Abstract
Background and purpose
Daily radiotherapy plan adaptation facilitated by a high field magnetic resonance linac (MRL) may potentially reduce the treated volume due to a reduction of the setup uncertainty. However, the technology also imposes limitations to the treatment technique compared to a standard linac. This study investigated the clinical quality of MRL treatment plans against current standard plans using identical planning target volume margins for high-risk prostate cancer patients.
Materials and methods
Twenty consecutive patients planned with our current clinical standard TPS and treated with single arc VMAT on standard linacs with 78 Gy in the prostate and 56 Gy for pelvic lymph nodes over 39 fractions were included. In addition, IMRT treatment plans for delivery by a 1.5 T MRL, using standard margins and dose objectives, were made in a dedicated TPS. Mean population dose volume histograms (DVH) and dose metrics were analyzed and clinical plan quality was evaluated by an oncologist.
Results
All MRL plans were considered clinically acceptable, and DVH analysis showed an overall high similarity to dose distributions of the clinically delivered plans. Mean target coverage was similar (78.0 Gy vs 77.8 Gy). Small but statistically significant differences were seen in doses to organs at risk; on average MRL plans reduced dose to the bladder (46.2 vs 48.3 Gy) compared to standard plans, while dose was higher to the bowel (29.2 vs 26.6 Gy) and penile bulb (16.5 vs 10.8 Gy).
Conclusion
MRL treatment plans were clinically acceptable and similar in quality to the current standard.
1. Introduction
Magnetic resonance (MR) images are routinely used in radiotherapy (RT) planning due to their superior soft tissue contrast compared to that of computed tomography (CT) [1]. With the development of MR guided radiotherapy treatment machines, such as MR-linacs (MRL), real-time MR imaging will be available at each treatment fraction [2], [3], [4]. The soft tissue contrast may allow more precise patient setup as well as online adaption of the initial dose plan to match the actual geometry of the patient. For patients with high-risk prostate cancer, MR-guided RT may lead to better visualization of the prostate and seminal vesicles with MR than CT [1] and implanted fiducial markers will no longer be required for set up verification [5]. Furthermore, daily adaptation of the treatment plan may facilitate a reduction of planning target volume (PTV) margin and thus the dose to organs at risk (OAR) [6].
The presence of a strong magnetic field in a MRL will cause secondary electrons to curl, a phenomenon called the electron return effect (ERE) [7]. The dose distribution therefore differs in patients treated in an MRL compared to a standard x-ray based linac. The collected ERE has been shown to be partially equalized in intensity modulated radiotherapy (IMRT) by contributions of opposing beams [8]. However, the ERE should be considered for the dose calculation, as demonstrated by Tseng et al. [9]. Furthermore, the geometry of an MRL differs from that of standard linacs, which also impact on dose distribution and restrict the treatment techniques available. At the time of the current study, only treatment planning systems (TPS) dedicated to the specific MRL type are able to take the ERE and specific geometry of the treatment machine into account and thus deliver treatment plans for these machines.
Volumetric modulated arc radiotherapy (VMAT) has become the standard treatment technique for prostate RT as IMRT typically require more monitor units (MU) to create conformal treatment plans [10]. However, VMAT is currently not available for the MRL, which may affect dose conformity and hence reduce MRL plan quality. At the time of this study, at least two MRL systems are commercially available and these machines differ in magnetic field strength. Studies reporting on high field 1.5 T MRL plan quality are still limited in number. However, feasibility studies have reported clinically acceptable MRL plan quality for stereotactic radiosurgery of brain metastasis [9], curative lung cancer [11] and rectum cancer [12]. Furthermore, Raaymakers et al. documented the feasibility of palliative treatment of lumbar bone metastasis, including high agreement between planned and delivered dose [13].
Although prostate cancer patients are likely to be among the first patient groups to be treated on the high field MRL, no studies reporting on prostate cancer MRL plan quality have currently been published. Besides eliminating the invasive process of implanting fiducial markers required at standard linacs, prostate cancer patients may also benefit from PTV margin reduction due to plan adaptation at the MRL. High risk prostate cancer patients constitute an especially interesting group for MR-guided RT, as the prostate inter-fractional motion is independent from the elective lymph node regions [14]. Both types of motion may be accounted for by daily MR-guided plan adaptation. The aim of the current study was to investigate the quality of treatment plans for these patients generated for the MRL. Treatment plans to be delivered on an MRL were created in a dedicated TPS and were compared to the current clinical standard both from a clinical point of view and through dose metrics. All plans were created without assuming reduction in PTV margins, thus the study considers baseline MRL plans, which may be improved in the future when daily plan adaptation schemes are introduced.
2. Method and materials
2.1. Patients
Twenty consecutive patients treated for high-risk prostate cancer on one type of linear accelerator were included in this planning study. All patients had biopsy verified adenocarcinoma T1-3, N0-1, M0, Gleason score ranged between 7 and 9, and PSA levels were below 70 ng/mL. All patients were referred for external beam radiotherapy and prescribed 78 Gy to the prostate and seminal vesicles and 56 Gy to pelvic lymph nodes in 39 fractions.
2.2. Anatomic data, volume definition and dose
Planning CTs were acquired using intravenous contrast in the arterial phase in a 50 cm FOV with 3 mm slice thickness and a 512 × 512 matrix. MR scans include an axial T2 weighted 2D small FOV (180 mm × 180 mm) 3 mm slice thickness without gap and a T2* weighted balanced Fast Field Echo (bFFE) sequence for detection of gold fiducials implanted in the prostate. Rigid co-registration of MR scans to the planning CT was performed manually for delineation of targets and organs at risk (OAR). CT and MR scans were acquired on the same day.
Contours and related margins from the clinical plans were used for all plans in this study. The prostate including the caudal 2 cm of the seminal vesicles constituted the CTV78. The PTV78 was defined as the CTV78 with a uniform 7 mm margin. The CTV56 was defined as the iliac lymph nodes caudal of the promontory and cranial of the acetabulum and proximal pre-sacral and internal iliac lymph nodes in fossa obturatoria [15]. The PTV56 was defined as the CTV56 + 5 mm margin left and right and 7 mm margin in all other directions. Volume characteristics of the target structures in the population are given in Table 1. Prescription followed the ICRU guidelines for homogeneous target doses, i.e. an allowed variation from prescribed dose ranging from 95 to 107% [16].
Table 1.
Population median volumes of target structures and their ranges.
| Structure | Median volume [cm3] | Range [cm3] |
|---|---|---|
| CTV78 | 40 | 28–64 |
| PTV78 | 130 | 94–172 |
| PTV78imrt | 119 | 87–164 |
| CTV56 | 343 | 251–433 |
| PTV56 | 859 | 700–1079 |
Help structures used in optimization are listed in the Table A.1 of the appendix.
Generally, the lowest possible dose should be obtained for all OARs. Objectives marked with (*) had higher priority than PTV coverage, and all others had lower priority. Rectum was defined from the sigmoid to the sphincter and had the following objectives (Vx is the volume of a given OAR receiving ≥ x Gy): V74 < 1 cm3 (*), V70 < 20% (*), V65 < 30%, V60 < 50% and V50 < 60%. The circumference of rectum was not allowed to be fully encompassed by the 50 Gy isodose line (*). The dose to the bowel bag including the sigmoid should not exceed V70 < 2 cm3, V50 < 200 cm3 and V35 < 40%. Objectives on bladder dose were V70 < 30%, V60 < 40% and V50 < 60%. Femoral heads, including the joints should meet Dmax < 52 Gy and V50 < 50% and penile bulb V40 < 50%.
2.3. Treatment planning
For each patient, two plans were created: The clinically delivered plan, which was used as reference, and a plan for treatment on the Elekta Unity (Elekta AB, Stockholm, Sweden) 1.5 T MRL.
Clinical plans were performed in Pinnacle version 14 (Philips Medical systems, Madison, WI, USA) using the Autoplan module, with a post-optimization performed by a dose planner for delivery on Elekta Versa HD Linacs with 5 mm MLC leaf width. Pinnacle autoplans (PAP) were optimized using the collapsed cone (CC) algorithm to generate a full, single arc VMAT plan with 6 MV. Collimator angulations were 20–35 degrees, 100 cm source-to-axis-distance (SAD) and 180 control points were used per arc.
Treatment plans for the MRL were created in Monaco research version 5.19.03 (Elekta AB, Stockholm, Sweden), taking a 1.5 T magnetic field into account and using the 7 MV beam available at the MRL. The planner was blinded to the clinical PAP plans. A template was developed to match our clinical standard and used to set up nine equidistant static beams for step-and-shoot IMRT (ssIMRT), as VMAT was not available in the current MRL version. Monaco default ssIMRT parameters allowing up to 225 segments with at least 4 MUs were used. The collimator was by design fixed in the MRL at 90 degrees. Due to an SAD of 143.5 cm the effective MLC leaf width at isocenter was 7 mm. The radiation field of view in caudo-cranial direction was 21 cm at isocenter potentially requiring abutting of fields to cover treatment volumes longer in this direction.
Both Monaco Unity plans (MUPs) and clinical PAPs were calculated using a 3 mm dose grid covering the entire scanned volume. The dose in MUP plans were calculated using the Monte Carlo (MC) algorithm GPUMCD with a statistical uncertainty of 1%. All machine models used for dose calculations were calibrated to deliver 1 Gy per 100 MU in a 10 cm × 10 cm field at 10 cm depth. Optimization parameters of PAPs and the template used for MUPs are given in Table A.2, Table A.3 of the appendix.
The optimization time and time spent by the dose planner was recorded for 13 MUPs. The clinical quality of all MRL plans was evaluated by an experienced oncologist. It was not possible to make evaluations blinded to plan type, since MC and CC derived isodose curves differed in appearance. Besides visual inspection of the dose distribution, 15 dose objectives were evaluated for each patient, a total of 300 objectives per plan type for the patient group. Plan quality was also evaluated by comparing dose volume histograms (DVHs) and dose metrics. Conformity indices (CI) were calculated for PTV78 and PTV56 as , where Vprescribed was the volume covered by the prescribed dose. Homogeneity indices (HI) were calculated for PTV78 and PTV56-PTV78 as , where Dprescribed was the dose prescribed to the given ROI.
2.4. Statistics
All differences were tested for statistical significance with a two-tailed paired t-test with significance level of 5% upon confirmed normality of the distribution of the difference between PAP and MUP values.
3. Results
An oncologist evaluated dose distributions in all MUPs as acceptable for clinical delivery and on per-patient basis the oncologist found no significant clinical differences between PAPs and MUPs.
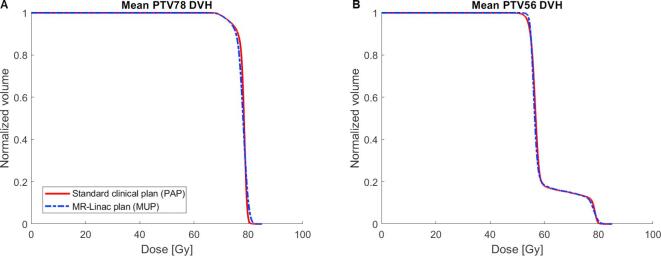
All plans were created without the use of abutted fields. An example of one typical patients’ PAP and MUP is shown in Fig. 1. Generally, slightly less steep DVH’s, indicating less uniform target coverage, were seen for PTV78 of MUPs compared to PAPs while the opposite was true for PTV56 (Fig. 2). These observations were confirmed by the mean V95 for PTV78, PTV56 and HI values given in Table 2.
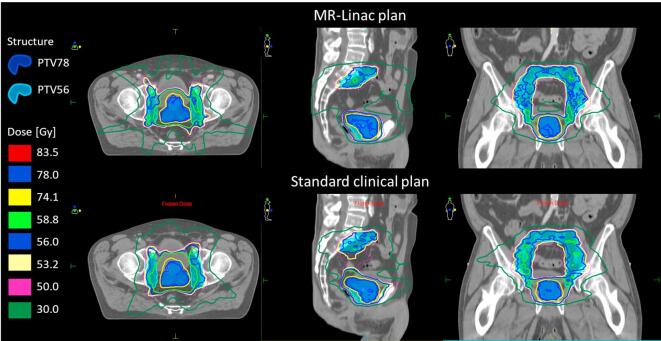
Fig. 1.
Example of PAP and MUP of a patient. Minor differences in dose distributions are seen.
Fig. 2.
Population mean DVHs for A) PTV78 and B) PTV56 of the group is shown for the standard PAPs (red) and MUPs (blue dashed). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Population mean dose metrics for PTV78 and PTV56, respectively for PAP and MUP.
| PAP |
MUP |
Dose difference |
|||||
|---|---|---|---|---|---|---|---|
| Target | Mean | SD | Mean | SD | Mean | SD | p-value |
| PTV78 | |||||||
| Mean [Gy] | 78.0 | 0.3 | 77.8 | 0.4 | 0.2 | 0.5 | 0.07 |
| V95 [%] | 94.9 | 1.8 | 94.1 | 2.4 | 0.8 | 1.0 | 0.002 |
| D2 [Gy] | 80.0 | 0.3 | 80.8 | 0.8 | −0.8 | 0.9 | <0.001 |
| D98 [Gy] | 71.2 | 1.1 | 71.3 | 1.4 | −0.1 | 0.5 | 0.28 |
| CI | 1.1 | 0.3 | 1.1 | 0.2 | 0.26 | ||
| HI | 0.1 | 0.02 | 0.1 | 0.01 | <0.01 | ||
| PTV56 | |||||||
| Mean [Gy] | 59.8 | 0.7 | 59.7 | 0.7 | 0.1 | 0.3 | 0.14 |
| V95 [%] | 97.8 | 1.5 | 99.7 | 0.3 | 0.1 | 1.6 | <0.001 |
| D2 [Gy] | 79.3 | 0.3 | 79.6 | 0.6 | −0.3 | 0.6 | 0.06 |
| D98 [Gy] | 53.2 | 0.8 | 54.2 | 0.3 | −1.0 | 0.8 | <0.001 |
| CI | 1.4 | 0.2 | 1.4 | 0.2 | 0.001 | ||
| PTV56-PTV78 | |||||||
| Mean [Gy] | 56.8 | 0.3 | 56.7 | 0.2 | 0.1 | 0.4 | 0.30 |
| HI | 0.2 | 0.03 | 0.2 | 0.03 | 0.13 | ||
The near maximum dose for PTV78 was observed 0.8 Gy higher for MUPs than PAPs (p = 0.002). The near minimum dose for PTV56 was 1.0 Gy lower for PAPs than MUPs. On average PAPs presented a more homogenous dose distribution in PTV78 (i.e. lower HI score) compared to MUPs.
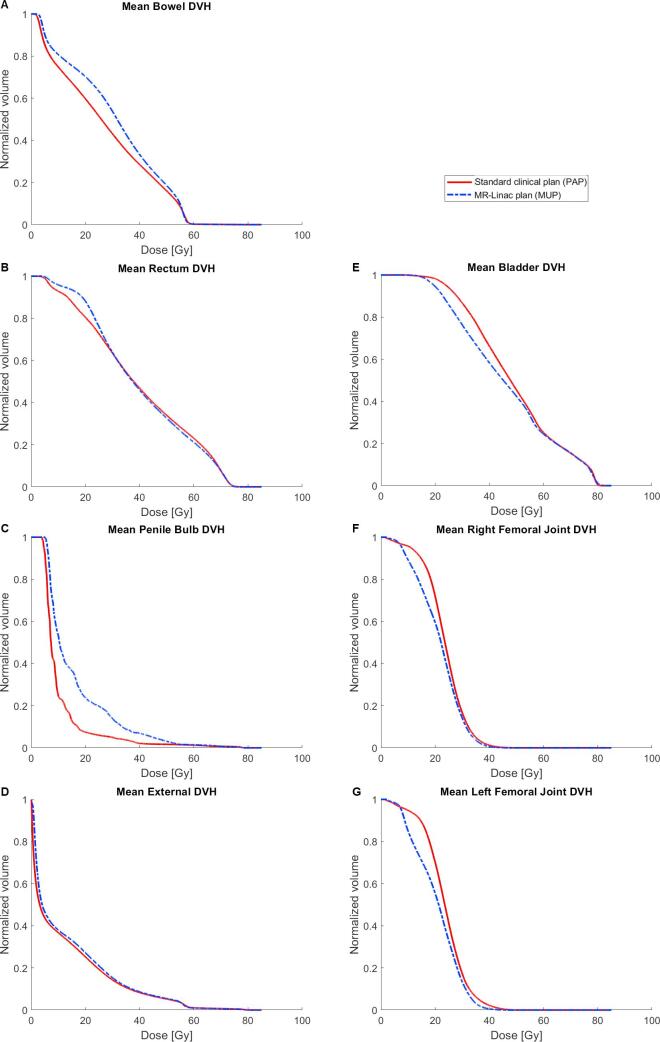
PAPs resulted in lower mean doses for some OARs; rectum (0.7 Gy), bowel (2.6 Gy) and penile bulb (5.7 Gy) (Fig. 3A–C)) while MUPs were superior for others; bladder (2.1 Gy) and femoral joints (2.1 and 3.0 Gy) (Fig. 3E–G)). Dose to the external structure (Fig. 3D), which included the entire scanned volume of the patient, received a mean dose 0.9 Gy higher, corresponding to 7%, in MUP compared to PAP. For objectives on rectum dose prioritized above target coverage a V74 was 0.4 cm3 for PAPs and 0.3 cm3 for MUPs (p = 0.08) and V70 = 7.5% for both PAPs and MUPs (p = 0.7). Some statistically significant differences were observed (Table 3).
Fig. 3.
Population mean DVHs for A) rectum, B) bowel, C) penile bulb, D) External contour, E) bladder, F) Right femoral joint and G) left femoral joint of the group is shown for the standard PAPs (red) and MUPs (blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Mean dose for all patients for each considered OAR and their standard deviations for PAPs and MUPs, respectively. Results of statistical analysis by paired t-test are given by their p-values.
| PAP |
MUP |
Dose difference |
|||||
|---|---|---|---|---|---|---|---|
| OAR | Mean [Gy] | SD [Gy] | Mean [Gy] | SD [Gy] | Mean [Gy] | SD [Gy] | p-value |
| Rectum | 39.6 | 4.3 | 40.3 | 2.6 | −0.7 | 2.4 | 0.3 |
| Bladder | 48.3 | 5.2 | 46.2 | 3.2 | 2.1 | 3.4 | 0.01 |
| Bowel | 26.6 | 5.4 | 29.2 | 5.1 | −2.6 | 1.6 | <0.001 |
| R. femoral joint | 23.5 | 3.6 | 21.4 | 2.8 | 2.1 | 3.6 | 0.02 |
| L. femoral joint | 23.6 | 4.4 | 20.6 | 2.5 | 3.0 | 3.6 | 0.001 |
| Penile bulb | 10.8 | 6.8 | 16.5 | 9.6 | −5.7 | 4.7 | <0.001 |
| External | 12.1 | 1.6 | 13.0 | 1.5 | −0.9 | 0.3 | <0.001 |
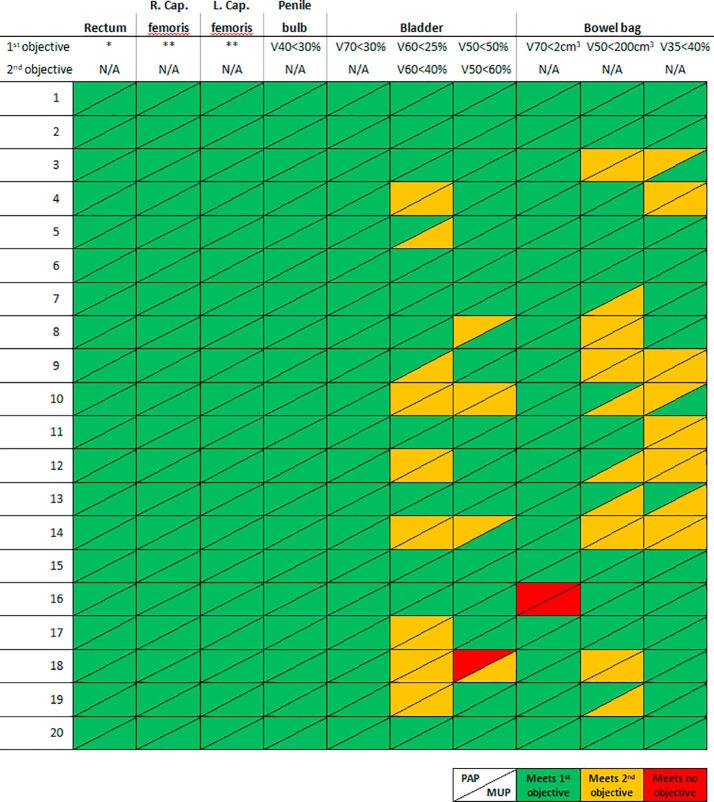
The specific objectives were met in 276 of 300 cases for PAPs and 272 of 300 for MUPs. An overview of passed and failed dose objectives for OAR can be seen in appendix Fig. A.1.
Fig. A.1.
Schematic representation of individually passed dose constraints for OAR for each patient planned with PAP and MUP respectively. Vx is the volume of the OAR receiving ≤ x Gy. * There are 4 objectives to be met for rectum (See Method & materials section). These have been collapsed to 1 column, as all were met in all patients. ** There are 2 objectives to be met for femoral heads (V50 < 50% and V52 > 0%). As both were met in all patients they are collapsed into 1 column.
MUPs required 137 segments on average (range 116–173) and 925 MU (range754-1179 MU). This was 80% more MUs than the average PAP plan (513 MU, range 476–562 MU). Mean MUP planning time was 68 min, of which 46 min were computer calculation time and 22 min spent by the operator on setup and optimization.
4. Discussion
This study investigated differences in quality between radiotherapy treatment plans used in clinical practice and plans created for MR-guided treatment in patients with high-risk prostate cancer. Differences were based on change of TPS, treatment technique, presence of the magnetic field, and the treatment machine geometry. Fractionation schemes and margins were identical for clinical and test plans, as the goal was to disentangle the effect that a new TPS and different treatment machine has on the treatment plan quality from other proposed impacts on the treatment, such as daily plan adaptation, reduced target margins and possibly hypo-fractionation regimes.
Comparison of PAPs and MUPs showed that no clinically relevant differences regarding target coverage or doses to OAR. When comparing current clinical standard treatment plans with MRL treatment plans a number of differences may contribute to the observed differences; presence of the magnetic field, differing TPS (dose engines, calculation algorithm, optimization technique), and geometric differences of accelerators (MLC width, collimator positioning, treatment technique, photon beam energy etc.). It was not possible to distinguish the exact contribution of each of these factors in the current study.
A main drawback of the current MRL planning and treatment chain was the lack of automated planning. Inter-planner variation has been shown to decrease, while plan quality and planning efficiency increases, for several indications, including prostate cancers, with automated planning such as PAP [17], [18], [19], [20], [21], [22]. However, a clinical version of automated treatment planning for localized prostate cancer has been developed and implemented in Monaco, with further development for MRL planning use in mind [23]. Another deficiency was the lacking VMAT functionality, since for most curative indications this has become standard due to its high conformability and efficient delivery [24], [25], [26]. MUP plans required substantially more MUs per plan than PAP and a high number of segments to achieve a conformal dose distribution. This may be due to the use of IMRT rather than VMAT, as was used for PAP. Similar findings were reported by Wolff et al. [27] and Ren et al. [10]. This could negatively affect delivery time and the dosimetric accuracy of the plans.
This study did not evaluate treatment delivery time or dosimetric accuracy in practice, as a clinical version of the high field MRL was not yet available at the time of the study. These factors are deciding of whether these plans will be clinically deliverable and must be evaluated and considered in a further commissioning process. Literature reporting clinical dosimetric accuracy of MRL treatments is limited; Raaymakers et al. found a mean difference in calculated (Monaco research ver. 5.19.02) and delivered dose of 0.4% for the first four patients treated in the lumbar spine by the pre-clinical version of the MRL [13].
The current feasibility study has shown that it was possible to create MRL step-and-shoot plans of similar clinical quality to PAP, taking a 1.5 T magnet field as well as limitations on beam directions and collimator angulation into account. This is in line with the findings of Van de Schoot et al. and Bainbridge et al. who reported MRL plans to be of clinically satisfactory standard for patients with rectum or lung cancer, respectively [11], [12]. Thus, the baseline for treatment planning for the MRL is similar to current standard provided by PAP. Improved IGRT by online MRI is expected to be used for treatment field adaptation to the current tumor size and position as well as the OAR configuration. This may permit a reduced PTV margin and in turn toxicity. Alternatively, increased target dose or hypo-fractionation schemes for the benefit of the patient may be possible, as demonstrated for lung patients by Bainbridge et al. [11].
The planning time used to create MUPs was considered feasible for clinical use and operator time comparable to that for autoplan setup and re-optimization for other treatment sites such as head and neck [22] and esophagus [19] treatment plans in our clinic. A distinction between the initial treatment planning time, as reported here, and the online adaption of that plan is important. Time consumption of online planning should be minimized to secure patient comfort. This could be achieved through either a simple shift of MLCs, warm start optimization or selecting an offline generated plan from a patient specific plan library [28].
The current study was limited by the risk of bias when clinical plans were compared to plans created using a novel technique. However, the clinical plans used in the current study were produced by experienced physicists and RTTs using PAP including a post-optimization step to fine-tune the final plan. Although the plans were produced by different planners, this process has been shown to consistently deliver high quality treatment plans both in terms of target coverage and sparing of OAR for several complex treatment sites, including the prostate [17], [18], [19], [22].
In summary, this study has demonstrated that it is possible to produce clinically acceptable treatment plans for patients with high-risk prostate cancer in presence of a 1.5 T magnetic field on the MRL. A baseline has been established for evaluation of the effect of future adaptive planning strategies. MUP planning time consumption was acceptable. Further studies are required in order to determine the deliverability of the MUPs in terms of treatment time and dosimetric agreement.
Conflict of interest statement
The authors have no conflict of interest to declare.
Acknowledgements
This work was supported by scholarships from the University of Southern Denmark and Odense University Hospital. The authors acknowledge support from AgeCare (Academy of Geriatric Cancer Research), an international research collaboration based at Odense University Hospital, Denmark. Elekta AB, Stockholm, Sweden provided research software tools.
Appendix
See Fig. A.1 and Table A.1, Table A.2, Table A.3
Table A.1.
Declaration of help structures used in plan optimization both in the PAP and MUP planning described in Table A.2, Table A.3. During PAP optimization a number of plan specific help structures are defined, e.g. cold spot ROIs.
| Structure | Contains |
|---|---|
| PTV78imrt | PTV78 – (Rectum + 2 mm) |
| RectumVclose | Nearest 3 mm of Rectum to PTV78imrt |
| Residual rectum | Rectum – (RectumVclose + PTV78) |
| Rectum posterior wall | Most posterior 2 mm of Rectum |
| PTV_prostata_imrt | Prostate + 7 mm – (Rectum + 2 mm) |
| PTV70imrt | PTV78 – PTV78imrt |
| PTV78LatRectum | Part of PTV78imrt lateral and posterior to anterior rectum wall |
| BladderIMRT | Bladder – PTV56 |
Table A.2.
PAP protocol parameters.
| OAR | Objective | Dose level [Gy] | Priority | Compromise |
|---|---|---|---|---|
| RectumVclose | Max DVH | 74 @ 25% | High | No |
| Bladder | Max DVH | 70 @ 20% | Medium | Yes |
| Mean dose | 30 | Medium | Yes | |
| Bowel bag | Max DVH | 70 @ 1% | High | Yes |
| Mean dose | 40 | Medium | Yes | |
| Right femoral joint | Max dose | 52 | Medium | Yes |
| Left femoral joint | Max dose | 52 | Medium | Yes |
| Residual rectum | Mean dose | 20 | High | Yes |
| Penile bulb | Mean dose | 40 | High | Yes |
| Rectum posterior wall | Max dose | 50 | High | No |
Table A.3.
Template for intial optimization parameters for MUP. Unlisted shrink margins, k-factor etc. may apply to some cost functions. The template was trained on a series of patients prior to the current study.
| Structure | Cost function | Reference dose [Gy] | Isoconstraint |
|---|---|---|---|
| PTV78imrt | Target Penalty | 78.3 | |
| Quadratic Overdose | 79.0 | 1.0 | |
| Target Penalty | 74.3 | ||
| PTV70imrt | Target Penalty | 68.0 | |
| Target Penalty | 66.8 | ||
| PTV78 | Target Penalty | 66.5 | |
| PTV_prostata_imrt | Target Penalty | 74.1 | |
| PTV56 | Target Penalty | 56.0 | |
| Quadratic Overdose | 74.1 | 0.02 | |
| Quadratic Overdose | 56.0 | 1.4 | |
| Target Penalty | 53.4 | ||
| PTV78LatRectum | Target Penalty | 74.1 | |
| PTV56-PTV78 | Quadratic Overdose | 75.0 | 0.1 |
| Quadratic Overdose | 56.0 | 3.0 | |
| Rectum | Serial | 55.0 | |
| Serial | 44.3 | ||
| Serial | 29.5 | ||
| Serial | 27.4 | ||
| Maximum Dose | 78.3 | ||
| Parallel | 41.5 | 45.0 | |
| Parallel | 41.5 | 50.0 | |
| RectumVclose | Serial | 69.9 | |
| RectumPostWall | Serial | 35.0 | |
| Bladder | Serial | 51.5 | |
| Serial | 37.5 | ||
| Serial | 30.0 | ||
| Parallel | 44.5 | 50.0 | |
| BladderIMRT | Serial | 50.0 | |
| Bowel bag | Parallel | 40.0 | 30.0 |
| Parallel | 30.0 | 50.0 | |
| Serial | 41.0 | ||
| Right femoral joint | Serial | 29.5 | |
| Maximum Dose | 40.0 | ||
| Parallel | 20.0 | 50.0 | |
| Left femoral joint | Serial | 29.5 | |
| Maximum Dose | 40.0 | ||
| Parallel | 20.0 | 50.0 | |
| Penile bulb | Parallel | 30.0 | 45.0 |
| Serial | 30.0 | ||
| External | Quadratic Overdose | 74.1 | 0.05 |
| Quadratic Overdose | 56.0 | 0.1 | |
| Quadratic Overdose | 45.0 | 0.3 | |
| Quadratic Overdose | 35.0 | 0.5 | |
| Maximum Dose | 83.0 |
References
- 1.Khoo V.S., Joon D.L. New developments in MRI for target volume delineation in radiotherapy. Br J Radiol. 2006;79 doi: 10.1259/bjr/41321492. Spec No 1:S2-15. [DOI] [PubMed] [Google Scholar]
- 2.Mutic S., Dempsey J.F. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Lagendijk J.J., Raaymakers B.W., Van den Berg C.A., Moerland M.A., Philippens M.E., van Vulpen M. MR guidance in radiotherapy. Phys Med Biol. 2014;59:R349–R369. doi: 10.1088/0031-9155/59/21/R349. [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe P., Liney G.P., Holloway L., Walker A., Barton M., Delaney G.P. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013;12:429–446. doi: 10.7785/tcrt.2012.500342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernchou U., Agergaard S.N., Brink C. Radiopaque marker motion during pre-treatment CBCT as a predictor of intra-fractional prostate movement. Acta Oncol. 2013;52:1168–1174. doi: 10.3109/0284186X.2012.747698. [DOI] [PubMed] [Google Scholar]
- 6.Kontaxis C., Bol G.H., Kerkmeijer L.G.W., Lagendijk J.J.W., Raaymakers B.W. Fast online replanning for interfraction rotation correction in prostate radiotherapy. Med Phys. 2017;44:5034–5042. doi: 10.1002/mp.12467. [DOI] [PubMed] [Google Scholar]
- 7.Raaijmakers A.J., Raaymakers B.W., Lagendijk J.J. Experimental verification of magnetic field dose effects for the MRI-accelerator. Phys Med Biol. 2007;52:4283–4291. doi: 10.1088/0031-9155/52/14/017. [DOI] [PubMed] [Google Scholar]
- 8.Raaijmakers A.J., Hardemark B., Raaymakers B.W., Raaijmakers C.P., Lagendijk J.J. Dose optimization for the MRI-accelerator: IMRT in the presence of a magnetic field. Phys Med Biol. 2007;52:7045–7054. doi: 10.1088/0031-9155/52/23/018. [DOI] [PubMed] [Google Scholar]
- 9.Tseng C.L., Eppinga W., Seravalli E., Hackett S., Brand E., Ruschin M. Dosimetric feasibility of the hybrid magnetic resonance imaging (MRI)-linac System (MRL) for brain metastases: the impact of the magnetic field. Radiother Oncol. 2017;125:273–279. doi: 10.1016/j.radonc.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Ren W., Sun C., Lu N., Xu Y., Han F., Liu Y.P. Dosimetric comparison of intensity-modulated radiotherapy and volumetric-modulated arc radiotherapy in patients with prostate cancer: a meta-analysis. J Appl Clin Med Phys. 2016;17:254–262. doi: 10.1120/jacmp.v17i6.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bainbridge H.E., Menten M.J., Fast M.F., Nill S. Oelfke U, McDonald F. Treating locally advanced lung cancer with a 1.5T MR-Linac – Effects of the magnetic field and irradiation geometry on conventionally fractionated and isotoxic dose-escalated radiotherapy. Radiother Oncol. 2017;125:280–285. doi: 10.1016/j.radonc.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van de Schoot A.J.A.J., Carbaat C., Van Triest B., Janssen T.M., Sonke J.J. PO-, 0838: Treatment planning for the MR-linac: plan quality compared with current clinical practice (Abstr.) Radiother Oncol. 2017;123:S452–S453. [Google Scholar]
- 13.Raaymakers B.W., Jurgenliemk-Schulz I.M., Bol G.H., Glitzner M., Kotte A., van Asselen B. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. 2017;62:L41–L50. doi: 10.1088/1361-6560/aa9517. [DOI] [PubMed] [Google Scholar]
- 14.Groher M., Kopp P., Drerup M., Deutschmann H., Sedlmayer F., Wolf F. An IGRT margin concept for pelvic lymph nodes in high-risk prostate cancer. Strahlenther Onkol. 2017;193:750–755. doi: 10.1007/s00066-017-1182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawton C.A., Michalski J., El-Naqa I., Buyyounouski M.K., Lee W.R., Menard C. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–387. doi: 10.1016/j.ijrobp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.I.C.R.U. Report No. 83: Prescribing, recording and reporting photonbeam intensity Modulated Radiation Therapy (IMRT). J ICRU 2010;10.
- 17.Agergaard S., Hansen C.R., Dysager L., Bertelsen A., Jensen H.R., Hansen S. EP-1525: Automatic treatment plan generation for Prostate Cancer (Abstr.). Radiother Oncol. 2017;123 S819-S20. [Google Scholar]
- 18.Hazell I., Bzdusek K., Kumar P., Hansen C.R., Bertelsen A., Eriksen J.G. Automatic planning of head and neck treatment plans. J Appl Clin Med Phys. 2016;17:272–282. doi: 10.1120/jacmp.v17i1.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen C.R., Nielsen M., Bertelsen A.S., Hazell I., Holtved E., Zukauskaite R. Automatic treatment planning facilitates fast generation of high-quality treatment plans for esophageal cancer. Acta Oncol. 2017;56:1495–1500. doi: 10.1080/0284186X.2017.1349928. [DOI] [PubMed] [Google Scholar]
- 20.Nawa K., Haga A., Nomoto A., Sarmiento R.A., Shiraishi K., Yamashita H. Evaluation of a commercial automatic treatment planning system for prostate cancers. Med Dosim. 2017;42:203–209. doi: 10.1016/j.meddos.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Krayenbuehl J., Norton I., Studer G., Guckenberger M. Evaluation of an automated knowledge based treatment planning system for head and neck. Radiat Oncol. 2015;10:226. doi: 10.1186/s13014-015-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen C.R., Bertelsen A., Hazell I., Zukauskaite R., Gyldenkerne N., Johansen J. Automatic treatment planning improves the clinical quality of head and neck cancer treatment plans. Clin Transl Radiat Oncol. 2016;1:2–8. doi: 10.1016/j.ctro.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winkel D., Bol G.H., van Asselen B., Hes J., Scholten V., Kerkmeijer L.G. Development and clinical introduction of automated radiotherapy treatment planning for prostate cancer. Phys Med Biol. 2016;61:8587–8595. doi: 10.1088/1361-6560/61/24/8587. [DOI] [PubMed] [Google Scholar]
- 24.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 25.Wiezorek T., Brachwitz T., Georg D., Blank E., Fotina I., Habl G. Rotational IMRT techniques compared to fixed gantry IMRT and tomotherapy: multi-institutional planning study for head-and-neck cases. Radiat Oncol. 2011;6:20. doi: 10.1186/1748-717X-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertelsen A., Hansen C.R., Johansen J., Brink C. Single arc volumetric modulated arc therapy of head and neck cancer. Radiother Oncol. 2010;95:142–148. doi: 10.1016/j.radonc.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Wolff D., Stieler F., Welzel G., Lorenz F., Abo-Madyan Y., Mai S. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol. 2009;93:226–233. doi: 10.1016/j.radonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Kontaxis C., Bol G.H., Lagendijk J.J., Raaymakers B.W. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys Med Biol. 2015;60:7485–7497. doi: 10.1088/0031-9155/60/19/7485. [DOI] [PubMed] [Google Scholar]