Highlights
-
•
Target motion assessment using rigid and non-rigid strategies significantly differ.
-
•
Geometric changes are not accounted for in margins using rigid approximation.
-
•
Rectal volume is moderately correlated with anterior/posterior target motion.
-
•
Substantial reductions in rectal volume result in underdosing of the target.
Abbreviations: EC, endometrial cancer; CBCT, cone beam CT; CTVv, clinical target volume vaginal vault and upper vagina; DVFD, deformation vector field displacement; RV, rectal volume; EBRT, external beam radiotherapy; IMRT, intensity modulated radiotherapy; pCT, planning CT; OARs, organs at risk; DIR, deformable image registration; σ, random; Σ, systematic; DSC, dice similarity coefficient; MDA, mean distance to agreement
Abstract
Background and purpose
Appropriate internal margins are essential to avoid a geographical miss in intensity-modulated radiation therapy (IMRT) for endometrial cancer (EC). This study evaluated interfraction target motion using rigid and non-rigid approximation strategies and calculated internal margins based on random and systematic errors using traditional rigid margin recipes. Dosimetric impact of target motion was also investigated.
Materials and methods
Cone beam CTs (CBCTs) were acquired days 1–4 and then weekly in 17 patients receiving adjuvant IMRT for EC; a total of 169 CBCTs were analysed. Interfraction motion for the clinical target volume vaginal vault and upper vagina (CTVv) was measured using bony landmarks and deformation vector field displacement (DVFD) within a 1 mm internal wall of CTVv. Patient and population systematic and random errors were estimated and margins calculated. Delivered dose to the CTVv and organs at risk was estimated.
Results
There was a significant difference in target motion assessment using the different registration strategies (p < 0.05). DVFD up to 30 mm occurred in the anterior/posterior direction, which was not accounted for in PTV margins using rigid margin recipes. Underdosing of CTVv D95% occurred in three patients who had substantial reductions in rectal volume (RV) during treatment. RV relative to the planning CT was moderately correlated with anterior/posterior displacement (r = 0.6) and mean relative RV during treatment was strongly correlated with mean relative RV at CBCT acquired days 1–3 (r = 0.8).
Conclusion
Complex and extensive geometric changes occur to the CTVv, which are not accounted for in margin recipes using rigid approximation. Contemporary margin recipes and adaptive treatment planning based on non-rigid approximation are recommended.
1. Introduction
External beam radiotherapy (EBRT) to the pelvic lymph nodes and vaginal vault following hysterectomy reduces local recurrence in intermediate and high-risk endometrial cancer (EC) [1], [2]. Compared to 3D conformal plans, intensity modulated radiotherapy (IMRT) in EC reduces dose to the small bowel and bladder and enables better target coverage and dose homogeneity [3], [4]. This translates into the clinical benefit of less acute and late gastrointestinal toxicity [5], [6], [7]. The tight conformity and sharp dose gradients achieved with IMRT mean that appropriate internal margins are essential to avoid a geographical miss.
The current assessment of IMRT plans and dose–volume histograms (DVHs) is based on the radiotherapy planning CT (pCT), a single image set taken at one point before the start of treatment. The dosimetry and DVHs do not necessarily represent the actual dose delivered to the target and organs at risk (OARs), because they do not account for motion and geometric changes occurring during treatment. Target motion can be evaluated geometrically or by measuring the impact that target motion has on delivered dose. Previous studies of IMRT in EC have largely focused on the magnitude of target motion. Rigid approximation has been studied to assess target motion, often with fiducial or centre of mass measurement. Internal margins are then calculated based on recipes that assume these structures are rigid [8], [9], [10], [11], [12], [13], [14], [15]. Non-rigid or deformable image registration (DIR) can account for motion at each point within a defined structure, potentially providing a better representation of changing geometry compared to rigid methods. DIR is necessary to accumulate the delivered dose in the context of changing target and OARs geometry over a treatment course. This is particularly relevant in the post-operative female pelvis where the central vaginal vault target is subject to compression and displacement by changes in the adjacent bladder and rectum. DIR is now commercially available, but it is not in widespread clinical use in radiotherapy treatment planning and delivery. It is limited by the model used to define the deformation, and its clinical application is challenging in the pelvis where complex anatomical variation occurs. In particular, there is difficulty accounting for tissue interfaces that are sliding such as the rectum, and tissues such as the bladder which show substantial elastic changes.
To our knowledge, no previous studies have investigated interfraction target motion using both rigid and non-rigid approximation strategies and no studies have evaluated the dosimetric impact of target motion in EC. The first aim of this study was to evaluate and compare interfraction target motion during IMRT for EC using rigid and non-rigid approximation strategies as assessed on Cone beam CT (CBCT) and to calculate internal margins based on the random (σ) and systematic (Σ) error observed using traditional rigid margin recipes. The second aim was to determine the dosimetric impact of target motion in EC using dose accumulation.
2. Materials and methods
2.1. Patients and image data
Seventeen patients were identified who underwent adjuvant pelvic IMRT following hysterectomy for EC from December 2011 to December 2012. Local protocol margins of 15 mm superiorly and inferiorly and 10 mm circumferentially were applied to the clinical target volume of the vaginal vault and upper vagina (CTVv). All patients were treated with 5–7 field IMRT single phase treatment including the CTVv and pelvic lymph nodes to a dose of 45 Gy in 25 fractions, delivered over 5 weeks.
Each patient underwent a pCT in supine position with IV contrast. Patients were instructed to drink 350 ml water after complete bladder voiding 1 hour before the pCT. There were no bowel preparation instructions. The pCT was acquired from the top of L4 to the perineum, with a slice thickness of 2.5 mm, in-plane resolution of 0.97 mm pixels and field of view (FOV) 500 mm. Kv CBCT images were acquired (M20 kV mAs Elekta Synergy v5.4 Crawley UK) immediately before treatment and an off-line correction protocol was used with Σ error based on bony registration. In all patients CBCTs were acquired days 1–3 and weekly thereafter unless there was an error of >5 mm, in which case they were acquired more frequently. All images acquired were transferred to the RayStation treatment planning system (TPS) (RayStation v6 Raysearch Laboratories) for contour delineation, motion assessment, and dose accumulation.
2.2. Target and organ at risk segmentation
The CTVv, rectum and bladder were manually contoured on each pCT and CBCT image set. The rectum was contoured from the anorectum junction to the rectosigmoid junction. Consensus delineations were performed by experienced radiation oncologists (IW and SL). Pearson’s correlation coefficient (r) was calculated to determine the correlation between both bladder and rectal volumes with time-point during treatment and target motion.
2.3. Image registration
Rigid registration between the pCT and each CBCT was defined by treatment position, which was based on bony anatomy. DIR was performed between the pCT and each CBCT using the Anaconda algorithm [16] with the CTVv and rectum as controlling regions of interest. The Anaconda algorithm expresses the registration problem as a non-linear optimisation problem and uses a hybrid algorithm which combines both a geometric and intensity-based approach [16]. The quality of DIR was quantified by dice similarity coefficient (DSC) and mean distance to agreement (MDA) of the CTVv and rectum contours, with the pCT representing the true contour. The CTVv mean DSC was 0.9 and MDA was 0.7 mm, the rectum DSC was 0.9 and MDA was 0.9 mm, all consistent with good agreement. The quality of these registrations was deliberately improved at the rectum and CTVv and would not be representative of the quality of DIR at more peripherally located structures.
2.4. Target motion assessment
Target motion was assessed using both rigid and non-rigid approximation strategies. A 1 mm internal wall of the CTVv was created and sub-divided into halves to generate structures representing the right, left, anterior and posterior walls of the CTVv (Fig. 1). This was motivated by the deformable nature of the CTVv, which showed extrinsic compression from the rectum posteriorly and the bladder anteriorly. We assumed a smooth expansion of the contour edge and no sharp discontinuity of motion. It is possible that one part of the subdivision is moving very differently to another part and overlap of the subdivisions could lead to over or underestimation of motion, but providing there is smooth contour expansion the impact this has on the determined target motion should be minimal. To determine internal motion in different directions only translations in the plane of interest were assessed. Rigid assessment of target motion was estimated by translation of the most extreme right, left, anterior and posterior positions of the CTVv structure, relative to the mid femoral head bony landmark. These measurements were taken at each 2.5 mm slice on which the structure was contoured and mean displacement in each direction was calculated across all slices for each CBCT. Target motion assessment using non-rigid approximation was achieved by calculating the mean deformation vector field displacement (DVFD) of all voxel displacements within each subdivision of the CTVv wall on each CBCT (Fig. 2). The mean deformation vector field displacement for each CBCT was used to calculate the mean and standard deviation (SD) voxel displacement within each subdivision of the CTVv wall for each patient. The mean target motion using both methods, calculated for each CBCT and each patient, was compared using the Wilcoxon rank test. Population Σ and σ error for internal target motion was calculated from the patient mean and SD internal target motion for both assessment strategies.
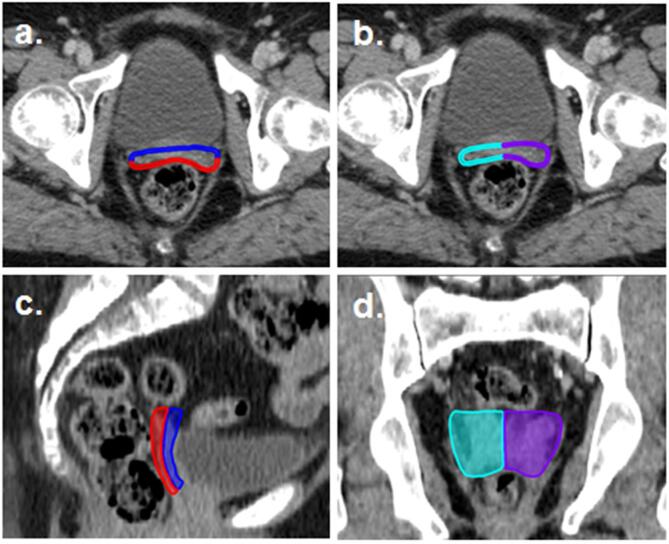
Fig. 1.
Sub-division of CTVv for non-rigid assessment of internal motion; a) Anterior and posterior wall and b) Right and left wall, both on axial view, c) Anterior and posterior wall on sagittal view and d) Right and left wall on coronal view. Anterior wall = blue, posterior = red, right = turquoise and left = purple. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
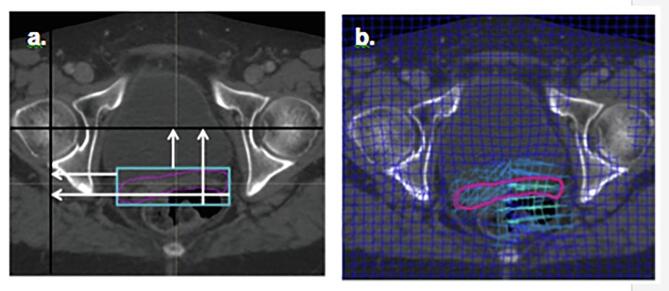
Fig. 2.
CTVv motion assessment; a) Rigid approximation strategy, b) Non-rigid approximation strategy.
Set up error was determined from bony displacement of the CBCT with the reference pCT and population Σ and σ error set up error were calculated. For the rigid approximation data, the van Herk formula (2.5Σ + 0.7σ) was used to calculate margins required to account for observed internal motion and set up errors [17]. CTV-PTV margins to encompass the population target motion and set-up error were determined by combining the margins for target motion and set-up error in quadrature [18].
2.5. Simulation of delivered dose
CBCT dose calculation was facilitated by bulk density assignment [19]. Bulk density assignments were defined on CBCT, dividing the image dataset into regions of air, lung, adipose, tissue, cartilage/bone and high density. The corresponding mass densities (g/cm3) were used for the dose calculations; air 0.00121, lung 0.26, adipose 0.95, tissue 1.05 and cartilage/bone 1.6. The clinically delivered plan was calculated on each CBCT and deformed to the pCT using the DIR. The deformed dose cubes were assigned weights according to the number of fractions delivered between each consecutive CBCT acquisition. The weighted deformed dose was summed and compared to the planned dose as calculated on the pCT. The planned dose to the CTVv (D99, D95, D50, D5 and D2), rectum (V40 Gy and V30 Gy) and bladder (mean dose) was compared to the simulated delivered dose using the Wilcoxon rank test. All statistical analyses were performed on GraphPad Prism 7 and p-values <0.05 were considered statistically significant.
3. Results
3.1. Rigid and non-rigid assessment of target motion
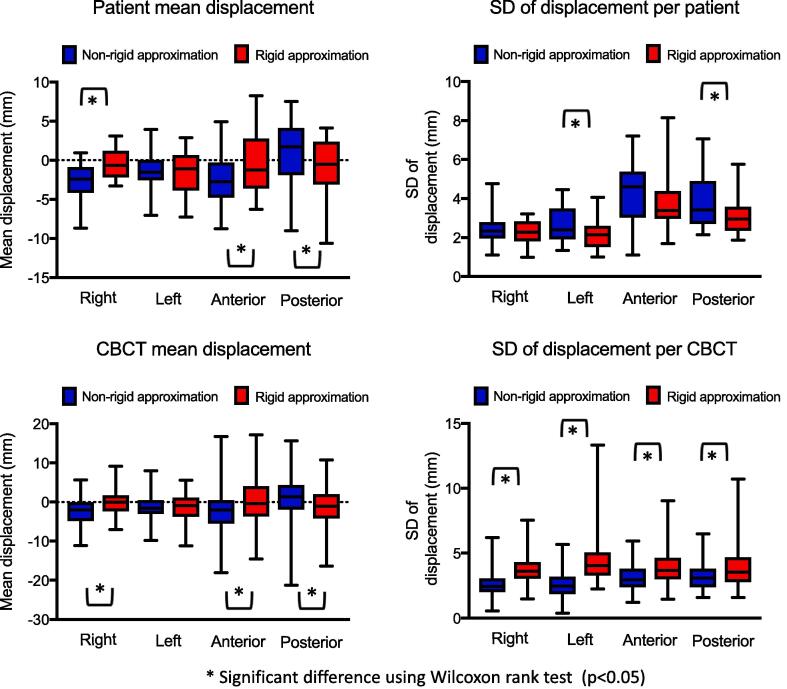
A total of 169 CBCTs were analysed. The mean CTVv at baseline on pCT was 47 cm3 (range, 25–72 cm3) and the maximal change in CTVv CBCT volume relative to baseline was 43–129%. Target motion was largest in anterior and posterior directions (Table 1). Based on measured motion using rigid approximation, the CTVv to PTV margins, calculated for IMRT in EC without the use of daily online imaging, were 10 mm in the right direction, 11 mm in the left direction, 14 mm anteriorly and 12 mm posteriorly, to ensure 95% of the prescribed dose is delivered to the CTVv in 90% of patients. DVFD of up to 30 mm occurred in the anterior direction in 1 patient and anterior or posterior DVFD >20 mm were seen in a further 8 patients. In 15/17 (88%) of patients (53/169 fractions) DVFD in the anterior/posterior directions were greater than margins calculated using the rigid approximation data and rigid margin recipes. In 16/17 (94%) of patients (60/169 fractions) DVFD in the left or right directions were larger than the calculated margins. Fig. 3 shows the distribution of mean and standard deviation (SD) target motion in individual patients and CBCTs for the rigid and non-rigid approximation strategies. There was a statistically significant difference in mean target motion using the different strategies for anterior, posterior and right directions (p < 0.05).
Table 1.
Target motion according to non-rigid and rigid approximation. Margins calculated for rigid approximation data to account for the observed errors using van Herk’s formula and PTV margins calculated to encompass the population target motion and set-up error.
| Movement | DVF displacement (mm) |
Rigid translation (mm) |
Setup error (mm) |
Rigid data CTVv to PTV (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean of mean | Σ | σ | Range | Mean of mean | Σ | σ | Range | Margin | Σ | σ | Margin | Margin | |
| Right | −2.7 | 2.5 | 2.4 | –22.8–19.1 | −0.5 | 1.9 | 2.2 | −20.0–21.6 | 6.6 | 2.1 | 3.3 | 7.6 | 10.1 |
| Left | −1.4 | 2.2 | 2.6 | −21.1–18.1 | −1.5 | 2.9 | 2.2 | −29.2–20.3 | 8.8 | 2.1 | 3.3 | 7.6 | 11.4 |
| Anterior | −2.5 | 3.4 | 4.0 | −29.3–30 | −0.4 | 4.1 | 3.8 | −27.0–23.4 | 12.9 | 1.1 | 2.0 | 4.2 | 13.5 |
| Posterior | 1.0 | 4.0 | 3.9 | −29.6–27.2 | −1.0 | 3.9 | 3.1 | −26.3–23.4 | 12.1 | 1.1 | 2.0 | 4.2 | 12.2 |
DVF = Deformation vector field.
Fig. 3.
Boxplots showing the distribution of mean and standard deviation (SD) target motion in individual patients and CBCTs for the rigid and non-rigid approximation strategies. Horizontal bars, boxes and whiskers represent mean, 5th, 95th percentiles, minimum and maximum.
3.2. Impact of rectal and bladder volume on target motion
Despite a bladder filling protocol to minimise changes in bladder volume during treatment, there were substantial variations in some patients. The maximal change in CBCT bladder volume relative to baseline was 9–260%. The maximal change in rectal volume (RV) relative to baseline was 32–249%. There was weak but statistically significant correlation between relative bladder volume and RV on CBCT and treatment time point, with a trend towards smaller volumes later in treatment (r = −0.3, p < 0.05 for bladder and r = −0.2, p < 0.05 for rectum). Mean % RV change, relative to the pCT, in CBCT acquired days 1–3 was strongly correlated with mean % RV change over all CBCT (r = +0.8) and CBCT acquired from day 4 onwards (r = +0.7) both p < 0.01.
In both the rigid and non-rigid approximation data, RV change relative to pCT was correlated with anterior and posterior target motion and this was statistically significant. For the non-rigid approximation, there was moderate to strong correlation r = −0.6 for posterior displacement of posterior CTVv wall and r = +0.6 for anterior displacement of anterior CTVv wall (both p < 0.01). For the rigid approximation data correlation was weak to moderate, r = −0.3 and r = +0.4 for posterior and anterior CTVv wall displacement in respective directions (both p < 0.05). For the rigid approximation data, bladder volume relative to pCT was very weakly correlated with anterior wall displacement (r = 0.2, p < 0.05), but there was no correlation with the non-rigid approximation data.
3.3. Dosimetric impact of target motion on simulated delivered dose
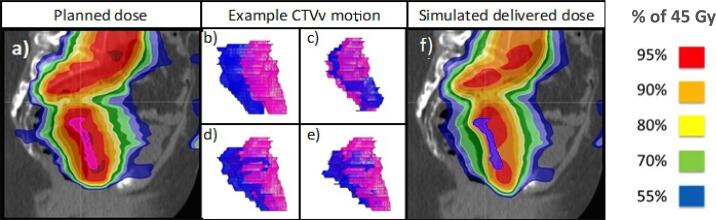
There were statistically significant differences in the planned and delivered dose for all target CTVv dose constraints (Table 2). There was underdosing of CTVv D95% in 3/17 (18%) of patients who did not receive the target dose constraint of 42.8 Gy (D95% 40.7 Gy, 41.4 Gy and 42.7 Gy). In these patients there was substantial reduction in RV during treatment compared to the reference pCT (mean RV during treatment, relative to baseline 51%, 58% and 61%), which resulted in posterior displacement of the CTVv (Fig. 4). Seven patients (41%) had underdosing of CTVv D50% which did not reach the dose constraint of 44.6–45.5 Gy. There were also statistically significant differences in delivered dose for the rectum V30 Gy and the mean bladder dose (Fig. 4). CTVv motion outside the target region occurred posteriorly in both superior and inferior halves of the CTVv.
Table 2.
Summary of dosimetric results for CTV and OAR planned and delivered dose.
| Dose constraint | Planned dose (Gy) |
Delivered dose (Gy) |
p-value | ||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | ||
| CTVv D95% | 43.9 (0.4) | 43.0–44.4 | 43.4 (1.1) | 40.7–45.0 | 0.02 |
| CTVv D99% | 43.5 (0.3) | 42.7–44.0 | 43.0 (1.2) | 39.5–44.6 | 0.04 |
| CTVv D50% | 45.1 (0.3) | 44.6–45.7 | 44.5 (0.7) | 42.9–45.7 | <0.01 |
| CTVv D5% | 46.3 (0.4) | 45.6–47.1 | 45.5 (0.7) | 44.0–46.8 | <0.01 |
| CTVv D2% | 46.6 (0.4) | 45.9–47.3 | 45.7 (0.7) | 44.2–47.0 | <0.01 |
| Rectum V40 Gy | 0.6 (0.2) | 0.3–0.9 | 0.5 (0.3) | 0.0–0.9 | 0.35 |
| Rectum V30 Gy | 0.8 (0.4) | 0.7–1.0 | 0.8 (0.1) | 0.6–1.0 | <0.01 |
| Bladder mean dose | 37.5 (2.4) | 31.3–41.4 | 37.7 (2.5) | 31.3–42.4 | 0.04 |
Fig. 4.
Sagittal images of patient with significant under dosing of CTVv; a) Planned dose with CTVv in pink, (b-e) CTVv geometry at treatment CBCTs (blue) compared with CTVv geometry at planning CT (pink) for weeks 1–4 respectively, (f) Simulated delivered dose with CTVv in blue. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We have demonstrated statistically significant differences in target motion assessment of CTVv and OAR in IMRT for EC using rigid versus non-rigid approximation. Normal tissue sparing with IMRT in EC is limited by geometric changes in the CTVv target and OARs. Accurate assessment of these changes is essential to generate appropriate margins and ensure adequate target coverage during treatment. The first aim of this study was to evaluate target motion using both rigid and non-rigid approximation strategies. To our knowledge, no studies have compared this before. Non-rigid approximation generated larger estimates of mean motion and σ error. Anterior/posterior target motion was moderately correlated with RV change relative to pCT for both motion assessment strategies, but correlation was stronger for the non-rigid data. This compares to other studies using rigid registration, which have only shown weak correlation between anterior/posterior target motion and RV change [8], [20].
Previous published data for target motion in EC has used rigid registration strategies and traditional margin recipes that assume these targets are rigid. However, we know these targets are deformable structures. Our data suggests that traditional rigid approximation strategies do not reflect the geometric complexity and underestimate the extent of motion. This results in generation of inadequate internal margins. Over 85% of patients studied had DVFD in the anterior/posterior and right/left direction, which were outside internal margins calculated using the rigid approximation data and rigid margin recipes. Non-rigid registration provides a much better representation of geometric change and has been validated for robustness and reproducibility [21]. The challenge now is to translate motion data acquired through deformable registration strategies into new internal margins, which improve target coverage and reduce normal tissue irradiation.
Both the rigid and non-rigid approximation data indicate that anisotropic margins are required. Calculated margins using rigid margin recipes are larger than the 10 mm circumferential margins we presently use clinically. Extent and variability in target CTVv motion was largest in the anterior/posterior direction with displacements of up to 30 mm observed. This is consistent with other published studies [8], [9], [10], [12].
The second aim of this study was to evaluate the dosimetric impact of target motion by comparing the planned dose with the simulated delivered dose. This has not been evaluated previously in patients receiving external beam radiotherapy for EC. We found statistically significant differences in the planned and simulated delivered dose. There was underdosing of the target CTVv D95% in three patients and underdosing of D50% in seven patients. Studies evaluating the dosimetric advantage of IMRT have not taken target and OAR geometric change into account and do not necessarily reflect the actual delivered dose. They may therefore under or over-estimate target coverage and OAR sparing. A previous study has evaluated dose accumulation using rigid and non-rigid deformable registration strategies in 19 patients receiving 3 or 5 fraction brachytherapy to the vaginal vault for EC [22]. They also found a statistically significant difference in the delivered dose to the bladder, but in comparison to our results they did not find a significant difference in the dose delivered to the rectum. They did not evaluate the dose delivered to the CTVv. DIR can account for the deformable nature of the CTVv, rectum and bladder, which makes it attractive for dose accumulation in the pelvis. But there are limitations and challenges in using DIR for dose accumulation. Large geometric changes in pelvic anatomy between image sets and difficulty interpreting sliding tissue interfaces can cause registration errors, and the image registration algorithm itself is limited by the model used to describe the deformation. These registration uncertainties lead to dose mapping uncertainty and even a very small registration error can lead to substantial error in dose accumulation at sites of sharp dose gradient or geometric uncertainty, such as those that occur between the target CTVv and the rectum. We quantitatively evaluated the registration error using DSC and MDA, which were within tolerance in keeping with the AAPM Task Group 132 report [23]. Solutions to estimate the impact on the dose distribution from uncertainties in the DIR algorithm have been presented [24], but there is no consensus on how this should be achieved and this was not further evaluated in our study.
There were no specific rectal filling instructions at pCT or during treatment and substantial variation in rectal filling was observed. We found moderate to strong correlation between RV and target anterior/posterior motion (r = 0.6) and patients who had a rectal diameter >50 mm at pCT had underdosing of CTVv D95%. In two patients with underdosing of CTVv D95%, anterior/posterior rectal diameter at pCT measured 56 mm and 52 mm, and there was substantial reduction in RV during treatment compared to the reference CT, which resulted in posterior displacement of the CTVv. The 3rd patient with underdosing of CTVv D95% also had posterior displacement of CTVv during treatment due to reduced RV, but this was not predictable based on rectal diameter at pCT, which was 36 mm. In all 3 patients with underdosing of CTVv D95%, RVs of less than 50% of the RV at pCT were seen within the first week of treatment. Anticipation of posterior displacement by limiting rectal diameter at pCT or increasing posterior margins in patients with large rectal diameter has been suggested. In our study a threshold of <50 mm rectal diameter would identify 2/3 patients with clinical underdosing of target, but rectal diameter was a poor surrogate for RV at pCT and RV variation during treatment. For example, 1 patient with rectal diameter of 49 mm at pCT had very stable RV throughout treatment with no underdosing of the target. We did find mean RV change, relative to the pCT, in CBCT acquired days 1–3 was strongly correlated with mean % RV change over all CBCT (r = +0.8) and CBCT acquired day 4 onwards (r = +0.7) both p < 0.01. This suggests that patients who could benefit from margin adaptation can be identified within the first week of treatment.
In a study including 15 post-operative cervix and endometrial patients, the impact of bladder and rectal filling on organ dose was evaluated [25]. Patients had two pCTs, one with an empty bladder and rectum and the second following bladder and rectum filling. There was no reduction in integral rectal dose with increased rectal filling. This compares to a study in 92 patients who received brachytherapy to the vaginal vault where there was a significant positive correlation between rectal volume and Dmax, D0.1cc and D1cc and D2cc [26]. In a study of 10 prostate patients, contouring the entire rectum, compared to contouring the proximal and distal rectal volumes separately, led to significantly increased dose to the distal rectum [27]. This increased for patients with large rectal volumes at pCT with a mean increase in dose of 31% ± 19 for D50.
RV variation and the range of anterior/posterior target motion is difficult to predict. Rectal filling strategies to reduce RV variation such as regular use of laxatives have been studied [28], [29], [30], [31], but in gynaecological radiotherapy these have failed to show benefit [30]. Taku et al. evaluated the use of rectal balloon and found that smaller 7 mm anterior/posterior margins were required as measured using fiducial makers and rigid registration techniques [13].
Based on the results presented here we suggest some strategies to improve IMRT in EC. Population based margins using non-rigid approximation and non-rigid margin recipes should be developed. Accounting for variations in target deformation through the whole volume may reduce the incidence of geographic miss and dose to OAR. This should be coupled with daily CBCT imaging to identify patients at most risk of RV variation during treatment. Our study suggests that these patients, who may require margin adaptation, can be identified within the first week of treatment. IMRT techniques take full advantage of normal tissue sparing whilst maintaining adequate target coverage, but additional adaptive strategies need to be developed. We suggest a plan selection strategy with variable anterior/posterior margins, which is where the largest variation in target motion was observed. Unless adaptive therapy is used on the basis of our rigid approximation analysis, margins of 11 mm in right and left directions, 14 mm anteriorly and 12 mm posteriorly are required in IMRT for EC without daily online imaging.
Limitations of our study include the relatively poor soft tissue contrast of CBCT for contour delineation and lack of imaging data for each treatment fraction. To reduce contour delineation variability and error all contouring was performed by the same experienced observer and reviewed by a second senior radiation oncologist. The DIR and dose accumulation results were driven by these contoured structures so delineation error will affect their accuracy. A comprehensive analysis of interobserver contouring variability was not addressed in this study. Previous studies have used MRI or diagnostic CT to evaluate target motion, but for dose accumulation CBCT was necessary to reflect patient target and OAR geometry at the time of treatment.
In conclusion, significant differences in target motion assessment were seen between rigid and non-rigid registration strategies with non-rigid approximation demonstrating complex and extensive geometric changes to the CTVv, which were not accounted for in margin recipes using rigid registration. Target and OAR motion was observed that exceeded the motion accounted for within our standard clinical margins and margins calculated using rigid margin recipes. This resulted in underdosing in some patients. RV relative to the pCT was moderately correlated with anterior/posterior displacement and could be predicted from CBCTs acquired days 1–3. EC patients need daily on-line image guidance. Contemporary margin recipes and adaptive treatment planning based on non-rigid approximation are recommended.
Acknowledgments
Acknowledgements
This work was undertaken in The Royal Marsden NHS Foundation Trust who received a proportion of its funding from the NHS Executive; the views expressed in this publication are those of the authors and not necessarily those of the NHS Executive. This work was supported by The Institute of Cancer Research and Cancer Research UK [CRUK] grant number C33589/A19727. We acknowledge NHS funding to the NIHR Biomedical Research Centre.
Conflicts of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2019.02.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Keys H.M., Roberts J.A., Brunetto V.L., Zaino R.J., Spirtos N.M., Bloss J.D. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 2.Creutzberg C.L., Nout R.A., Lybeert M.L., Warlam-Rodenhuis C.C., Jobsen J.J., Mens J.W. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e631–e638. doi: 10.1016/j.ijrobp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Roeske J.C., Lujan A., Rotmensch J., Waggoner S.E., Yamada D., Mundt A.J. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2000;48:1613–1621. doi: 10.1016/s0360-3016(00)00771-9. [DOI] [PubMed] [Google Scholar]
- 4.Guo S., Ennis R.D., Bhatia S., Trichter F., Bashist B., Shah J. Assessment of nodal target definition and dosimetry using three different techniques: implications for re-defining the optimal pelvic field in endometrial cancer. Radiat Oncol. 2010:59. doi: 10.1186/1748-717X-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mundt A.J., Mell L.K., Roeske J.C. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:1354–1360. doi: 10.1016/s0360-3016(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen M.F., Tseng C.J., Tseng C.C., Kuo Y.C., Yu C.Y., Chen W.C. Clinical outcome in posthysterectomy cervical cancer patients treated with concurrent Cisplatin and intensity-modulated pelvic radiotherapy: comparison with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1438–1444. doi: 10.1016/j.ijrobp.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Beriwal S., Heron D.E., Kim H., King G., Shogan J., Bahri S. Intensity-modulated radiotherapy for the treatment of vulvar carcinoma: a comparative dosimetric study with early clinical outcome. Int J Radiat Oncol Biol Phys. 2006;64:1395–1400. doi: 10.1016/j.ijrobp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Jurgenliemk-Schulz I.M., Toet-Bosma M.Z., de Kort G.A., Schreuder H.W., Roesink J.M., Tersteeg R.J. Internal motion of the vagina after hysterectomy for gynaecological cancer. Radiother Oncol. 2011;98:244–248. doi: 10.1016/j.radonc.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Ma D.J., Michaletz-Lorenz M., Goddu S.M., Grigsby P.W. Magnitude of interfractional vaginal cuff movement: implications for external irradiation. Int J Radiat Oncol Biol Phys. 2012;82:1439–1444. doi: 10.1016/j.ijrobp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Jhingran A., Salehpour M., Sam M., Levy L., Eifel P.J. Vaginal motion and bladder and rectal volumes during pelvic intensity-modulated radiation therapy after hysterectomy. Int J Radiat Oncol Biol Phys. 2012;82:256–262. doi: 10.1016/j.ijrobp.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Harris E.E., Latifi K., Rusthoven C., Javedan K., Forster K. Assessment of organ motion in postoperative endometrial and cervical cancer patients treated with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:e645–e650. doi: 10.1016/j.ijrobp.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Rash D., Hagar Y., Cui J., Hunt J.P., Valicenti R., Mayadev J. Interfraction motion of the vaginal apex during postoperative intensity modulated radiation therapy: are we missing the target? Int J Gynecol Cancer. 2013;23:385–392. doi: 10.1097/IGC.0b013e3182791f24. [DOI] [PubMed] [Google Scholar]
- 13.Taku N., Dise J., Kenton O., Yin L., Teo B.K., Lin L.L. Quantification of vaginal motion associated with daily endorectal balloon placement during whole pelvis radiotherapy for gynecologic cancers. Radiother Oncol. 2016;120:532–536. doi: 10.1016/j.radonc.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto H., Murakami N., Carvajal C.C., Miura Y., Wakita A., Nakamura S. Positional uncertainty of vaginal cuff and feasibility of implementing portable bladder scanner in postoperative cervical cancer patients. Phys Med. 2018;45:1–5. doi: 10.1016/j.ejmp.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Wu C.C., Wuu Y.R., Yanagihara T., Jani A., Xanthopoulos E.P., Tiwari A. Rectal balloon use limits vaginal displacement, rectal dose, and rectal toxicity in patients receiving IMRT for postoperative gynecological malignancies. Med Dosim. 2018;43:23–29. doi: 10.1016/j.meddos.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Weistrand O., Svensson S. The ANACONDA algorithm for deformable image registration in radiotherapy. Med Phys. 2015;42:40–53. doi: 10.1118/1.4894702. [DOI] [PubMed] [Google Scholar]
- 17.van Herk M., Remeijer P., Rasch C., Lebesque J.V. The probability of correct target dosage: dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 18.Measurements. ICtRUa. ICRU report 62: Prescribing, recording, and reporting photon beam therapy (supplements to ICRU report 50).1999.
- 19.Dunlop A., McQuaid D., Nill S., Murray J., Poludniowski G., Hansen V.N. Comparison of CT number calibration techniques for CBCT-based dose calculation. Strahlenther Onkol. 2015;191:970–978. doi: 10.1007/s00066-015-0890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Bunt L., Jurgenliemk-Schulz I.M., de Kort G.A., Roesink J.M., Tersteeg R.J., van der Heide U.A. Motion and deformation of the target volumes during IMRT for cervical cancer: what margins do we need? Radiother Oncol. 2008;88:233–240. doi: 10.1016/j.radonc.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Oh S., Kim S. Deformable image registration in radiation therapy. Radiat Oncol J. 2017;35:101–111. doi: 10.3857/roj.2017.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabater S., Andres I., Sevillano M., Berenguer R., Machin-Hamalainen S., Arenas M. Dose accumulation during vaginal cuff brachytherapy based on rigid/deformable registration vs. single plan addition. Brachytherapy. 2014;13:343–351. doi: 10.1016/j.brachy.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Brock K.K., Mutic S., McNutt T.R., Li H., Kessler M.L. Use of image registration and fusion algorithms and techniques in radiotherapy: report of the AAPM Radiation Therapy Committee Task Group No. 132. Med Phys. 2017;44:e43–e76. doi: 10.1002/mp.12256. [DOI] [PubMed] [Google Scholar]
- 24.Salguero F.J., Saleh-Sayah N.K., Yan C., Siebers J.V. Estimation of three-dimensional intrinsic dosimetric uncertainties resulting from using deformable image registration for dose mapping. Med Phys. 2011;38:343–353. doi: 10.1118/1.3528201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchali A., Koswig S., Dinges S., Rosenthal P., Salk J., Lackner G. Impact of the filling status of the bladder and rectum on their integral dose distribution and the movement of the uterus in the treatment planning of gynaecological cancer. Radiother Oncol. 1999;52:29–34. doi: 10.1016/s0167-8140(99)00068-7. [DOI] [PubMed] [Google Scholar]
- 26.Sabater S., Arenas M., Berenguer R., Machin-Hamalainen S., Andres I., Sevillano M.M. Dosimetric analysis of rectal filling on rectal doses during vaginal cuff brachytherapy. Brachytherapy. 2015;14:458–463. doi: 10.1016/j.brachy.2015.02.391. [DOI] [PubMed] [Google Scholar]
- 27.Guckenberger M., Pohl F., Baier K., Meyer J., Vordermark D., Flentje M. Adverse effect of a distended rectum in intensity-modulated radiotherapy (IMRT) treatment planning of prostate cancer. Radiother Oncol. 2006;79:59–64. doi: 10.1016/j.radonc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 28.McNair H.A., Wedlake L., McVey G.P., Thomas K., Andreyev J., Dearnaley D.P. Can diet combined with treatment scheduling achieve consistency of rectal filling in patients receiving radiotherapy to the prostate? Radiother Oncol. 2011;101:471–478. doi: 10.1016/j.radonc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 29.McNair H.A., Wedlake L., Lips I.M., Andreyev J., Van Vulpen M., Dearnaley D. A systematic review: effectiveness of rectal emptying preparation in prostate cancer patients. Pract Radiat Oncol. 2014;4:437–447. doi: 10.1016/j.prro.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Lim K., Kelly V., Stewart J., Xie J., Cho Y.B., Moseley J. Pelvic radiotherapy for cancer of the cervix: is what you plan actually what you deliver? Int J Radiat Oncol Biol Phys. 2009;74:304–312. doi: 10.1016/j.ijrobp.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 31.Andres I., Gutierrez-Perez M., Rodriguez-Vela M.P., Berenguer R., Sevillano M., Aguayo M. The usefulness of fleet rectal enemas on high-dose-rate intracavitary cervical cancer brachytherapy. A prospective trial. J Contemp Brachytherapy. 2017;9:224–229. doi: 10.5114/jcb.2017.68135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.