Abstract
Background and purpose
Functional avoidance radiation therapy (RT) aims at sparing functional lung regions. The purpose of this simulation study was to evaluate the feasibility of functional lung avoidance methodology in RT of lung cancer and to characterize the achievable dosimetry of single photon emission computed tomography (SPECT) guided treatment planning.
Materials and methods
Fifteen consecutive lung cancer patients were included and planned for definitive RT of 60–66 Gy in 2-Gy fractions. Two plans were optimized: a standard CT-plan, and functional SPECT-plan. The objective was to reduce dose to the highly functional lung subvolumes without compromising tumour coverage, and respecting dose to other organs at risk. For each patient a 3D-conformal, intensity modulated radiation therapy (IMRT) and volumetric modulated arc therapy plans were created for standard and functional avoidance. Standard versus functional dose-volume parameters for functional lung (FL) subvolumes, organs at risk and tumour coverage were compared.
Results
The largest dose reduction was achieved with IMRT plans. Functional plans resulted in dose reduction from 9.0 Gy to 6.7 Gy (mean reduction of 2.3 Gy or 26%) to the highest functional subvolume FL80% (95%CI 1.1; 3.5). Dose to FL40% was reduced from 13.3 Gy to 11.6 Gy with functional planning. Dose reduction to FL40% was 1.7 Gy (95%CI 0.9; 2.6). Functional volume of lung receiving over 20 Gy improved by 5% (standard 22%, functional 17%). Dose to organs at risk and tumour coverage were not significantly different between plans.
Conclusions
SPECT/CT-guided planning resulted in improved dose-volumetric outcomes for functional lung. This methodology may lead to potential reduction in radiation-induced lung toxicity.
Keywords: Perfusion SPECT/CT, Functional avoidance, Functional lung, Non-small-cell lung cancer, Radiation therapy
1. Introduction
Despite diagnostic and treatment advances, non-small-cell lung cancer (NSCLC) remains one of the leading causes of cancer death due to poor prognosis and high rate of local failure [1]. About 25% of patients with lung cancer have locally advanced stage III disease, which is inoperable and potentially curable with radiation therapy (RT) combined with chemotherapy [2], [3]. Strategies to improve local tumour control are warranted, as it remains suboptimal in patients treated with conventional chemo-RT. A potential for improvement has been shown with dose escalation, but proved to be inferior in the randomised controlled trials due to increased toxicity [4], [5]. The recent data suggest that patients with locally advanced NSCLC treated with definitive chemo-RT may benefit from additional immunotherapy, which emphasises the need for strategies to minimize lung toxicity for these patients [6]. Functional avoidance, implying the use of functional information from single photon emission computed tomography (SPECT/CT) in the optimisation of RT plans to spare the well-functional lung, has a potential to reduce the damage to functional lung and consequently, improve the toxicity outcomes for the patients.
In the previous prospective study, SPECT-derived dose-volume parameters demonstrated improved predictive results for radiation-induced toxicity [7], [8]. A set of functional dose-volume constraints was defined from the prospective lung patients’ cohort, treated with definitive chemo-RT [7]. In the present planning study, these constraints were applied to SPECT-determined functional lung.
The purpose of this study was to evaluate the clinical feasibility of functional avoidance planning and to characterise the achievable dosimetry of target and normal tissues of SPECT-guided dose redistribution in a prospective sample of consecutive lung cancer patients using different planning techniques.
2. Materials and methods
2.1. Study population and SPECT/CT scans acquisition
Informed consent was obtained for all participants prior to inclusion in the trial. The study was approved by Human Research Ethics Committee of Western Sydney Local Health District (reference number 5452). All patients received 60–66 Gy in 30–33 fractions of external beam RT, 5 fractions per week with cone-beam CT imaging using departmental protocol and national guidelines [9]. Pre-treatment target position is verified by daily KV images and weekly cone beam CT. RT was delivered without the use of functional data. Lung perfusion images were acquired on a Siemens Intevo BOLD SPECT/CT (Siemens, Knoxville, TN, USA) after the intravenous administration of 200 MBq of 99mTc-labeled macroaggregated albumin (MAA) particles, with the following acquisition parameters: counts were acquired into primary energy window of 140 keV ±7.5%, with a secondary scatter window of 15%; an acquisition matrix of 256 × 256 px with a 1.0× zoom applied; 60 acquisitions of 20 s durations were made in step and shoot mode equally distributed over 360°. A low-dose CT was also acquired using 110 kVp and CareDose, with image quality reference of 80 mAs. Images were reconstructed using the Siemens xSPECT algorithm with a 10.0 mm Gaussian filter applied, iterative reconstruction with resolution, scatter, and attenuation correction. Free breathing SPECT/CT scans were performed in the treatment position on the same day as simulation 4D CT scan (Varian respiratory tracking system, 125 kVp energy photons at 10 mA, 5 mm slice thickness, Varian Medical Systems, Palo Alto, California USA).
Functional lung (FL) threshold volumes were defined on perfusion Tc-MAA SPECT of the lung fused with the dose planning CT. FL 20–80% volumes corresponded to thresholds of >20–80% respectively, of maximum perfusion intensity within total lung minus gross tumour volume (GTV) (supplementary material Fig. S1). Functional lung contours were transferred from SPECT/CT to the dose planning CT with rigid and deformable registrations using MIM software (version 6.4.4 Inc. Cleveland, OH, USA). Dosimetric parameters, such as mean lung dose (MLD), volume of lung receiving 5 Gy (V5), volume of lung receiving 20 Gy (V20), and volume of lung receiving 30 Gy (V30) were calculated within threshold functional lung volumes.
2.2. RT planning and optimisation technique
Two dose plans were optimized: 1) a standard reference (SR) plan, based on CT alone, blinded to functional structures, and 2) functional avoidance (FA) SPECT-plan, imposing higher priority on functional levels. Functional avoidance RT planning objectives were:
-
1.
Multiple levels of non-overlapping regions of FL was created (FL20%-FL80%), with higher objectives given to regions of higher function. FL 20–80% were each given ascending priority;
-
2.
Further optimisation for FL40% was done with the following constraints: mean dose to FL40% ≤ 16 Gy, V20 ≤ 30% and V30 ≤ 23% [7];
-
3.
Planning target volume (PTV) coverage and dose to other organs at risk were maintained.
For each patient a 3D-conformal, intensity modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) plans were created for both SR and FA plans. In all six plans were produced per patient by a dedicated radiotherapy planner using Eclipse treatment planning system version 11 (Varian Medical Systems, Palo Alto, California USA). Doses were calculated with the AAA algorithm [10]. Anatomical versus functional dose-volume parameters for functional lung subvolumes, and other organs at risk were compared. The organs at risk included oesophagus, heart, spinal canal, and the lung volume relevant to each plan. Plan quality was assessed through PTV volume coverage, plan heterogeneity and conformity indices for each standard and functional avoidance plan [11].
2.3. Statistics
A repeated measures ANOVA with a Greenhouse-Geisser correction was used to compare dose-volume parameters and tumour coverage across the different planning techniques. Post hoc tests were performed using the Bonferroni correction. Lung subvolumes were assessed using Pearson correlation statistics for each treatment technique. Paired Student’s t-tests were performed to determine the statistical significance of differences between standard and functional avoidance plans. Mean dose, V5, V20 and V30 were compared between SR and FA plans for the whole lung and functional lung sub-structures. Statistical significance was defined as p < 0.05 for a 2-tailed test. Statistical analysis was performed in SPSS Statistics version 22 (IBM Corp., Armonk, NY, USA).
3. Results
Between June and September 2018, 15 consecutive patients were included in the study. Detailed patient, tumour and treatment characteristics are presented in Table 1.
Table 1.
Patient characteristics (n = 15).
| Parameter | n (%) or median (range) | |
|---|---|---|
| Age | 71 (58–81) | |
| Sex | ||
| Male | 11 (73) | |
| Female | 4 (27) | |
| Histology | ||
| SCC | 5 (33) | |
| Adenocarcinoma | 7 (47) | |
| NOS | 2 (13) | |
| Small cell | 1 (7) | |
| Stage | ||
| I-II | 3 (20) | |
| IIIa-IIIb | 10 (67) | |
| IV* | 2 (13) | |
| ECOG performance status | ||
| 0–1 | 13 (87) | |
| 2 | 2 (13) | |
| COPD | ||
| Yes | 7 (47) | |
| No | 8 (53) | |
| Smoking | ||
| Nonsmoker | 2 (13) | |
| Current smoker | 3 (20) | |
| Ex-smoker | 10 (67) | |
| Chemotherapy | ||
| Concurrent | 11 (73) | |
| Sequential | 1 (7) | |
| No | 3 (20) | |
*Isolated solitary brain metastasis resected.
SCC squamous cell carcinoma.
NOS not otherwise specified.
ECOG Eastern Cooperative Oncology Group.
COPD chronic obstructive pulmonary disorder.
Majority of patients had stage III disease. Mean tumour volume was 375 cm3. Mean anatomical lung volume was 3703 cm3 (±809 SD). The mean FL20% volume was 2727 (±735), FL40%- 1082 (±402), FL60%- 237 (±139) and FL80%- 26 (±18) cm3. The ratio of functional to anatomical lung volume was 0.31 (±0.14). There was weak correlation between anatomical and functional lung volumes within patients (Pearson r = 0.14–0.4), due to perfusion defects variations (tumour, emphysema). The correlation was high between FL20% and FL40% (r = 0.8), FL40% and FL60% (r = 0.8), and FL60% and FL80% (r = 0.7).
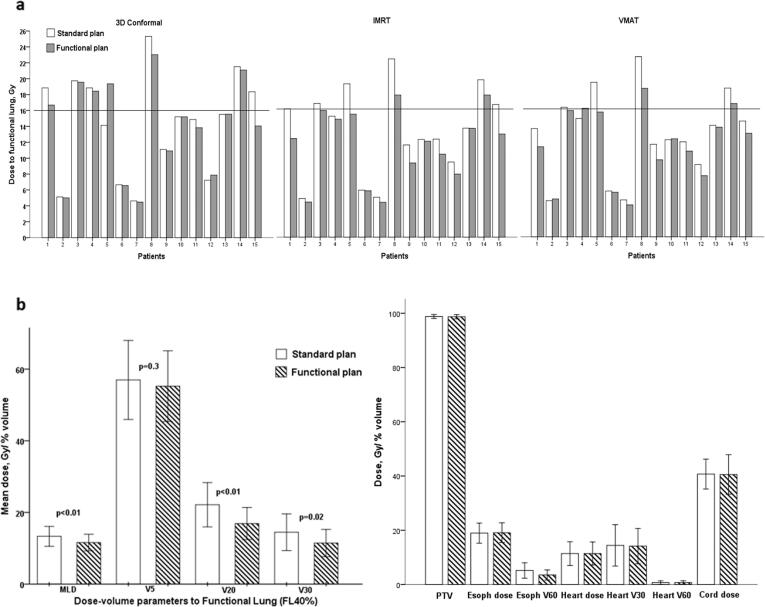
An example of FA plan and SR plan, and amount of functional lung spared for radiation is presented on Fig. 1. Planning strategies optimized to avoid regions of FL resulted in improved dose-volume parameters (Fig. 2). Tables 1–3 in supplementary material provide the details of standard and functional parameters for the 3D-conformal, IMRT and VMAT planning techniques, respectively. The largest dose-volume reduction was achieved by IMRT technique (Fig. 2). Dose to FL40% was reduced by a mean of 1.7 Gy (13%) [95% CI 0.9; 2.6]. Even larger reduction of 2.3 Gy (26%) [95% CI 1.1; 3.5] was achieved for FL80%. Functional avoidance IMRT plans produced significant reduction in V20 (mean reduction in V20 of 5%, p = 0.002) and V30 (mean reduction 3%, p = 0.02), while V5 reduction was not statistically significant (2%, p = 0.3). In the whole cohort, over 50% of patients had a significant reduction (>10% reduction) in dose-volume parameters to FL. Clinically significant dose parameters reduction of 15% and higher was achieved for 20–47% of patients.
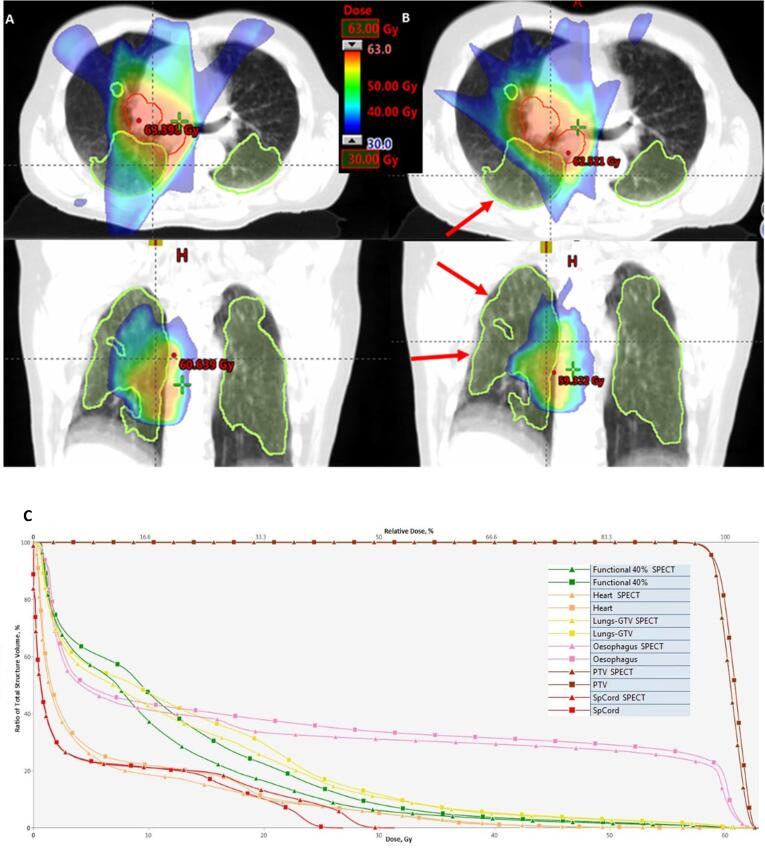
Fig. 1.
Differences in dose distribution with intensity-modulated radiation therapy plan optimised to anatomical lung contour (a) and functional avoidance plan (b) optimised to FL40% subvolume. Functional lung spared for radiation dose as result of functional avoidance (arrows). Clinical target volume in red, FL40% in lime, dose in colour wash. (c) Dose-volume histogram for planning target volume (dark red), oesophagus (pink), both lungs (yellow), heart (orange), FL40% subvolume (green), spinal cord (red). Squares represent standard reference plan, triangles-functional avoidance plan. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
a. Comparison of functional and standard plans for each patient. Dose to functional lung FL40% for 15 patients, planned with 3D Conformal, intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT). Functional mean lung dose constraint of 16 Gy is marked. Coloured bars represent functional plans. b. Mean dose (Gy) and dose-volume parameters, volume of lung receiving 5 Gy (V5), volume of lung receiving 20 Gy (V20) and volume of lung receiving 30 Gy (V30) (%) for functional lung FL40%. Comparison of standard and functionally adapted (coloured bars) intensity-modulated radiation therapy plans. Comparison of anatomical and functional dose to organs at risk and planning target volume (PTV) coverage for standard and functionally optimised (coloured bars) intensity-modulated radiation therapy plans.
In all planning techniques, standard and functional, dose to the whole lung without the functional data minus GTV, was not significantly different (Fig. 2; Supplementary Table S1). Radiation doses to other organs at risk were similar in FA plans and SR plans, except for V60 to oesophagus, which was significantly lower in functional plans with mean difference of 2%. PTV coverage was similar in SR and FA plans. Dose and percent volume parameters for oesophagus, heart and spinal cord produced by SR and FA plans, are described in details in supplementary material Table S1 and presented in Fig. 2b.
4. Discussion
This study demonstrates the benefit of SPECT/CT-guided FA planning for the reduction of dose to the functional lung subvolumes. FA planning with SPECT-guided dose redistribution proved to be clinically feasible with the achievable dosimetry of target and normal tissues. This is a novel study comparing three clinically available modern planning techniques in terms of functional lung sparing. To our knowledge, this study is the first to quantify the amount of functional lung sparing achievable with image-guided RT. In several previous studies different planning techniques have been used individually: 3DC, IMRT and VMAT depending on the clinical availability. In our study we compared three techniques in terms of which technique yields the largest functional lung sparing.
Several planning studies sparing highly functional lung regions for radiation have been conducted [12], [13], [14], [15], [16], [17], [18], [19], [20]. In these studies, various functional imaging modalities have been used to target perfusion, ventilation or both [12], [21]. In one of the earlier attempts to incorporate SPECT functional lung imaging into IMRT for non-small cell lung cancer from Royal Marsden, UK, the authors demonstrated that incorporation of functional information into standard anatomical 3DCRT or IMRT planning can allow reductions in the dose to the functional lung [17], [19], [20]. IMRT plans achieved significant reduction of mean functional V20 and functional MLD for patients with locally advanced disease and non-uniform perfusion defects with larger volumes of functional lung close to PTV [19].
The study of Matuszak et al. have identified the potential to reduce the dose delivered to highly functional lung regions as assessed by perfusion SPECT using generalised equivalent uniform functional dose approach [15]. The authors report 2.7 Gy mean lung dose reduction from mean 12.6 Gy ± 4.9 Gy to mean 9.9 Gy ± 4.3 Gy. Even though the reduction in lung dose was achieved at the cost of dose increase to spinal cord (5.4 Gy ± 3.9 Gy), oesophagus (3 Gy ± 3.7 Gy), and heart (2.3 Gy ± 2.6 Gy), this was not exceeding conventional limits for organs at risk. Researchers from Duke University Medical Center, NC describe a methodology for using perfusion SPECT to reduce IMRT dose to functioning lung and the role of beam arrangement on both low and high doses in the functional lung [13], [16]. In the five patients studied here, it was possible to achieve large reductions in irradiated lung function above the radiation-pneumonitis significant doses of 20 Gy (13.7% ±5.3%) and 30 Gy (10.6% ±5.8%).
Previous reports on SPECT-guided avoidance demonstrated the ability of FA planning techniques to spare regions of functional lung [14], [18], [22], [23]. Lee et al report on VMAT plans for eight patients, created to spare the dose to the highly perfused lung (FL 70% of max SPECT count). FA planning yielded a 7.6 Gy reduction in MLD (MLD of 7.3 Gy with FA planning versus 14.9 Gy on SR planning). Meanwhile, dose to the heart dose was 9.4 versus 5.8 Gy, and maximum dose to spinal cord was 50.1 versus 44.6 Gy relative to reference VMAT plans [22]. Shioyama et al. performed a study with the purpose to preserve functional lung using perfusion imaging and IMRT for advanced-stage NSCLC [24]. While incorporating perfusion information in IMRT planning, the median reductions in the mean doses to the F50 (FL 50% of max SPECT count) and F90 (FL 90% of max SPECT count) in the functional plan were 2.2 Gy and 4.2 Gy, respectively, compared with those in the standard anatomic plans. The median reductions in the percentage of volume irradiated with >5 Gy, >10 Gy, and >20 Gy in the functional plans were 7.1%, 6.0%, and 5.1%, respectively, for F50 lung, and 11.7%, 12.0%, and 6.8%, respectively, for F90 lung. The difference in achievable dosimetry for functional avoidance between these studies and our data may be explained by the definition of functional lung and percent point cut-off used, as well as patient selection and dose-volume constraints used for organs at risk.
Our results are consistent with the recent systematic review and meta-analysis on functional lung imaging potential in RT planning conducted by Bucknell et al. [12]. With the plans optimised to spare functional lung, the authors report mean functional V20 reduced by 4.2% (95% CI: 2.3; 6.0), and MLD by 2.2 Gy (95% CI: 1.2; 3.3) when plans were optimised to spare functional lung.
Comparing with the previous literature, the strength of our study is the use of new modern SPECT/CT imaging with xSPECT reconstruction algorithm to accurately define functional lung regions for RT planning. Strict organs at risk constraints and target coverage used in the planning process contributed to high plan quality assessment. However, this was also most likely the reason for reduced opportunities to spare dose to the functional lung areas, especially areas in the close proximity to the target.
The potential role of dose to the functional substructures in radiation-induced toxicity development has not been clinically verified. In different studies the ranges of functional lung definitions are reported from 20% to 90% with some studies using multiple functional lung definitions. Our group has previously investigated and published a clinical rationale for using a certain threshold to define functional lung [7]. Our results suggested that 40% of maximum perfusion threshold for perfusion SPECT imaging was mostly associated with radiation pneumonitis toxicity outcome, and therefore was used in the present FA planning study. The exact location of the functional subvolumes in relation to the tumour target plays a significant role in the potential to reduce the dose. Therefore, all levels of SPECT perfusion from 20% to 80% were used in ascending priority for FA planning.
Whereas the largest sparing of functional lung was produced by IMRT and VMAT techniques, the quantification of the benefit for the percentage of the patients is clinically important. Thus, 47% of patients had over 15% dose reduction to functional lung, while 60% and 53% had >15% reduction in V20 and V30, respectively, being clinically significant dose reduction. Even larger (>20%) dose reduction to FL was seen in 20% of the patients. Over 20% reduction in V20 and V30 was achieved in 47% of the patients.
Rationale for integrating SPECT images in RT planning is to avoid dose to highly functional lung, and consequently reduce the risk of radiation-induced toxicity. Minimising radiation induced lung injury is the major potential clinical benefit of the individualised approach of FA planning for the NSCLC patients. Along with the wider availability and proven benefit of immunotherapy for lung cancer patients, there are ongoing trials investigating concurrent immunotherapy and radio-chemotherapy [6], [25]. The lung toxicity profile of multimodality treatments is yet to be established, as the combination of different treatment modalities could potentially be more toxic. Even though several attempts of dose escalations were not successful due to increased toxicity, there is a potential in equitoxic image-guided dose escalation [4], [5]. FDG- PET dose escalation is currently being tested in a number of precision radiation oncology trials [22], [26]. However, this approach will also require measures to identify the patients who are at greater risk of radiation-induced toxicity. Therefore, improved methods to reduce radiation-induced toxicity are necessary to offer the lung cancer patients improved multimodality treatment, as well as to utilise the benefit of dose escalation. As a part of this initiative, our collaborative group from Denmark and Australia has initiated a prospective randomised phase II study of SPECT/CT image-guided treatment planning, with the reduction in radiation induced lung toxicity as the major potential clinical benefit.
SPECT/CT is a simple and widely available investigation which can be routinely incorporated in the planning pathways of lung cancer patients. Functional avoidance planning optimised to perfused lung volumes identified with SPECT resulted in improved dose volumetric outcomes for functional lung. This methodology may lead to potential reduction in radiation-induced lung toxicity in patients treated with definitive lung RT, and subsequently offers potential for targeted dose escalation. The development of clinical trials is underway to incorporate perfusion SPECT/CT imaging to spare functional lung in patients undergoing definitive lung RT.
Acknowledgments
Acknowledgement
The authors thank Professor Val Gebski, The University of Sydney School of Medicine, for statistical advice.
Funding
Katherina P. Farr is supported by The Danish Cancer Society Grant No. R146-A9182-16-S2.
Compliance with ethical standards
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Human Research Ethics Committee of Western Sydney Local Health District ref. nr 5452) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not describe any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2019.08.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.De Ruysscher D., van Elmpt W., Lambin P. Radiotherapy with curative intent for lung cancer: a continuing success story. Radiother Oncol. 2011;101:237–239. doi: 10.1016/j.radonc.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Fournel P., Robinet G., Thomas P., Souquet P.J., Lena H., Vergnenegre A. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 4.Bradley J., Graham M.V., Winter K., Purdy J.A., Komaki R., Roa W.H. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 5.Hallqvist A., Bergstrom S., Bjorkestrand H., Svard A.M., Ekman S., Lundin E. Dose escalation to 84 Gy with concurrent chemotherapy in stage III NSCLC appears excessively toxic: results from a prematurely terminated randomized phase II trial. Lung Cancer. 2018;122:180–186. doi: 10.1016/j.lungcan.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 7.Farr K.P., Kallehauge J.F., Moller D.S., Khalil A.A., Kramer S., Bluhme H. Inclusion of functional information from perfusion SPECT improves predictive value of dose-volume parameters in lung toxicity outcome after radiotherapy for non-small cell lung cancer: a prospective study. Radiother Oncol. 2015;117:9–16. doi: 10.1016/j.radonc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Farr K.P., Kramer S., Khalil A.A., Morsing A., Grau C. Role of perfusion SPECT in prediction and measurement of pulmonary complications after radiotherapy for lung cancer. Eur J Nucl Med Mol Imaging. 2015;42:1315–1324. doi: 10.1007/s00259-015-3052-3. [DOI] [PubMed] [Google Scholar]
- 9.Islam S.M., Vinod S.K., Lehman M., Siva S., Kron T., Dwyer P.M. Lung cancer radiation therapy in Australia and New Zealand: Patterns of practice. J Med Imaging Radiat Oncol. 2016;60:677–685. doi: 10.1111/1754-9485.12475. [DOI] [PubMed] [Google Scholar]
- 10.Fogliata A., Nicolini G., Vanetti E., Clivio A., Cozzi L. Dosimetric validation of the anisotropic analytical algorithm for photon dose calculation: fundamental characterization in water. Phys Med Biol. 2006;51:1421–1438. doi: 10.1088/0031-9155/51/6/004. [DOI] [PubMed] [Google Scholar]
- 11.Murshed H., Liu H.H., Liao Z., Barker J.L., Wang X., Tucker S.L. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1258–1267. doi: 10.1016/j.ijrobp.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 12.Bucknell N.W., Hardcastle N., Bressel M., Hofman M.S., Kron T., Ball D. Functional lung imaging in radiation therapy for lung cancer: a systematic review and meta-analysis. Radiother Oncol. 2018;129:196–208. doi: 10.1016/j.radonc.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 13.McGuire S.M., Zhou S., Marks L.B., Dewhirst M., Yin F.F., Das S.K. A methodology for using SPECT to reduce intensity-modulated radiation therapy (IMRT) dose to functioning lung. Int J Radiat Oncol Biol Phys. 2006;66:1543–1552. doi: 10.1016/j.ijrobp.2006.07.1377. [DOI] [PubMed] [Google Scholar]
- 14.Siva S., Thomas R., Callahan J., Hardcastle N., Pham D., Kron T. High-resolution pulmonary ventilation and perfusion PET/CT allows for functionally adapted intensity modulated radiotherapy in lung cancer. Radiother Oncol. 2015;115:157–162. doi: 10.1016/j.radonc.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Matuszak M.M., Matrosic C., Jarema D., McShan D.L., Stenmark M.H., Owen D. Priority-driven plan optimization in locally advanced lung patients based on perfusion SPECT imaging. Adv Radiat Oncol. 2016;1:281–289. doi: 10.1016/j.adro.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuire S.M., Marks L.B., Yin F.F., Das S.K. A methodology for selecting the beam arrangement to reduce the intensity-modulated radiation therapy (IMRT) dose to the SPECT-defined functioning lung. Phys Med Biol. 2010;55:403–416. doi: 10.1088/0031-9155/55/2/005. [DOI] [PubMed] [Google Scholar]
- 17.Christian J.A., Partridge M., Nioutsikou E., Cook G., McNair H.A., Cronin B. The incorporation of SPECT functional lung imaging into inverse radiotherapy planning for non-small cell lung cancer. Radiother Oncol. 2005;77:271–277. doi: 10.1016/j.radonc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Munawar I., Yaremko B.P., Craig J., Oliver M., Gaede S., Rodrigues G. Intensity modulated radiotherapy of non-small-cell lung cancer incorporating SPECT ventilation imaging. Med Phys. 2010;37:1863–1872. doi: 10.1118/1.3358128. [DOI] [PubMed] [Google Scholar]
- 19.Lavrenkov K., Singh S., Christian J.A., Partridge M., Nioutsikou E., Cook G. Effective avoidance of a functional spect-perfused lung using intensity modulated radiotherapy (IMRT) for non-small cell lung cancer (NSCLC): an update of a planning study. Radiother Oncol. 2009;91:349–352. doi: 10.1016/j.radonc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Lavrenkov K., Christian J.A., Partridge M., Niotsikou E., Cook G., Parker M. A potential to reduce pulmonary toxicity: the use of perfusion SPECT with IMRT for functional lung avoidance in radiotherapy of non-small cell lung cancer. Radiother Oncol. 2007;83:156–162. doi: 10.1016/j.radonc.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.De Bari B., Deantonio L., Bourhis J., Prior J.O., Ozsahin M. Should we include SPECT lung perfusion in radiotherapy treatment plans of thoracic targets? Evidences from the literature. Crit Rev Oncol Hematol. 2016;102:111–117. doi: 10.1016/j.critrevonc.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Lee E., Zeng J., Miyaoka R.S., Saini J., Kinahan P.E., Sandison G.A. Functional lung avoidance and response-adaptive escalation (FLARE) RT: multimodality plan dosimetry of a precision radiation oncology strategy. Med Phys. 2017;44:3418–3429. doi: 10.1002/mp.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waxweiler T., Schubert L., Diot Q., Faught A., Stuhr K., Castillo R. A complete 4DCT-ventilation functional avoidance virtual trial: developing strategies for prospective clinical trials. J Appl Clin Med Phys. 2017;18:144–152. doi: 10.1002/acm2.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shioyama Y., Jang S.Y., Liu H.H., Guerrero T., Wang X., Gayed I.W. Preserving functional lung using perfusion imaging and intensity-modulated radiation therapy for advanced-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;68:1349–1358. doi: 10.1016/j.ijrobp.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Filippi A.R., Di Muzio J., Badellino S., Mantovani C., Ricardi U. Locally-advanced non-small cell lung cancer: shall immunotherapy be a new chance? J Thorac Dis. 2018;10:S1461–S1467. doi: 10.21037/jtd.2017.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moller D.S., Nielsen T.B., Brink C., Hoffmann L., Lutz C.M., Drogemuller Lund M. Heterogeneous FDG-guided dose-escalation for locally advanced NSCLC (the NARLAL2 trial): design and early dosimetric results of a randomized, multi-centre phase-III study. Radiother Oncol. 2017;124:311–317. doi: 10.1016/j.radonc.2017.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.