differ from the planned dose. Among the many reasons for this are inaccuracies in the calculations of treatment planning systems (TPSs), errors in plan transfer to the accelerator or in beam delivery, and differences in patient geometry between the planning and treatment stages. The three aims of in vivo dosimetry (IVD) [1] are to catch treatment errors, assist in treatment adaptation, and record the actual dose delivered to the patient. Therefore, one would expect that IVD is already an essential link in the clinical workflow of modern radiotherapy. However, very few radiotherapy centers perform IVD during beam delivery [2]. The current standard is to perform dosimetry checks using pretreatment dose measurements in phantoms, which requires major resources but cannot catch errors related to patient geometry or in beam delivery during the actual treatment [3].
The purpose of this report is to analyze why IVD currently is not routinely performed, which methods are available, which methods need more development, and what needs to be done to augment clinical acceptance of the various methods. Although IVD is a widely applied term, its use easily causes misunderstandings not only between vendors and users but also within the research community. In order to prevent future misinterpretations, a concise definition of IVD in the scope of EBRT is provided in the Methods section. According to this definition, point detectors placed on the patient’s skin in the treatment field such as thermoluminescent detectors, silicon diodes, metal-oxide semiconductor field-effect transistors, optically stimulated luminescence dosimeters, and electronic portal imaging devices (EPIDs) [4], [5] are the main commercially available IVD methods. Due to the limitations of point detectors for large-scale implementation of IVD in modern EBRT, the emphasis of this report lies on EPID-based IVD (EIVD) systems. EPIDs are nowadays ubiquitous on modern accelerators, they are easy to use, they have potential for automation and they can perform dosimetric verification in 2D or 3D. The use of EPIDs for dosimetric measurements has matured for both pre-treatment patient-specific quality assurance (QA) [6] and IVD [7], [8], [9], [10]. The various EPID dosimetry approaches have been discussed in a comprehensive way in a review article by van Elmpt et al. [11], and have been discussed further in the updated literature review by McCurdy et al. [12]. Currently, commercial EPID dosimetry software products are available from several vendors. While vendors generally provide specific guidelines and overall support for the implementation of their EIVD system, the user has an important role in commissioning and implementation of these systems. However, there are currently no specific guidelines for users of EIVD systems on the potential, limitations and correct utilization of EIVD systems.
This report identifies system, software, hardware and user requirements needed for the widespread clinical implementation of EIVD systems. While mainly directed at vendors, these requirements should be of help in bridging the current vendor-user gap. To ensure a clear understanding of these requirements, the main concepts and basic terminology for the current types of EIVD methods are introduced. Finally, further directions for development are proposed to vendors and researchers in areas where improvements are needed for a wider adoption of EIVD systems. The report focuses on the use of EIVD systems for common external photon beam technology. Other IVD systems are only briefly discussed.
2. Methods and materials
2.1. IVD definition
IVD is a radiation measurement that is acquired while the patient is being treated containing information related to the absorbed dose in the patient. This definition implies that an IVD system must be able to capture errors due to equipment failure, errors in dose calculation, patient positioning errors, and patient anatomy changes.
The definition excludes all ‘transmission dosimetry’ methods that capture only the accelerator exit dose/energy fluence (or related quantities or metrics) before the beam reaches the patient, even if these are combined with a cone beam computed tomography (CBCT) image acquired right before treatment delivery. Using an accelerator log file, even in combination with a CBCT image, is also not considered IVD. The aforementioned systems can, however, complement IVD methods. Also, the comparison of an EPID image made during a specific fraction of a patient treatment with a reference EPID image, e.g., of the first fraction, may miss dose errors present in the reference image and is therefore a constancy check but not in vivo dosimetry. While such methods can be valuable tools for patient-specific QA, they are currently not categorized as IVD.
2.2. Structure of task group report
While the emphasis of this report lies on EIVD systems, the report presents first a brief state-of-the-art overview of the use of point detectors where their future role and limitations are recognized. As was the case with the definition of IVD, and to avoid misinterpretations, this report covers the main concepts and defines a basic terminology for the current types of EIVD systems. This terminology is used to guide readers through the next sections.
The report identifies a list of system, software, hardware and user requirements needed for the widespread clinical implementation of EIVD systems. Requirements are presented for the overall EIVD system, as well as for the different subsystems: EPID imager, image acquisition software and EIVD software. Specific requirements for automation are also provided as they are key to guarantee an optimal balance between resources and performance. The requirements list was elaborated first by the members of the task group with experience implementing EIVD systems and then reviewed by the rest of the task group members following an iterative process. Further recommendations are made for vendors and researchers in areas where improvements are needed for a wider clinical adoption of EIVD systems.
The references in the manuscript were selected to assist in the narrative of the report either by providing evidence for particular statements or by pointing to extra sources for further technical details but are not needed to understand the text. The references were screened for their inclusion by all members of the task group first and later by the advisory group.
3. Current IVD methods
3.1. Point detectors
Many types of point dose detector systems are available for IVD. The most commonly used are diodes, thermoluminescent dosimeters, and metal-oxidesemiconductor field-effect transistors. Optically stimulated luminescent dosimeters and plastic scintillation detectors have recently come into use for IVD [13], [14], [15], [16], [17], [18], [19]. The use of detectors for IVD in EBRT requires calibration methods to correlate the detector reading with the delivered dose [20], [21]. Point detectors, either placed on the skin of the patient or embedded in the immobilization mask or frame, are typically used to measure entrance dose and/or exit dose. In conformal beam radiotherapy, entrance IVD can detect major errors, mainly those caused by incorrect beam parameter settings (data transfer between planning and delivery) or machine malfunctions [22]. Before the widespread use of record-and-verify systems and the automatic transfer of all beam parameters from the TPS to the treatment units, entrance IVD was shown to detect data transfer errors [23]. By combining entrance with exit measurements, differences in patient anatomy between planning and delivery can also be detected [24]. However, the transition from simple conformal fields to intensity-modulated techniques such as intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) has limited the usefulness of point measurements [25], [26], particularly when bearing in mind the additional uncertainty due to placement error, which can substantially impact measured-to-planned dose agreement of point detectors in high gradient regions, demonstrating the criticality of accurate dosimeter placement for IMRT and VMAT treatments [27].
Point detectors that can be placed inside a catheter or implanted near the tumor can provide direct verification of the actual dose delivered to the tumor or organ at risk [15], [28], [29]. The main limitation of these detectors for clinical implementation is their invasiveness. Even when placement of the detector near the tumor is possible, the location of the detector, combined with changes in patient anatomy, means that the results may have large uncertainties. In addition, the dose can be checked at only one or a few points. Finally, point detectors may perturb the dose.
Point detectors are useful in determining skin dose and peripheral dose (e.g., contralateral breast, lens of eye, scrotum), as most TPSs do not provide accurate dose calculations in these situations. Point detectors can also confirm that cardiovascular implantable electronic devices (e.g., pacemakers) receive a minimal dose (below the manufacturer’s specified dose limits) [30]. In the absence of TPSs, such as for total body irradiation or intraoperative radiation therapy, IVD with point detectors has proven to be valuable [31], [32], [33], [34].
Even if point detectors still have a role for 3D conformal beam radiotherapy, for skin and peripheral dose measurements, for special techniques, and for dosimetry audits for clinical trials [35], their limitations of for large-scale implementation of IVD are obvious. First, point dosimetry is insufficient for patient-specific QA in modern EBRT. Point detectors are cumbersome and intrusive, add extra time to treatment delivery, cannot be automated and require well-trained staff. Due to the recognized limitations of point detectors for a widespread implementation of IVD, the focus of this report is on the use of EPID dosimetry for IVD.
3.2. EPID dosimetry for IVD
Although initially developed for patient setup verification, EPIDs show also useful dosimetric characteristics [36], [37], [38], [39] that have made them suitable for the implementation of IVD verification solutions [8], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]. Beginning in the early 2000 s, the current generation of amorphous-silicon flat-panel EPID technology became commercially available from major linac manufacturers and today is a ubiquitous choice on newly purchased linacs. The primary layers of an amorphous silicon EPID detection structure are a buildup or interaction layer, which is usually copper; a scintillating phosphor layer, usually gadolinium oxysulfide; and the photo-diode matrix, whose support material is amorphous silicon. Owing to the high atomic number (Z) of the materials and the inherent beam-energy spectrum, the response of the EPID will differ from ion-chamber measurements. Other factors that modify the spectrum, such as patient attenuation, field size, off-axis distance, and patient-to-EPID distance, also contribute to this difference. All EIVD systems use the measured image signal from the transmission energy fluence that impinges on the detector. This is affected by: (1) the incident beam fluence from the linac impinging on the patient, (2) primary fluence attenuation in the patient, (3) scatter from the patient, and (4) the response of the EPID to (2) and (3).
Typically, EIVD assessments are performed by combining the data of all EPID frames acquired during delivery. Time-resolved analysis refers to assessments using subsets of frames or cumulative frame signals. These frames can be grouped according to time, control point, or gantry angle. Offline assessment is performed after delivery and can be performed using both time-resolved and non-time-resolved methods [7], [44]. Online assessment is performed in real time using time-resolved analysis so that assessment is made before the total dose has been delivered to the patient, with the aim to interrupt treatment [51], [52]. Supplementary Table S7 provides a brief overview of currently available EIVD systems. These systems do not all comply with all of the requirements formulated in this report.
3.2.1. Forward systems at the EPID level
A direct method of EIVD is to predict and compare the measured portal image signals to a predicted portal image. This method uses the treatment plan and planning CT with a physics model that typically includes an incident fluence model, a patient attenuation and scatter model, a treatment couch attenuation model, and an EPID energy deposition model. Monte Carlo and analytical techniques have been used in this approach [46], [53], [54], [55]. Another closely related method is to predict the dose to a water slab at the position of the EPID, i.e., a predicted portal dose image. This method requires an algorithm to convert the measured signals to dose in water or measured portal dose image including corrections for energy-dependent response [56].
Most implementations to date employ non-time-resolved methods using the integrated image signal; however, for VMAT, this obviously has major limitations. Time-resolved analysis for VMAT deliveries can be performed following alignment of predicted and measured images [44], [57]. Experience with an online system using comparison of predicted to measured portal images has been reported [51].
A disadvantage of these types of EIVD methods at the plane of the EPID is that the comparisons are not intuitive and cannot easily be related to clinically relevant comparison metrics such as patients’ dose volume histograms. However, they do not contain any less information on the delivery than back-projection systems. Generally, 2D planar gamma analysis is employed, but other metrics have also been investigated [58].
3.2.2. Back-projection systems within the patient: dose reconstructed within a patient model
These systems estimate a point dose or dose distribution within a patient model from the measured EPID image. When the patient model is a CBCT scan acquired immediately prior to treatment, then the dose is an estimate of the delivered dose for that treatment fraction as it accounts for the anatomy of the patient before the delivery [45], [59]. When, as is more common, the patient model is the planning CT scan, then the estimated dose in this model can be used to determine a change in the delivery from the planned delivery. A recent paper studied the effect of the choice of patient model on the performance of in vivo 3D EPID dosimetry [60]. In this paper, it was concluded that with planning CT images as patient model, EPID dose reconstructions underestimate the dosimetric effects caused by errors in patient positioning and overestimate the dosimetric effects caused by changes in patient anatomy.
For direct back-projection systems the primary fluence at the plane of the EPID is usually derived by correction of EPID scatter and patient scatter. This fluence is back-projected through the patient model and combined with dose-deposition kernels to determine the dose distribution [42], [61]. Solutions have been presented to compensate the suboptimal support for density inhomogeneities in these simple dose calculations [62], [63]. Other empirically based approaches have been developed to determine dose at a point or a plane in the patient model from the EPID image [64], [65]. For indirect back-projection systems, the primary fluence is back-projected through the patient model to determine the incident fluence to the patient. This is then used with a conventional patient dose-calculation engine and analytical and Monte Carlo methods have been employed [47], [66], [67].
The clear advantages of back-projection systems are that the estimated dose is more intuitive and that differences can be evaluated by direct comparison to the planned dose distribution in the patient model using gamma or dose volume histogram evaluations. The majority of implementations have been offline, although an online system has also been reported [52]. For back-projection systems for VMAT, a time-resolved acquisition and back-projection method is required; however, the analysis is typically based on the integrated back-projected signal [43]. A brief overview of the currently available EIVD methods is presented in Table 1.
Table 1.
Currently available types of EIVD systems.
| System type | Comparison location | Prediction | Measured/EPID-reconstructed |
|---|---|---|---|
| Forward systems (EPID) | EPID plane | Predicted portal image. Grayscale values. | Measured portal image. Grayscale values. |
| Forward systems (dose) | EPID plane | Predicted portal dose to water slab. | Measured portal dose to water slab. |
| Back-projection systems (direct) | Within patient model | Treatment planning system. Dose in patient model. | Dose back-projection directly into patient model. |
| Back-projection systems (indirect) | Within patient model | Treatment planning system. Dose in patient model. | Dose back-projection through patient to incident fluence. Calculate dose in patient model. |
4. Requirements for EPID-based IVD systems
Detailed software and hardware requirements regarding the overall system performance, as well as EPID imager, MV image acquisition software and IVD software are presented as Supplementary Material, see Tables S1–S4.
Furthermore, for a successful large-scale implementation, EIVD systems must ensure a manageable and configurable workload to guarantee an optimal balance between resources and performance [7], [8], [48], [68]. Automation is essential for large-scale clinical implementation of EIVD systems because it reduces the number of labor-intensive, time-consuming, and error-prone tasks [69], [70]. Automation also allows for more frequent use of the system, e.g., for all fractions. See Supplementary Table S5 for the specific list of requirements on the automation steps. In case automation is only partly available, the whole verification process must be carefully described, and an estimation of the workload required to perform the non-automated tasks must be provided. Note that full automation is a requisite for online assessments.
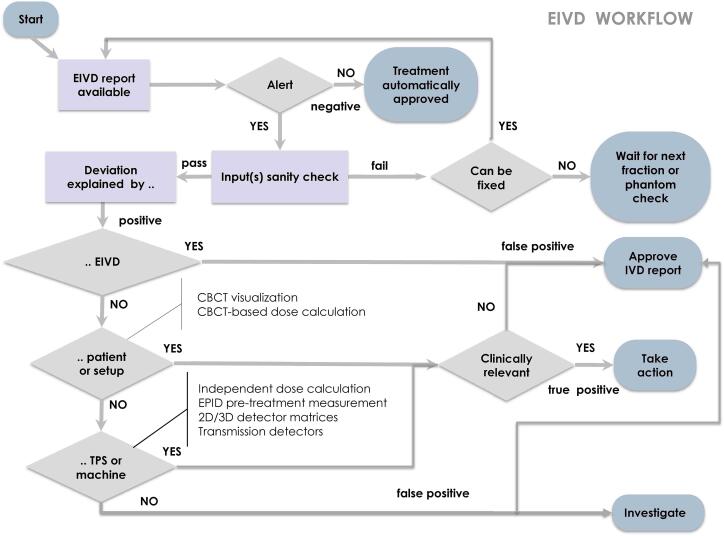
In an automated environment, the inspection of alerts becomes the only remaining work. Vendors are expected to facilitate a streamlined workspace for the timely inspection of alerts (Requirements S5.7 and S5.8) and additional tools to aid in the alert inspection work (S4.13–15). An estimate of the average per-treatment alert-management workload must also be given. Fig. 1 displays a flow chart illustrating the basics of an EIVD alert-inspection workflow.
Fig. 1.
Basics of an EIVD alert-inspection workflow.
The commissioning of the EIVD system also demands resources and manpower. Vendors are expected to provide information about the commissioning equipment and the measurement procedures, expected workload, acceptance tests and acceptance criteria [71], [72], [73]. Regarding periodic QA procedures, the test frequency, equipment, expected workload and automatic possibilities must also be known.
5. Future directions for research, development and clinical practice
5.1. Uncertainties
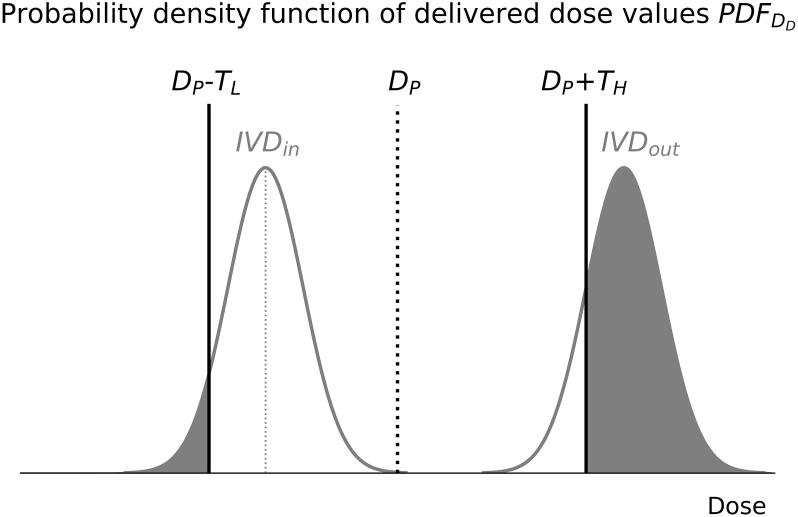
IVD systems need to assess whether the actual dose delivered to the patient DD lies within agreed-upon dosimetric lower and higher tolerance limit values (TL, TH) with respect to the originally intended planned dose value DP [74]. In this assessment, the measured in vivo dose value DIVD is used for an estimation of DD. The uncertainty UIVD in the DIVD measurement defines the range of values (and their probability) within which DD is expected to lie. The probability density function of delivered dose values PDFDD is used to calculate the likelihood that the delivered dose deviation will exceed the tolerance limit values (see Fig. 2) and needs to be taken into account when defining dosimetric action limits for IVD systems. Note that for EIVD, the meaning of ‘dose’ differs among systems, and ‘value’ is often not a point dose value but a 2D/3D dose distribution.
Fig. 2.
Probability density functions of delivered dose values corresponding to two DIVD measurements, one inside (IVDin) and one outside (IVDout) the dosimetric tolerance limit values (DP − TL, DP + TH). The graph illustrates how the uncertainty of the IVD system influences the likelihood that the actual delivered dose deviation will exceed the tolerance limits (gray shaded areas). For simplicity, UIVD is assumed to follow a normal distribution without bias.
EIVD vendors, possibly in collaboration with users across multiple institutions utilizing different technologies, should put efforts into determining UIVD for all dose comparison indicators and clinically relevant combinations of delivery techniques and treatment disease sites, see Requirement S1.13. The determination can be made by comparing DIVD dose values against reference values that can be reasonably used as DD or by simply considering the inherent spread of DIVD dose values in nominally “error-free” deliveries. It may be insightful to evaluate the uncertainty of the system for increasing levels of complexity: (i) simple plans, e.g. square fields, on homogeneous phantoms, (ii) complex plans on homogeneous phantoms and (iii) complex plans on anthropomorphic phantoms. In addition, extra uncertainties in case of uncorrected errors in patient positioning and/or anatomy changes if daily patient imaging is not used in the reconstruction should be evaluated.
The presence of a bias in UIVD distributions would suggest that there is a systematic error in the determination of DIVD dose values that needs to be corrected.
Furthermore, EIVD vendors need to explain the main causes for uncertainty and put efforts into not only assessing but also reducing the uncertainty.
5.2. Error detection
The ultimate goal of IVD is error detection, i.e., to detect deviations between planned dose distributions and delivered dose values exceeding specified tolerance limits. An overview with the errors that EIVD systems are expected to detect is presented as Supplementary Material, see Table S6. The detectability of a specific type of error depends very much on the specificity and sensitivity of a particular EIVD system for that type of error, as discussed in the next section. Mijnheer [75] presented an overview of the different types of errors detected by various groups using both in-house-developed and commercial EIVD systems. In this overview, examples of point-dose errors, errors in the 2D or 3D features of leaf sequencing, dose calculation errors, and patient-related errors are shown. Bojechko et al. [76] analyzed a series of near-miss incidents with high potential severity at their institute. Most of these errors cannot be detected by means of pretreatment dose verification, highlighting the importance of IVD.
5.3. Specificity and sensitivity
IVD systems act as binary classifiers where treatments are identified either as positive (alerted) or negative (not alerted). Ideally, a treatment should be classified as a positive only if the actual delivered dose deviation is relevant to the outcome of the treatment; and otherwise it should be considered a negative. In practice, however, treatments are sometimes incorrectly identified: e.g., non-relevant dose deviations classified as positives or relevant dose deviations classified as negatives. False positives lead to unnecessary extra inspection work, while false negatives hide errors not detected by the system. The reasons for incorrect classification are EIVD uncertainties, EIVD limitations and the inappropriate choice of action limits.
Ultimately, the error detectability of an IVD system is expressed in terms of its sensitivity and specificity, i.e., the true positive rate and true negative rate, respectively. EIVD vendors are requested to help the user community determine the sensitivity and specificity of the system for a set of representative clinical situations, for instance through large-scale trials and information gathering initiatives. This includes an assessment of the dependency on treatment site, delivery technique, and/or indicator used. These studies would help in determining optimal action limits to detect errors of a given magnitude and in elucidating the possibilities and limitations of the IVD system, e.g., situations where clinically relevant deviations do not significantly change the recorded signal at the EPID level.
Receiver operating characteristic (ROC) curves can be used to evaluate the ability of IVD systems to correctly classify observed dose deviations and to determine optimal action limits [77]. ROC analysis, however, requires a statistically significant size of error and no-error samples as input. The experimental acquisition of EPID measurements to produce such samples is typically a cumbersome process, which explains why there are only a few studies on the topic in the IVD literature [78], [79], [80], [81]. Recently, use has been made of synthetic EPID images to eliminate the need for phantom error introduction and positioning [82]. Another alternative is to model possible errors by introducing modifications in the TPS [83]. Vendors, in collaboration with the clinical community, are requested to promote research activities related to the error detectability of their IVD systems and to facilitate collaboration within a user group. Additional research is also required to investigate the use of alternative measurement analysis techniques, e.g., use of exploratory data analysis, radiomics, and/or machine learning [84], [85], [86].
5.4. Online systems
Online EIVD is performed in real time using time-resolved analysis with the aim to interrupt treatment before the total dose has been delivered to the patient [51], [52]. There are additional challenges with online EIVD systems such as speed, latency, robustness, specificity, and tolerances for real-time analysis that require further research.
5.5. EPID technology
The main limitation of the use of standard amorphous-silicon EPID technology for in vivo dosimetry is the non-water-equivalent response which demands extra commissioning steps and software corrections to effectively model the dose response characteristics of the EPID imager [87], [88]. Although research efforts have been made to modify current EPID designs to make them more water-equivalent [89], [90], [91], [92], none of these configurations have been adopted for clinical use yet. One of the reasons is that EPID technology developments over recent decades have not been driven by the demands of IVD, but by the needs for improved patient positioning.
5.6. Other IVD systems
In this section, several new developments of IVD in radiotherapy are discussed using systems other than EIVD. These systems need further investigation before they can be used with the required accuracy.
The use of Gafchromic film for in vivo entrance dose determination can be advantageous compared to point detectors if a higher resolution is needed. Gafchromic film can furthermore be used to determine the skin dose when introducing a new treatment technique or during total skin electron therapy. Several studies have also shown the usefulness of Gafchromic film during intraoperative radiotherapy, both with electron beams and with low-energy X-ray beams [93]. Improvements regarding film reading characteristics are still needed to increase the accuracy of dose measurements for these types of IVD measurements.
PRESAGE, which is an optically transparent radiochromic dosimeter, has a comparable resolution to Gafchromic film. PRESAGE sheets have the same dosimetric capability as Gafchromic film but are softer and more flexible to conform to the patient’s skin, which makes them in principle a valuable tool for IVD [94], [95]. However, currently there is only limited experience with PRESAGE sheets.
Recently, Cherenkov imaging has been shown to allow high-resolution, video-rate imaging of radiation delivery to tissue using a gated camera system. The Cherenkov emission can be used to evaluate the surface dose received by the patient in real time. However, the relationship between the video signal and the actual in vivo dose is complex. Cherenkov radiation emission in radiotherapy is affected by tissue optical properties (e.g., pigmentation, thickness of tissue), entrance/exit geometry, and imaging angles. Despite the limitations of Cherenkov imaging, several interesting applications have been reported. These include in vivo surface dose measurements during total skin electron therapy [96] and imaging during breast treatments to monitor beam shape in real time on the patient’s skin throughout the treatment [97].
Recent in vivo investigations have shown that short pulses of radiation at very-high-dose rates (several hundred Gy/s) are less harmful to healthy tissue but just as efficient as conventional dose-rate radiation at inhibiting tumor growth. A first patient treatment using this so-called FLASH effect has recently been described [98]. IVD during FLASH radiotherapy is strongly recommended due to the uncertainties in beam calibration and beam monitoring. It seems, therefore, worthwhile to investigate whether existing IVD detectors can be used for this purpose after determination of their dosimetric characteristics in very-high-dose-rate fields [99], [100], [101].
IVD should also be accepted as an essential part of translational and preclinical research, where the dose uncertainties are often large [102]. The potential use of EIVD systems in a similar way to that used during patient EBRT treatments has been reported for the verification of small animal kilovoltage X-ray irradiations [103], [104], but this needs further investigation.
Finally, it should be noted that there are several EBRT systems where IVD is not used such as the Cyberknife, Tomotherapy, Gamma Knife and ViewRay MR-linac. Also in proton therapy IVD is not used routinely, although various techniques for in vivo verification of the delivered dose or the beam range have been proposed and in some cases already clinically investigated [105]. For these EBRT systems new techniques for IVD should be developed.
6. Discussion
This report has documented IVD methods used in EBRT, focusing on EPID-based approaches. The report provides detailed requirements to vendors for overall EIVD system performance, as well as for the EPID imager, image acquisition software, and IVD analysis software. Further recommendations are made for vendors and researchers to improve the understanding and reduce the uncertainties in IVD systems and to estimate the sensitivity and specificity of the system. Finally, the report recommends that automation of all aspects of EIVD is needed to help facilitate clinical adoption, including automation of image acquisition, analysis, result interpretation, and reporting/documentation.
In some cases, requirements are stated without any numerical specifications. This is because specific tolerance values for some of the criteria will depend on the application. A few of these requirements are elementary and are being satisfied by today’s technology, while others are not available yet. The goal of this report is to raise awareness for the need of each of the proposed requirements and to prompt users of EIVD systems to ask vendors for details about their fulfilment, using quantitative information whenever applicable. An essential requirement that has been identified for the seamless integration of the different subsystems is the use of open, free and non-proprietary formats and interfaces, e.g. Requirement S3.11. The fulfilment of this requirement would simplify the implementation of EIVD systems and would allow researchers to gain easy access to EPID data in their studies. Another example would be Requirement S3.8 where it is stated that “each integrated image and each cine mode image frame must be tagged with real-time treatment delivery information”. But in order to achieve this, linac manufacturers must provide an open interface for access to the raw portal image data and related real-time information which would be beneficial to both potential new vendors of MV acquisition software and researchers.
Task Group 307 (TG307) has been recently formed by the American Association of Physicists in Medicine (AAPM) to review the use of EPIDs for Patient-Specific IMRT and VMAT QA. This task group aims to provide an extensive review on existing EPID products, methods and algorithms for both pre-treatment and in vivo dosimetric verification. It is hoped that the proposed requirements in this EBRT IVD report will not only complement the ongoing TG307 work, but will also contribute to raise the awareness of the community about the required capabilities of EIVD systems and inspire further related work. For example, it may lead to recommendations for a set of customer acceptance tests to evaluate and compare the basic performance of EIVD systems to detect errors.
In practice, and owing to the presence of false positives and false negatives, EIVD systems are typically implemented in combination with other patient-specific QA systems. False negatives are of particular importance because they hide relevant errors that are not being detected [82]. To detect these errors, EIVD must be used in combination with image-guided radiation therapy procedures such as CBCT visualization and/or CBCT-based dose calculations. Similarly, the use of linac log files or transmission detectors mounted on the linac head may show additional value in the detection of, or confirmation of the absence of, machine delivery errors. Other QA systems, such as 2D/3D detector matrices or independent dose calculations, can help to discriminate between false and true positives during the inspection of EIVD alerts (see Fig. 2). Table S8 lists several commercial systems that may complement EIVD methods. Finally, note that the sensitivity and specificity of EIVD highly conditions the feasibility of clinical workflows. For instance, if a specific EIVD system is very sensitive to changes in patient anatomy for a specific treatment site, then EIVD assessments could be used to trigger the acquisition of CBCTs in case that these are not acquired daily.
One aspect that has received little or no attention so far is auditing of IVD systems by an external organization, possibly with dedicated phantoms. This may require customized audits for specific EIVD systems. An interesting test could be to combine IVD dosimetry with a regular dosimetric audit when the auditing phantom is irradiated according to the auditing protocol. This would provide information about the agreement between IVD, the audit result, and the TPS for that specific treatment technique. This would only be relevant for IVD methods that reconstruct dose in a phantom.
Finally, it is hoped that widespread clinical use of IVD for EBRT will be significantly accelerated with the guidance of this report.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: I Olaciregui-Ruiz and B Mijnheer declare that their department licensed portal dosimetry software to Elekta Oncology Systems Ltd. for the development of the iViewDose product. This product is currently not commercially available. P Greer declares research collaborations with Varian Medical Systems. B McCurdy declares funded research collaborations on EPID dosimetry with Varian Medical Systems. N. Jornet declares that she is member of the European scientific advisory board of Sun Nuclear. F Verhaegen declares research collaborations and a patent on in vivo dosimetry with Varian Medical Systems.
Acknowledgments
The final version of the manuscript was shared with an advisory group consisting of vendor representatives and selected academic scholars. The authors would like to thank Jie Shi, Christof Baltes, François Husson, Eric DeWerd, Wouter van Elmpt and other members of the advisory board for their advice and comments. The authors, however, remain solely responsible for the contents of this report.
Footnotes
During the 1st ESTRO Physics Workshop celebrated in November 2017 in Glasgow, Scotland, a task group was created to stimulate the wider adoption of in vivo dosimetry for external beam photon radiotherapy. The members of this task group, authors of this report, were selected on the basis of their expertise to contribute relevant input to the area of study and their long-term experience in the clinical implementation of in vivo dosimetry systems.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.phro.2020.08.003.
Appendix A. Supplementary data
References
- 1.Mijnheer B., Beddar S., Izewska J., Reft C. In vivo dosimetry in external beam radiotherapy. Med Phys. 2013;40 doi: 10.1118/1.4811216. [DOI] [PubMed] [Google Scholar]
- 2.MacDougall N.D., Graveling M., Hansen V.N., Brownsword K., Morgan A. In vivo dosimetry in UK external beam radiotherapy: Current and future usage. Br J Radiol. 2017;90:1–14. doi: 10.1259/bjr.20160915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miften M, Olch A, Mihailidis D, Moran J, Pawlicki T, Molineu A, et al. Tolerance limits and methodologies for IMRT measurement-based verification QA: Recommendations of AAPM Task Group No. 218. Med Phys 2018;45:e53-83. doi: 10.1002/mp.12810. [DOI] [PubMed]
- 4.International Atomic Energy Agency. Development of Procedures for In Vivo Dosimetry in Radiotherapy. IAEA Human Health Report No. 8. Vienna: International Atomic Energy Agency; 2013. doi: 10.1016/0167-8140(90)90102-3.
- 5.Esposito M., Villaggi E., Bresciani S., Cilla S., Daniela Falco M., Garibaldi C. Estimating dose delivery accuracy in stereotactic body radiation therapy: a review of in-vivo measurement methods. Radiother Oncol. 2020 doi: 10.1016/j.radonc.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Olaciregui-Ruiz I., Vivas-Maiques B., Kaas J., Perik T., Wittkamper F., Mijnheer B. Transit and non-transit 3D EPID dosimetry versus detector arrays for patient specific QA. J Appl Clin Med Phys. 2019;20(6):79–90. doi: 10.1002/acm2.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mijnheer B.J., González P., Olaciregui-Ruiz I., Rozendaal R.A., van Herk M., Mans A. Overview of 3-year experience with large-scale electronic portal imaging device-based 3-dimensional transit dosimetry. Pract Radiat Oncol. 2015;5:e679–e687. doi: 10.1016/j.prro.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Celi S., Costa E., Wessels C., Mazal A., Fourquet A., Francois P. EPID based in vivo dosimetry system: clinical experience and results. J Appl Clin Med Phys. 2016;17(3):262–276. doi: 10.1120/jacmp.v17i3.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidanzio A., Azario L., Greco F., Cilla S., Piermattei A. Routine EPID in-vivo dosimetry in a reference point for conformal radiotherapy treatments. Phys Med Biol. 2015;60:N141–N150. doi: 10.1088/0031-9155/60/8/N141. [DOI] [PubMed] [Google Scholar]
- 10.McCurdy B.M.C., McCowan P.M. In vivo dosimetry for lung radiotherapy including SBRT. Phys Medica. 2017;44:123–130. doi: 10.1016/j.ejmp.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 11.van Elmpt W., McDermott L., Nijsten S., Wendling M., Lambin P., Mijnheer B. A literature review of electronic portal imaging for radiotherapy dosimetry. Radiother Oncol. 2008;88:289–309. doi: 10.1016/j.radonc.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 12.McCurdy B., Greer P., Bedford J. Electronic portal imaging device dosimetry. In: Mijnheer B., editor. Clinical 3D dosimetry in modern radiation therapy. CRC Press; Boca Raton, FL: 2017. pp. 169–198. [Google Scholar]
- 13.Jursinic P.A., Yahnke C.J. In vivo dosimetry with optically stimulated luminescent dosimeters, OSLDs, compared to diodes; The effects of buildup cap thickness and fabrication material. Med Phys. 2011;38:5432–5440. doi: 10.1118/1.3633939. [DOI] [PubMed] [Google Scholar]
- 14.Archambault L., Briere T.M., Pönisch F., Beaulieu L., Kuban D.A., Lee A. Toward a real-time in vivo dosimetry system using plastic scintillation detectors. Int J Radiat Oncol Biol Phys. 2010;78:280–287. doi: 10.1016/j.ijrobp.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wootton L., Kudchadker R., Lee A., Beddar S. Real-time in vivo rectal wall dosimetry using plastic scintillation detectors for patients with prostate cancer. Phys Med Biol. 2014;59:647–660. doi: 10.1088/0031-9155/59/3/647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jornet N., Carrasco P., Jurado D., Ruiz A., Eudaldo T., Ribas M. Comparison study of MOSFET detectors and diodes for entrance in vivo dosimetry in 18 MV x-ray beams. Med Phys. 2004;31:2534–2542. doi: 10.1118/1.1785452. [DOI] [PubMed] [Google Scholar]
- 17.Beaulieu L., Beddar S. Review of plastic and liquid scintillation dosimetry for photon, electron, and proton therapy. Phys Med Biol. 2016;61:R305–R343. doi: 10.1088/0031-9155/61/20/R305. [DOI] [PubMed] [Google Scholar]
- 18.Beddar A.S., Mackie T.R., Attix F.H. Water-equivalent plastic scintillation detectors for high-energy beam dosimetry: II. Properties and measurements. Phys Med Biol. 1992;37:1901–1913. doi: 10.1088/0031-9155/37/10/007. [DOI] [PubMed] [Google Scholar]
- 19.Beddar A.S., Mackie T.R., Attix F.H. Water-equivalent plastic scintillation detectors for high-energy beam dosimetry: I. Physical characteristics and theoretical considerations. Phys Med Biol. 1992;37(1883–900) doi: 10.1088/0031-9155/37/10/006. [DOI] [PubMed] [Google Scholar]
- 20.Huyskens D, Bogaerts R, Verstraete J, Lööf M, Nyström H et al. Practical Guidelines for the Implementation of In Vivo Dosimetry with Diodes in External Radiotherapy with Photon Beams (Entrance Dose). ESTRO Booklet No. 5. Brussels: European Society for Radiotherapy and Oncology; 2001.
- 21.Costa A, Lisbona A, Noel A. Guide pour la mise en œuvre en radiothérapie externe de l’assurance de qualité par mesures in vivo par dosimètres thermoluminescents et semi-conducteurs. Rapport SFPM no. 18-2000. Paris: Société Française de Physique Médicale; 2000:5-26.
- 22.Fiorino C., Corletto D., Mangili P., Broggi S., Bonini A., Cattaneo G.M. Quality assurance by systematic in vivo dosimetry: Results on a large cohort of patients. Radiother Oncol. 2000;56:85–95. doi: 10.1016/S0167-8140(00)00195-X. [DOI] [PubMed] [Google Scholar]
- 23.Klein E.E., Drzymala R.E., Purdy J.A., Michalski J. Errors in radiation oncology: a study in pathways and dosimetric impact. J Appl Clin Med Phys. 2005;6(3):81–94. doi: 10.1120/jacmp.v6i3.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dam J, Marinello G. Methods for in Vivo Dosimetry in External Radiotherapy. ESTRO Booklet No. 1, First edition: 1994 Second edition: 2006. Brussels: European Society for Radiotherapy and Oncology; 2006.
- 25.Chuang C.F., Verhey L.J., Xia P. Investigation of the use of MOSFET for clinical IMRT dosimetric verification. Med Phys. 2002;29:1109–1115. doi: 10.1118/1.1481520. [DOI] [PubMed] [Google Scholar]
- 26.Kadesjö N., Nyholm T., Olofsson J. A practical approach to diode based in vivo dosimetry for intensity modulated radiotherapy. Radiother Oncol. 2011;98:378–381. doi: 10.1016/j.radonc.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Tariq M., Gomez C., Riegel A.C. Dosimetric impact of placement errors in optically stimulated luminescent in vivo dosimetry in radiotherapy. Phys Imag Radiat Oncol. 2019;11:63–68. doi: 10.1016/j.phro.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briere T.M., Gillin M.T., Beddar A.S. Implantable MOSFET detectors: evaluation of a new design. Med Phys. 2007;34:4585–4590. doi: 10.1118/1.2799578. [DOI] [PubMed] [Google Scholar]
- 29.Beyer G.P., Scarantino C.W., Prestidge B.R., Sadeghi A.G., Anscher M.S., Miften M. Technical evaluation of radiation dose delivered in prostate cancer patients as measured by an implantable MOSFET dosimeter. Int J Radiat Oncol Biol Phys. 2007;69:925–935. doi: 10.1016/j.ijrobp.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 30.Miften M., Mihailidis D., Kry S.F., Reft C., Esquivel C., Farr J. Management of radiotherapy patients with implanted cardiac pacemakers and defibrillators: a report of the AAPM TG-203. Med Phys. 2019;46:e757–e788. doi: 10.1002/mp.13838. [DOI] [PubMed] [Google Scholar]
- 31.Duch M.A., Ginjaume M., Chakkor H., Ortega X., Jornet N., Ribas M. Thermoluminescence dosimetry applied to in vivo dose measurements for total body irradiation techniques. Radiother Oncol. 1998;47:319–324. doi: 10.1016/S0167-8140(98)00013-9. [DOI] [PubMed] [Google Scholar]
- 32.Ribas M., Jornet N., Eudaldo T., Carabante D., Duch M.A., Ginjaume M. Midplane dose determination during total body irradiation using in vivo dosimetry. Radiother Oncol. 1998;49:91–98. doi: 10.1016/S0167-8140(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 33.Soriani A., Landoni V., Marzi S., Iaccarino G., Saracino B., Arcangeli G. Setup verification and in vivo dosimetry during intraoperative radiation therapy (IORT) for prostate cancer. Med Phys. 2007;34:3205–3210. doi: 10.1118/1.2750965. [DOI] [PubMed] [Google Scholar]
- 34.Bloemen-van Gurp E.J., Mijnheer B.J., Verschueren T.A.M., Lambin P. Total body irradiation, toward optimal individual delivery: dose evaluation with metal oxide field effect transistors, thermoluminescence detectors, and a treatment planning system. Int J Radiat Oncol Biol Phys. 2007;69:1297–1304. doi: 10.1016/j.ijrobp.2007.07.2334. [DOI] [PubMed] [Google Scholar]
- 35.Swinnen A., Verstraete J., Huyskens D.P. Feasibility study of entrance in vivo dose measurements with mailed thermoluminescence detectors. Radiother Oncol. 2004;73:89–96. doi: 10.1016/j.radonc.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Greer P.B., Popescu C.C. Dosimetric properties of an amorphous silicon electronic portal imaging device for verification of dynamic intensity modulated radiation therapy. Med Phys. 2003;30:1618–1627. doi: 10.1118/1.1582469. [DOI] [PubMed] [Google Scholar]
- 37.McDermott L.N., Louwe R.J.W., Sonke J.-J., van Herk M.B., Mijnheer B.J. Dose-response and ghosting effects of an amorphous silicon electronic portal imaging device. Med Phys. 2004;31:285–295. doi: 10.1118/1.1637969. [DOI] [PubMed] [Google Scholar]
- 38.Winkler P., Hefner A., Georg D. Implementation and validation of portal dosimetry with an amorphous silicon EPID in the energy range from 6 to 25 MV. Phys Med Biol. 2007;52:N355–N365. doi: 10.1088/0031-9155/52/15/N05. [DOI] [PubMed] [Google Scholar]
- 39.Greer P.B. Correction of pixel sensitivity variation and off-axis response for amorphous silicon EPID dosimetry. Med Phys. 2005;32:3558–3568. doi: 10.1118/1.2128498. [DOI] [PubMed] [Google Scholar]
- 40.McCowan P.M., Asuni G., Van Beek T., Van Uytven E., Kujanpaa K., McCurdy B.M.C. A model-based 3D patient-specific pre-treatment QA method for VMAT using the EPID. Phys Med Biol. 2017;62:1600–1612. doi: 10.1088/1361-6560/aa590a. [DOI] [PubMed] [Google Scholar]
- 41.Bedford J.L., Hanson I.M., Hansen V.N. Portal dosimetry for VMAT using integrated images obtained during treatment. Med Phys. 2014;41:021725–21814. doi: 10.1118/1.4862515. [DOI] [PubMed] [Google Scholar]
- 42.Wendling M., McDermott L.N., Mans A., Sonke J.-J., van Herk M., Mijnheer B.J. A simple backprojection algorithm for 3D in vivo EPID dosimetry of IMRT treatments. Med Phys. 2009;36:3310–3321. doi: 10.1118/1.3148482. [DOI] [PubMed] [Google Scholar]
- 43.Mans A., Remeijer P., Olaciregui-Ruiz I., Wendling M., Sonke J.-J., Mijnheer B. 3D dosimetric verification of volumetric-modulated arc therapy by portal dosimetry. Radiother Oncol. 2010;94:181–187. doi: 10.1016/j.radonc.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 44.Persoon LCGG, Podesta M, Nijsten SMJJG, Troost EGC, Verhaegen F. Time-Resolved Versus Integrated Transit Planar Dosimetry for Volumetric Modulated Arc Therapy: Patient-Specific Dose Differences During Treatment, a Proof of Principle. Technol Cancer Res Treat 2016;15:NP79-N87. doi: 10.1177/1533034615617668. [DOI] [PubMed]
- 45.van Elmpt W., Nijsten S., Petit S., Mijnheer B., Lambin P., Dekker A. 3D in vivo dosimetry using megavoltage cone-beam CT and EPID dosimetry. Int J Radiat Oncol Biol Phys. 2009;73:1580–1587. doi: 10.1016/j.ijrobp.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 46.Berry S.L., Polvorosa C., Cheng S., Deutsch I., Chao K.S.C., Wuu C.S. Initial clinical experience performing patient treatment verification with an electronic portal imaging device transit dosimeter. Int J Radiat Oncol Biol Phys. 2014;88:204–209. doi: 10.1016/j.ijrobp.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 47.Van Uytven E., Van Beek T., McCowan P.M., Chytyk-Praznik K., Greer P.B., McCurdy B.M.C. Validation of a method for in vivo 3D dose reconstruction for IMRT and VMAT treatments using on-treatment EPID images and a model-based forward-calculation algorithm. Med Phys. 2015;42:6945–6954. doi: 10.1118/1.4935199. [DOI] [PubMed] [Google Scholar]
- 48.Nailon W.H., Welsh D., McDonald K., Burns D., Forsyth J., Cooke G. EPID-based in vivo dosimetry using Dosimetry CheckTM: Overview and clinical experience in a 5-yr study including breast, lung, prostate, and head and neck cancer patients. J Appl Clin Med Phys. 2019;20(1):6–16. doi: 10.1002/acm2.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bedford J.L., Hanson I.M., Hansen V.N. Comparison of forward- and back-projection in vivo EPID dosimetry for VMAT treatment of the prostate. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aa9c60. [DOI] [PubMed] [Google Scholar]
- 50.Cilla S., Ianiro A., Craus M., Viola P., Deodato F., Macchia G. Epid-based in vivo dose verification for lung stereotactic treatments delivered with multiple breath-hold segmented volumetric modulated arc therapy. J Appl Clin Med Phys. 2019;20(3):37–44. doi: 10.1002/acm2.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodruff H.C., Fuangrod T., Van Uytven E., McCurdy B.M.C., Van Beek T., Bhatia S. First experience with real-time EPID-based delivery verification during IMRT and VMAT sessions. Int J Radiat Oncol Biol Phys. 2015;93:516–522. doi: 10.1016/j.ijrobp.2015.07.2271. [DOI] [PubMed] [Google Scholar]
- 52.Spreeuw H., Rozendaal R., Olaciregui-Ruiz I., González P., Mans A., Mijnheer B. Online 3D EPID-based dose verification: proof of concept. Med Phys. 2016;43:3969–3974. doi: 10.1118/1.4952729. [DOI] [PubMed] [Google Scholar]
- 53.Chytyk-Praznik K., Vanuytven E., Vanbeek T.A., Greer P.B., McCurdy B.M.C. Model-based prediction of portal dose images during patient treatment. Med Phys. 2013;40 doi: 10.1118/1.4792203. [DOI] [PubMed] [Google Scholar]
- 54.Juste B., Miró R., Díez S., Campayo J.M., Verdú G. Dosimetric capabilities of the Iview GT portal imager using MCNP5 Monte Carlo simulations. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3743–3746. doi: 10.1109/IEMBS.2009.5334899. [DOI] [PubMed] [Google Scholar]
- 55.Yoon J., Jung J.W., Kim J.O., Yeo I. A Monte Carlo calculation model of electronic portal imaging device for transit dosimetry through heterogeneous media. Med Phys. 2016;43:2242. doi: 10.1118/1.4945276. [DOI] [PubMed] [Google Scholar]
- 56.Nijsten S.M.J.J.G., van Elmpt W.J.C., Jacobs M., Mijnheer B.J., Dekker A.L.A.J., Lambin P. A global calibration model for a-Si EPIDs used for transit dosimetry. Med Phys. 2007;34:3872–3884. doi: 10.1118/1.2776244. [DOI] [PubMed] [Google Scholar]
- 57.Podesta M., Nijsten S.M.J.J.G., Persoon L.C.G.G., Scheib S.G., Baltes C., Verhaegen F. Time dependent pre-treatment EPID dosimetry for standard and FFF VMAT. Phys Med Biol. 2014;59:4749–4768. doi: 10.1088/0031-9155/59/16/4749. [DOI] [PubMed] [Google Scholar]
- 58.Podesta M., Persoon L.C.G.G., Verhaegen F. A novel time dependent gamma evaluation function for dynamic 2D and 3D dose distributions. Phys Med Biol. 2014;59:5973–5985. doi: 10.1088/0031-9155/59/20/5973. [DOI] [PubMed] [Google Scholar]
- 59.McDermott L.N., Wendling M., Nijkamp J., Mans A., Sonke J.-J., Mijnheer B.J. 3D in vivo dose verification of entire hypo-fractionated IMRT treatments using an EPID and cone-beam CT. Radiother Oncol. 2008;86:35–42. doi: 10.1016/j.radonc.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 60.Olaciregui-Ruiz I., Rozendaal R., van Kranen S., Mijnheer B., Mans A. The effect of the choice of patient model on the performance of in vivo 3D EPID dosimetry to detect variations in patient position and anatomy. Med Phys. 2019;47:171–180. doi: 10.1002/mp.13893. [DOI] [PubMed] [Google Scholar]
- 61.Wendling M., Louwe R.J.W., McDermott L.N., Sonke J.-J., van Herk M., Mijnheer B.J. Accurate two-dimensional IMRT verification using a back-projection EPID dosimetry method. Med Phys. 2006;33:259–273. doi: 10.1118/1.2147744. [DOI] [PubMed] [Google Scholar]
- 62.Wendling M., McDermott L.N., Mans A., Olaciregui-Ruiz I., Pecharromán-Gallego R., Sonke J.-J. In aqua vivo EPID dosimetry. Med Phys. 2012;39:367–377. doi: 10.1118/1.3665709. [DOI] [PubMed] [Google Scholar]
- 63.Sterckx B., Steinseifer I., Wendling M. In vivo dosimetry with an electronic portal imaging device for prostate cancer radiotherapy with an endorectal balloon. Phys Imag Radiat Oncol. 2019;12:7–9. doi: 10.1016/j.phro.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piermattei A., Fidanzio A., Azario L., Grimaldi L., D’Onofrio G., Cilla S. Application of a practical method for the isocenter point in vivo dosimetry by a transit signal. Phys Med Biol. 2007;52:5101–5117. doi: 10.1088/0031-9155/52/16/026. [DOI] [PubMed] [Google Scholar]
- 65.Francois P., Boissard P., Berger L., Mazal A. In vivo dose verification from back projection of a transit dose measurement on the central axis of photon beams. Phys Medica. 2011;27:1–10. doi: 10.1016/j.ejmp.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 66.van Elmpt W., Nijsten S., Mijnheer B., Dekker A., Lambin P. The next step in patient-specific QA: 3D dose verification of conformal and intensity-modulated RT based on EPID dosimetry and Monte Carlo dose calculations. Radiother Oncol. 2008;86:86–92. doi: 10.1016/j.radonc.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Jarry G., Verhaegen F. Patient-specific dosimetry of conventional and intensity modulated radiation therapy using a novel full Monte Carlo phase space reconstruction method from electronic portal images. Phys Med Biol. 2007;52:2277–2299. doi: 10.1088/0031-9155/52/8/016. [DOI] [PubMed] [Google Scholar]
- 68.Li J., Piermattei A., Wang P., Kang S., Xiao M., Tang B. Setup in a clinical workflow and results of in vivo dosimetry procedure in an overload radiation therapy department. Int J Radiat Oncol Biol Phys. 2017;99:E736. doi: 10.1016/j.ijrobp.2017.06.2371. [DOI] [Google Scholar]
- 69.Olaciregui-Ruiz I, Rozendaal R, Mijnheer B, van Herk M, Mans a. Automatic in vivo portal dosimetry of all treatments. Phys Med Biol 2013;58:8253-64. doi: 10.1088/0031-9155/58/22/8253. [DOI] [PubMed]
- 70.Piermattei A., Greco F., Grusio M., Menna S., Azario L., Stimato G. A validation study of a dedicated software for an automated in vivo dosimetry control in radiotherapy. Med Biol Eng Comput. 2018;56:1939–1947. doi: 10.1007/s11517-018-1822-3. [DOI] [PubMed] [Google Scholar]
- 71.Hanson I.M., Hansen V.N., Olaciregui-Ruiz I., Van Herk M. Clinical implementation and rapid commissioning of an EPID based in-vivo dosimetry system. Phys Med Biol. 2014;59:N171–N179. doi: 10.1088/0031-9155/59/19/N171. [DOI] [PubMed] [Google Scholar]
- 72.Chuter R.W., Rixham P.A., Weston S.J., Cosgrove V.P. Feasibility of portal dosimetry for flattening filter-free radiotherapy. J Appl Clin Med Phys. 2016;17(1):112–120. doi: 10.1120/jacmp.v17i1.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Held M., Cheung J., Perez Andujar A., Husson F., Morin O. Commissioning and evaluation of an electronic portal imaging device-based in-vivo dosimetry software. Cureus. 2018:10. doi: 10.7759/cureus.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karlsson M, Georg, D. Ahnesjö A. Nyholm T OJ. Independent Dose Calculations: Concepts and Models. ESTRO Booklet 10. Brussels: European Society for Radiotherapy and Oncology; 2010.
- 75.Mijnheer B. Patient specific QA: in vivo 3D dose verification. In: Mijnheer B., editor. Clinical 3D dosimetry in modern radiation therapy. CRC Press; Boca Raton, FL: 2017. pp. 461–490. [Google Scholar]
- 76.Bojechko C., Phillps M., Kalet A., Ford E.C. A quantification of the effectiveness of EPID dosimetry and software-based plan verification systems in detecting incidents in radiotherapy. Med Phys. 2015;42:5363–5369. doi: 10.1118/1.4928601. [DOI] [PubMed] [Google Scholar]
- 77.Carlone M., Cruje C., Rangel A., McCabe R., Nielsen M., MacPherson M. ROC analysis in patient specific quality assurance. Med Phys. 2013;40:1–7. doi: 10.1118/1.4795757. [DOI] [PubMed] [Google Scholar]
- 78.Bedford J.L., Chajecka-Szczygielska H., Thomas M.D.R. Quality control of VMAT synchronization using portal imaging. J Appl Clin Med Phys. 2015;16(1):284–297. doi: 10.1120/jacmp.v16i1.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mijnheer B., Jomehzadeh A., Gonzalez P., Olaciregui-Ruiz I., Rozendaak R., Shokrani P. Error detection during VMAT delivery using EPID-based 3D transit dosimetry. Phys Medica. 2018;54:137–145. doi: 10.1016/j.ejmp.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 80.Zhuang A.H., Olch A.J. Sensitivity study of an automated system for daily patient QA using EPID exit dose images. J Appl Clin Med Phys. 2018;19(3):114–124. doi: 10.1002/jacm2.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piron O., Varfalvy N., Archambault L. Establishing action threshold for change in patient anatomy using EPID gamma analysis and PTV coverage for head and neck radiotherapy treatment. Med Phys. 2018;45:3534–3545. doi: 10.1002/mp.13045. [DOI] [PubMed] [Google Scholar]
- 82.Olaciregui-Ruiz I., Rozendaal R., Mijnheer B., Mans A. Site-specific alert criteria to detect patient-related errors with 3D EPID transit dosimetry. Med Phys. 2019;46:45–55. doi: 10.1002/mp.13265. [DOI] [PubMed] [Google Scholar]
- 83.Bojechko C., Ford E.C. Quantifying the performance of in vivo portal dosimetry in detecting four types of treatment parameter variations. Med Phys. 2015;42:6912–6918. doi: 10.1118/1.4935093. [DOI] [PubMed] [Google Scholar]
- 84.Wootton L.S., Nyflot M.J., Chaovalitwongse W.A., Ford E. Error detection in intensity-modulated radiation therapy quality assurance using radiomic analysis of gamma distributions. Int J Radiat Oncol Biol Phys. 2018;102:219–228. doi: 10.1016/j.ijrobp.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 85.Varfalvy N., Piron O., Cyr M.F., Dagnault A., Archambault L. Classification of changes occurring in lung patient during radiotherapy using relative γ analysis and hidden Markov models. Med Phys. 2017;44:5043–5050. doi: 10.1002/mp.12488. [DOI] [PubMed] [Google Scholar]
- 86.Lam D., Zhang X., Li H., Deshan Y., Schott B., Zhao T. Predicting gamma passing rates for portal dosimetry-based IMRT QA using machine learning. Med Phys. 2019;46:4666–4675. doi: 10.1002/mp.13752. [DOI] [PubMed] [Google Scholar]
- 87.Kirkby C., Sloboda R. Consequences of the spectral response of an a-Si EPID and implications for dosimetric calibration. Med Phys. 2005;32:2649–2658. doi: 10.1118/1.1984335. [DOI] [PubMed] [Google Scholar]
- 88.Deshpande S., Xing A., Holloway L., Metcalfe P., Vial P. Dose calibration of EPIDs for segmented IMRT dosimetry. J Appl Clin Med Phys. 2014;15(6):103–118. doi: 10.1120/jacmp.v15i6.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blake S.J., McNamara A.L., Deshpande S., Holloway L., Greer P.B., Kuncic Z. Characterization of a novel EPID designed for simultaneous imaging and dose verification in radiotherapy. Med Phys. 2013;40:1–11. doi: 10.1118/1.4816657. [DOI] [PubMed] [Google Scholar]
- 90.Sabet M., Menk F.W., Greer P.B. Evaluation of an a-Si EPID in direct detection configuration as a water-equivalent dosimeter for transit dosimetry. Med Phys. 2010;37:1459–1467. doi: 10.1118/1.3327456. [DOI] [PubMed] [Google Scholar]
- 91.Vial P., Gustafsson H., Oliver L., Baldock C., Greer P.B. Direct-detection EPID dosimetry: investigation of a potential clinical configuration for IMRT verification. Phys Med Biol. 2009;54:7151–7169. doi: 10.1088/0031-9155/54/23/008. [DOI] [PubMed] [Google Scholar]
- 92.Deshpande S., McNamara A.L., Holloway L., Metcalfe P., Vial P. Feasibility study of a dual detector configuration concept for simultaneous megavoltage imaging and dose verification in radiotherapy. Med Phys. 2015;42:1753–1764. doi: 10.1118/1.4907966. [DOI] [PubMed] [Google Scholar]
- 93.Das IJ. Radiochromic Film: Role and Applications In Radiation Dosimetry. Boca Raton, FL: CRC Press;2017. doi: 10.1201/b20964.
- 94.Collins C., Kodra J., Yoon S.W., Coakley R., Adamovics J., Oldham M. Preliminary investigation of a reusable radiochromic sheet for radiation dosimetry. J Phys Conf Ser. 2019;1305 doi: 10.1088/1742-6596/1305/1/012032. [DOI] [Google Scholar]
- 95.Wang Y.-F., Liu K., Adamovics J., Wuu C.-S. An Investigation of dosimetric accuracy of a novel PRESAGE radiochromic sheet and its clinical applications. J Phys Conf Ser. 2019;1305 doi: 10.1088/1742-6596/1305/1/012041. [DOI] [Google Scholar]
- 96.Zhu T.C. Cherenkov imaging of total skin electron irradiation (TSEI) J Phys Conf Ser. 2019;1305 doi: 10.1088/1742-6596/1305/1/012016. [DOI] [Google Scholar]
- 97.Pogue B.W., Zhang R., Glaser A., Andreozzi J.M., Bruza P., Gladstone D.J. Cherenkov imaging in the potential roles of radiotherapy QA and delivery. J Phys Conf Ser. 2017;847 doi: 10.1088/1742-6596/847/1/012046. [DOI] [Google Scholar]
- 98.Bourhis J., Sozzi W.J., Jorge P.G., Gaide O., Bailat C., Duclos F. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 2019;139:18–22. doi: 10.1016/j.radonc.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 99.Jorge P.G., Jaccard M., Petersson K., Gondré M., Durán M.T., Desorgher L. Dosimetric and preparation procedures for irradiating biological models with pulsed electron beam at ultra-high dose-rate. Radiother Oncol. 2019;139:34–39. doi: 10.1016/j.radonc.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 100.Jarry G., Verhaegen F. TH-C-J-6B-03: electron beam treatment verification using measured and Monte Carlo predicted portal images. Med Phys. 2005;50:4977–4994. doi: 10.1118/1.1998647. [DOI] [PubMed] [Google Scholar]
- 101.Schüler E., Trovati S., King G., Lartey F., Rafat M., Villegas M. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int J Radiat Oncol Biol Phys. 2017;97:195–203. doi: 10.1016/j.ijrobp.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 102.Verhaegen F., Dubois L., Gianolini S., Hill M.A., Karger C.P., Lauber K. ESTRO ACROP: technology for precision small animal radiotherapy research: Optimal use and challenges. Radiother Oncol. 2018;126:471–478. doi: 10.1016/j.radonc.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 103.Granton P.V., Podesta M., Landry G., Nijsten S., Bootsma G., Verhaegen F. A combined dose calculation and verification method for a small animal precision irradiator based on onboard imaging. Med Phys. 2012;39:4155–4166. doi: 10.1118/1.4725710. [DOI] [PubMed] [Google Scholar]
- 104.Anvari A., Poirier Y., Sawant A. Kilovoltage transit and exit dosimetry for a small animal image-guided radiotherapy system using built-in EPID. Med Phys. 2018;45:4642–4651. doi: 10.1002/mp.13134. [DOI] [PubMed] [Google Scholar]
- 105.Parodi K. Dose verification of proton and carbon ion beam treatments. In: Mijnheer B, ed. Clinical 3D Dosimetry in Modern Radiation Therapy. Boca Raton, FL: CRC Press; 2017, p. 589-613.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.