Highlights
-
•
Very high patient position accuracy can be achieved in spine SBRT treatments.
-
•
Short beam-on time reduces the need for intrefractional imaging.
-
•
Adding an extra verification CBCT prior to treatment increases position accuracy.
Keywords: Stereotactic body radiotherapy, Spine, Intrafractional motion, Cone beam computed tomography, Image guided radiotherapy, Flattening filter free
Abstract
Background and purpose
Spine stereotactic body radiotherapy (SBRT) requires a high degree of accuracy due to steep dose gradients close to the spinal cord. This study aimed to (1) evaluate intrafractional motion in spine SBRT utilizing flattening filter free (FFF) beam delivery and cone beam computed tomography (CBCT) image guidance and (2) evaluate if adding another CBCT acquisition and corrections prior to treatment improves the overall position accuracy.
Materials and methods
Intrafractional motion was retrospectively analyzed for 78 fractions in 54 patients. All patients were immobilized with an evacuated cushion. Before treatment, a CBCT was acquired, a bony fusion with the planning CT was performed and translational and rotational errors were corrected. For 30 of the patients (39 fractions) acquisition of another CBCT and corrections were performed before treatment. A post treatment CBCT was acquired for all patients, and translational and rotational errors measured by fusion of the post treatment CBCT with the planning CT were recorded to calculate means and standard deviations (SDs).
Results
The positional errors were significantly smaller in 4 out of 6 error values in the patient group treated with verification CBCT. In this group, translational and rotational SDs ranged from 0.5 to 0.6 mm and 0.3°, respectively. Corresponding values in the group treated without verification CBCT were 0.7–1.0 mm and 0.4–0.7°.
Conclusion
With proper CBCT image guidance, patient immobilization and FFF-beam delivery, one can obtain very high patient position accuracy in spine SBRT. Inclusion of a verification CBCT prior to treatment increases the overall position accuracy.
1. Introduction
The spine is one of the most common sites of metastatic cancer disease, and it has been estimated that approximately 10% of cancer patients will develop spine metastases [1]. The standard treatment option for these patients has traditionally been conventional palliative fractionated external-beam radiotherapy (EBRT). This treatment approach is, however, associated with relatively low rates of complete tumor response and limited pain relief after 3–6 months [2], [3], [4], [5], [6], [7], [8].
In recent years, stereotactic body radiation therapy (SBRT) has emerged as an alternative approach in the treatment of spinal metastases. In spine SBRT, high doses are delivered to a confined target volume in one or a few fractions while keeping the dose to the spinal cord within tolerance. Published data suggests that this approach results in high rates of local control and pain response and low rates of toxicity [9]. SBRT of spine metastases is technically challenging because of the spinal cord tolerance dose. Due to this constraint the dose distribution has a steep dose gradient close to the spinal cord. The aim of delivering a high dose to the target volume while keeping the spinal cord dose within tolerance poses high technical requirements for treatment planning, patient immobilization and image guidance. It has been shown that small errors in patient positioning (≤2 mm) can lead to significant increase in spinal cord dose [10], [11], [12]. An excessive dose to the spinal cord is associated with increased risk of radiation myelopathy [13], [14]. Myelopathy is a feared side effect that can significantly influence the patient’s quality of life and in the worst case lead to death.
Several studies have reported on patient setup accuracy and intrafractional motion in image guided spine SBRT [15], [16], [17], [18], [19], [20]. In all these studies the entire treatment duration for one fraction, including patient setup, imaging and beam delivery, was relatively long: more than 60 min in some cases. This is related to time-consuming patient positioning, often highly modulated treatment plans and the high number of monitor units required to deliver high doses. Longer treatment durations increase the risk of unwanted patient movements [21] and hence intrafractional imaging and patient position correction are often used to improve accuracy.
Recently, linear accelerators with the ability to deliver beams without a flattening filter (FF) have become commercially available. The use of flattening filter free (FFF) beams leads to a significant increase in dose rate, which reduces beam-on time considerably in SBRT [22], [23], [24]. In addition, many accelerators are equipped with a six degrees of freedom (6DoF) couch and patients position errors are typically corrected by the use of cone beam computed tomography (CBCT). When applying the positional corrections, even small couch movements may be very noticeable for the patient, especially rotations along the x- and y-axis (pitch and roll). We hypothesize that when these corrections are applied, some patients may do a countermovement to stabilize themselves. Acquisition of another CBCT and corrections prior to treatment could improve the overall patient position accuracy.
The purpose of this study was (1) to evaluate intrafractional patient motion in spine SBRT treatment utilizing fast FFF beam delivery, CBCT image guidance and patient position correction in six degrees of freedom; and (2) to evaluate the difference in overall patient positional accuracy when implementing another verification CBCT and corrections prior to treatment.
2. Methods and materials
2.1. Patient characteristics
Fifty-four patients treated with seventy-eight fractions of spine SBRT between March 2015 and April 2018 were retrospectively included in this analysis. Treatment sites included thoracic, lumbar and sacral vertebras. To ensure immobilization homogeneity in the patient group, patients with cervical metastases were not included in the analysis, since they are immobilized differently than patients with metastases in other vertebrae segments (thermoplastic mask versus evacuated cushion). Patient characteristics are summarized in Table 1.
Table 1.
Patient and treatment characteristics.
| Characteristic | Parameter | No. of patients |
|---|---|---|
| Tumor location | Thoracic | 36 |
| Lumbar | 19 | |
| Sacral | 3 | |
| Involved vertebral bodies | 1 | 47 |
| 2 | 7 | |
| Technique | VMAT | 48 |
| IMRT | 2 | |
| 3DCRT | 4 | |
2.2. Immobilization and treatment planning
Patients were immobilized in an evacuated cushion (BlueBAG; Elekta, Stockholm, Sweden) which is shaped around the patient to make a precise mold of the patient’s position. Patients were treated in supine position with their arms abducted above the head.
CT images with slice thickness of 1.5 mm were acquired and imported into Oncentra External Beam v.4.3 (Elekta, Stockholm, Sweden) or RayStation v.5.0 (RaySearch Laboratories, Stockholm, Sweden) for delineation and treatment planning. An axial T2-weighted magnetic resonance imaging (MRI) scan with a slice thickness of 1 mm was also acquired and manually co-registered to the planning CT to guide tumor and organ at risk (OAR) delineation. The MRI was not acquired with the patients in treatment position. However, we do not think this will affect the accuracy significantly since the registration to the CT was done focusing only on a very limited part of the patient’s body, i. e., the involved vertebra(es). Gross tumor volume (GTV) and clinical target volume (CTV) were manually contoured. To create a planning target volume (PTV), the CTV was expanded only in areas necessary to create a 3 mm margin from the GTV. A 3 mm margin was added to the spinal cord to create a planning OAR volume (PRV). Patients were typically treated with 16 Gy in 1 fraction or 27 Gy in 3 fractions prescribed to the 67% isodoseline.
Planning techniques included volumetric modulated arc therapy (VMAT), intensity modulated radiotherapy (IMRT) and 3D conformal radiotherapy (3DCRT) with the vast majority of plans made with VMAT. The 3DCRT planning was based on a beam template with nine static beams and manual adjustment of the leafs was used in order to fulfill spinal cord dose constraints. This technique was used for only four patients in the first phase of the project. Treatment techniques are summarized in Table 1.
2.3. Image guided treatment
All patients were treated on a Varian TrueBeam STx linear accelerator (Varian Medical Systems, Inc., Palo Alto, USA). The TrueBeam STx is equipped with a HD120 Multileaf Collimator (MLC), a kilovoltage CBCT image-guidance system and a PerfectPitch 6DoF couch. Treatments were delivered with 10 MV FFF beams with a maximum dose rate of 2400 monitor units (MU) per minute.
Initial setup was based on patient tattoos and/or marks on the BlueBAG. The subsequent image guidance can be divided into two patient cohorts: cohort 1 consists of 39 fractions from 24 patients treated between March 2015 and October 2016. After initial setup, a CBCT was acquired (CBCTsetup) and translational and rotational deviations from the planning CT were corrected. If the ±3° rotational couch tolerance was exceeded, the patients were manually repositioned and the process was reinitiated. Immediately after treatment, another CBCT (CBCTposttr) was acquired to assess intrafractional patient movement. Cohort 2 consists of 39 fractions from 30 patients treated between December 2016 and April 2018. For these patients we included acquisition of a verification CBCT (CBCTverif) with corrections immediately after CBCTsetup with corrections. CBCTposttr was acquired in the same way as in cohort 1. In both cohorts, all patient position deviations were corrected.
2.4. Data analysis
A total of 78 setup, 39 verification and 78 post-treatment CBCT series were evaluated.
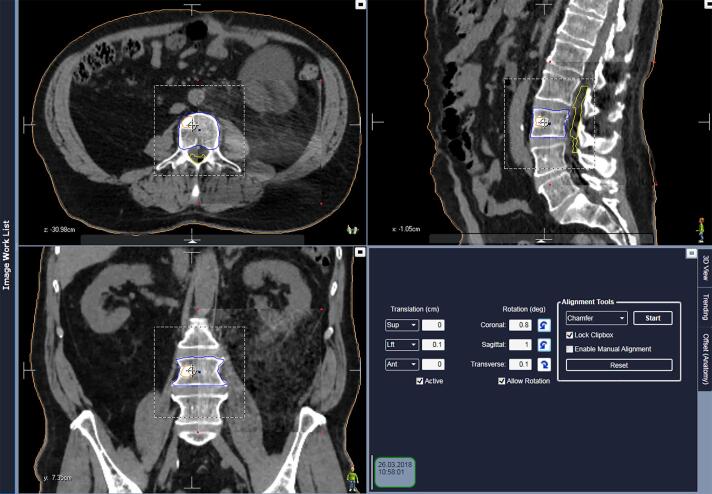
Each of the CBCTs was compared with the planning CT and three translational and three rotational deviations in position error were recorded per CBCT series. Deviations for CBCTsetup and CBCTverif were recorded directly from the online fusion performed at the time of treatment. This was done by experienced treatment therapists. Deviations for CBCTposttr were retrospectively recorded from automatic offline fusion using a chamfer algorithm in the Image Review Workspace in Mosaiq v.2.64 (Elekta, Stockholm Sweden) (Fig. 1). In all cases a rigid, bony-registration was used with the clipbox covering the involved vertebrae with a margin. Typically the two adjacent vertebras and a lateral and anterior/posterior margin of 2–3 cm were included in the clipbox. All CBCTposttr were reviewed by the same medical physicist.
Fig. 1.
Example of offline fusion in Mosaiq of a patient with a metastasis in L3. Cauda equine is outlined in yellow, GTV in orange and PTV in blue. The figure shows planning CT and post treatment CBCT in rectangular spyglass (corners marked with red dots). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Since all the deviations between the planning CT and the CBCTsetup and CBCTverif were corrected, ideally there should not be any deviation between the planning CT and the CBCTposttr. Therefore, intrafractional motions were quantified by calculating this latter deviation for the vertical, longitudinal and lateral directions as well as for the yaw, pitch and roll for each of the two cohorts. Systematic deviations within each cohort were evaluated by testing the mean values for these parameters against zero using Student T-test after normal distribution of the data was confirmed (Normal Q-Q-plots). Furthermore, the standard deviations (SD) were compared using Fischer’s exact test to evaluate differences in random errors between the two cohorts. SPSS version 25 was used for statistical calculation, and statistical significance was considered at p < 0.008 (after Bonferroni correction).
3. Results
Stereotactic treatment was successfully completed in all patients without any need for manual repositioning. The mean time ± SD between start of CBCTsetup and start of CBCTposttr was 8.5 ± 2.1 and 10.7 ± 1.7 min in cohort 1 and cohort 2, respectively. The mean values of the positional errors from the CBCTsetup in the two cohorts were not significantly different in any of the six translational or rotational directions, i.e., no difference in initial patient setup accuracy between the two cohorts was found. The mean translation deviations ± SD between CBCTverif and the planning CT for patients in cohort 2 were 0.1 ± 0.5 mm (range: −1 to 2 mm) in the vertical, 0.0 ± 0.4 mm (range: −1 to 1 mm) in the longitudinal and 0.0 ± 0.5 mm (range: −1 to 1 mm) in the lateral direction. Rotational deviations were −0.1 ± 0.2° (range: −0.9 to 0.4°) along yaw, 0.0 ± 0.4° (range: −0.9 to 1.3°) along pitch and 0.0 ± 0.3° (range: −0.8 to 0.4°) along roll.
The mean values and SDs for all translational and rotational deviations between the planning CT and the CBCTposttr are presented in Table 2 for both cohorts, including absolute values. In cohort 1, the mean deviation ranged from −0.2 to 0.2 mm for translational errors and from 0 to 0.1° for rotational errors, while corresponding figures for cohort 2 were −0.2 to 0.1 mm and −0.2 to 0°. Neither of these mean values were significant different from zero. SDs (not absolute values) ranged from 0.7 to 1.0 mm for translational errors and 0.4 to 0.7° for rotational errors in cohort 1 and 0.5 to 0.6 mm and 0.3° in cohort 2. Pairwise comparison of the SDs in the two cohorts showed a significant difference between the longitudinal and lateral displacement as well as for the rotations around the z-axis (yaw) and x-axis (pitch) (p < 0.008). For the two remaining parameters, the difference was just not significant.
Table 2.
Summary of translational and rotational deviations from the CBCTposttr in both patient cohorts. Summary of absolute values are also shown.
| Parameter | Translational deviations (mm) |
Rotational deviations (degrees) |
Time* (min) | ||||
|---|---|---|---|---|---|---|---|
| Vertical | Longitudinal | Lateral | Yaw | Pitch | Roll | ||
| Cohort 1 (n = 39) | 8.5 ± 2.1 | ||||||
| Mean | 0.2 | 0.2 | −0.2 | 0.0 | 0.0 | 0.1 | |
| SD | 0.7 | 1.0 | 0.9 | 0.4 | 0.7 | 0.5 | |
| Mean_abs | 0.5 | 0.7 | 0.6 | 0.3 | 0.4 | 0.4 | |
| SD_abs | 0.6 | 0.7 | 0.7 | 0.3 | 0.6 | 0.3 | |
| Cohort 2 (n = 39) | 10.7 ± 1.7 | ||||||
| Mean | −0.2 | 0.1 | 0.0 | 0.0 | −0.1 | −0.2 | |
| SD | 0.5 | 0.5 | 0.6 | 0.3 | 0.3 | 0.3 | |
| Mean_abs | 0.2 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | |
| SD_abs | 0.5 | 0.4 | 0.5 | 0.2 | 0.2 | 0.2 | |
| p-Value** | 0.051 | <0.001 | 0.001 | 0.002 | <0.001 | 0.053 | |
*Time is between start of CBCTsetup and start of CBCTposttr.
**Fischer exact test comparing SD for the two cohorts.
Fig. 2 illustrates the translational and rotational error distributions from CBCTposttr in both cohorts. For cohort 1, in 30 of 39 fractions (77%), patients had a translational error within 1 mm in any direction and in 37 of 39 fractions (95%) within 2 mm. In 35 of 39 fractions (90%), patients had a rotational error within 1° around any axis and in 38 of 39 fractions (97%) within 2°. One patient treated with fractionated SBRT to Th2 had a lateral error of −3 mm on one fraction and a pitch error of 3.3° on another fraction. Another patient also treated with fractionated SBRT to Th8 had a longitudinal error of 3 mm on one fraction. For none of the patients were the positions consistently off. Corresponding values for patients in cohort 2 were 37 of 39 fractions (95%) within 1 mm, 39 of 39 fractions (100%) within 2 mm and 39 of 39 fractions (100%) within 1°. In this cohort, two different patients had a vertical error of 2 mm.
Fig. 2.
Distribution of translational errors (A) and rotational errors (B) from CBCTposttr in both patient cohorts.
4. Discussion
We have in this study shown that intrafractional positional error is small when treating spine SBRT patients with the very short beam-on time achieved using FFF beam delivery. The positional errors as measured on the post treatment CBCT (compared with planning CT) were significantly smaller in the longitudinal and lateral direction as well as rotation around the z-axis (yaw) and x-axis (pitch) in the patient group were we included the acquisition of a verification CBCT before treatment. These results suggest that the inclusion of a verification CBCT in the treatment procedures will somewhat improve the overall patient positional accuracy. Certainly not all patients will clinically benefit from this extra CBCT and positional corrections. However, based on the results of this study, we believe that some patients will do a countermovement when corrections from the CBCTsetup are applied. This justifies the slight increase in time used to acquire an extra CBCT, registration with the planning CT and applying corrections (approximately 2 min) and the minimal increase in radiation dose due to an extra CBCT. Identifying for which patients inclusion of a verification CBCT should be done is not easy. We therefore recommend doing it for all patients.
Our results are in good agreement with previously reported data on spine SBRT and patient position accuracy [15], [16], [17], [18], [19], [20]. There are, however, two major differences between our study and these others. (1) We used FFF-beams in our treatment resulting in a significant decrease in beam-on time compared to FF-beams. We were unable to quantify this difference in beam-on time since all our patients were treated with FFF-beams. However, several other studies have done such comparisons [22], [23], [24]. For instance, Nalichowski et al. [24] reported average beam-on times of 4.4 and 9.5 min when comparing RapidArc spine SBRT plans with and without FFF, respectively. (2) We did not use any kind of intrafractional imaging. Chang et al. [15] and Hyde et al. [19] both reported on spine SBRT treatment using BodyFIX (Elekta, Stockholm, Sweden) and a 6D robotic couch with a total treatment duration of up to 60 min or more. BodyFIX combines a vacuum cushion with a plastic fixation sheet, which is presumably more rigid than only the evacuated cushion. In both studies, 1–2 intrafractional CBCTs were acquired and patient position corrections were applied according to their specific action level. They reported absolute translational and rotational mean values of 0.4–0.9 mm and 0.2–0.6°, respectively, with SDs of 0.3–1.3 mm and 0.1–0.5° when comparing post treatment CBCT to planning CT. Li et al. [20] did an analysis comparing three different immobilization devices (evacuated cushion, BodyFIX and S frame Mask). However, at the time of the study, they did not have a robotic couch that could correct rotational offsets; thus, rotations ≤2° were not corrected. The reported translational mean values as recorded on post treatment CBCT compared to planning CT were 0.2–0.4 mm with SDs of 0.7–1.3. The reported mean time (min:s) ± SD (min:s) from localization to post-treatment CBCT was 34:22 ± 7:17.
Comparing our results with previous studies, we have shown that with the use of FFF-beams and the resulting very short beam-on time, one can obtain the same or better patient position accuracy without any intrafractional imaging as compared to treatment using FF-beams and some sort of intrafractional imaging. The use of a fixation sheet in addition to a vacuum cushion seems less important with such fast beam delivery. It is clear that a reduction in treatment duration will reduce the risk of unwanted patient movement, which is also supported by the study by Hoogeman et al. [21]. In the case of high-precision radiation treatment with small margins, e.g. spine SBRT with treatment durations of 15 min or longer, they concluded that repeated intrafractional imaging and patient setup correction with an interval of less than 5 min may adequately reduce the error associated with intrafractional patient movement. A typical spine SBRT treatment at our institution takes 10–20 min depending on patient condition and time needed for setup and imaging procedures. The beam-on time is typically as little as 2–3 min. The need for intrafractional imaging with such a short beam-on time seems to be much less important than for longer treatments.
In addition to FFF-beams, the use of VMAT-technique is a considerable factor when considering treatment efficiency. It has not been the purpose of this study to compare VMAT with other treatment techniques. However, several previous studies have reported significantly shorter beam-on times using VMAT compared to other techniques such as static intensity modulated radiotherapy (IMRT) [25], [26], [27], [28]. The use of VMAT and FFF-beams is an ideal combination to obtain very short beam-on times and minimize the risk of unwanted patient movements.
Patient position accuracy is used to determine appropriate PTV/PRV margins. Adequate spinal cord PRV margin is often a safety priority to avoid overdosing of the spinal cord. This results in a relative underdosing of parts of the target volume close to the spinal cord. Minimal reduction in PRV-margin could be clinically significant, because it would likely lead to lower rates of local failure by allowing higher dose in the epidural space surrounding the spinal cord. The epidural space has been recognized as a common site of treatment failure [29], [30], [31], [32], [33], [34]. For that reason it is important not to apply an excessive spinal cord PRV margin. The results from this study and other similar studies should be used to determine appropriate PTV/PRV-margins. We do not recommend any specific margins since these margins should be determined based on analysis of uncertainties throughout the whole treatment chain, not only intrafractional patient motion.
An analysis of intrafractional motion as a function of treatment site was not done in our work. One might assume that there is a difference in target intrafractional motion between lumbar and thoracic sites, however, several studies have shown that this is not the case [16], [17], [19].
Although there are many studies, including this, that have shown the feasibility and accuracy of spine SBRT, there is at the time of writing no high-level clinical evidence favoring SBRT over conventional EBRT for spine lesions, as there is no published randomized data comparing the two. However, there are several publications showing promising results with spine SBRT. In a review by Husain et al. from 2017 including 14 articles on spine SBRT [9], the authors reported local control rates of 90% at 1 year, complete pain response in >50% and low rates of toxicity for patients with de novo spinal metastases after SBRT. Currently, there are two ongoing randomized studies that compare SBRT and conventional EBRT for spine lesions. The Radiation Oncology Group (RTOG) 0631 trial (ClinicalTrials.gov Identifier NCT00922974) is a Phase II/III study comparing conventional palliative irradiation of 8 Gy in 1 fx and SBRT of 16–18 Gy in 1 fx. In addition, a randomized Phase II/III study from the National Cancer Institute of Canada (ClinicalTrials.gov Identifier NCT02512965) compares outcomes between 20 Gy in 5 fx of conventional palliative irradiation with 24 Gy in 2 fx of SBRT. Hopefully these trials will provide high-level evidence for the benefits of SBRT in comparison with conventional EBRT in the treatment of spine lesions.
In conclusion, with proper CBCT image guidance, patient immobilization, VMAT- and FFF-beam delivery, one can obtain very high patient position accuracy in spine SBRT treatment. Adding an extra verification CBCT prior to treatment increases the overall position accuracy. With the inclusion of a verification CBCT, patients in 95% of the treatment fractions had a positional error of ≤1 mm/1° as measured on the post treatment CBCT. With the very short beam-on time needed when utilizing VMAT and FFF-beams, the need for intrafractional imaging seems to be less important compared to longer treatments to obtain adequate precision in patient position and dose delivery
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank Sondre Lie for his assistance with editing the manuscript.
References
- 1.Greco C., Pares O., Pimentel N., Moser E., Louro V., Morales X. Spinal metastases: From conventional fractionated radiotherapy to single-dose SBRT. Rep Pract Oncol Radiother. 2015;20:454–463. doi: 10.1016/j.rpor.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaze M.N., Kelly C.G., Kerr G.R., Cull A., Cowie V.J., Gregor A. Pain relief and quality of life following radiotherapy for bone metastases: a randomised trial of two fractionation schedules. Radiother Oncol. 1997;45:109–116. doi: 10.1016/S0167-8140(97)00101-1. [DOI] [PubMed] [Google Scholar]
- 3.Hartsell W.F., Scott C.B., Bruner D.W., Scarantino C.W., Ivker R.A., Roach M. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen O.S., Bentzen S.M., Sandberg E., Gadeberg C.C., Timothy A.R. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol. 1998;47:233–240. doi: 10.1016/S0167-8140(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 5.Price P., Hoskin P.J., Easton D., Austin D., Palmer S.G., Yarnold J.R. Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiother Oncol. 1986;6:247–255. doi: 10.1016/S0167-8140(86)80191-8. [DOI] [PubMed] [Google Scholar]
- 6.Roos D.E., Turner S.L., O'Brien P.C., Smith J.G., Spry N.A., Burmeister B.H. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05) Radiother Oncol. 2005;75:54–63. doi: 10.1016/j.radonc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Steenland E., Leer J.W., van Houwelingen H., Post W.J., van den Hout W.B., Kievit J. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/S0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 8.Rich S.E., Chow R., Raman S., Liang Zeng K., Lutz S., Lam H. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother Oncol. 2018;126:547–557. doi: 10.1016/j.radonc.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Husain Z.A., Sahgal A., De Salles A., Funaro M., Glover J., Hayashi M. Stereotactic body radiotherapy for de novo spinal metastases: systematic review. J Neurosurg Spine. 2017;27:295–302. doi: 10.3171/2017.1.SPINE16684. [DOI] [PubMed] [Google Scholar]
- 10.Chuang C., Sahgal A., Lee L., Larson D., Huang K., Petti P. Effects of residual target motion for image-tracked spine radiosurgery. Med Phys. 2007;34:4484–4490. doi: 10.1118/1.2790587. [DOI] [PubMed] [Google Scholar]
- 11.Guckenberger M., Meyer J., Wilbert J., Baier K., Bratengeier K., Vordermark D. Precision required for dose-escalated treatment of spinal metastases and implications for image-guided radiation therapy (IGRT) Radiother Oncol. 2007;84:56–63. doi: 10.1016/j.radonc.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Wang H., Shiu A., Wang C., O'Daniel J., Mahajan A., Woo S. Dosimetric effect of translational and rotational errors for patients undergoing image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2008;71:1261–1271. doi: 10.1016/j.ijrobp.2008.02.074. [DOI] [PubMed] [Google Scholar]
- 13.Sahgal A., Ma L., Gibbs I., Gerszten P.C., Ryu S., Soltys S. Spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:548–553. doi: 10.1016/j.ijrobp.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Sahgal A., Weinberg V., Ma L., Chang E., Chao S., Muacevic A. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85:341–347. doi: 10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Chang J.H., Sangha A., Hyde D., Soliman H., Myrehaug S., Ruschin M. Positional accuracy of treating multiple versus single vertebral metastases with stereotactic body radiotherapy. Technol Cancer Res Treat. 2017;16:231–237. doi: 10.1177/1533034616681674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finnigan R., Lamprecht B., Barry T., Jones K., Boyd J., Pullar A. Inter- and intra-fraction motion in stereotactic body radiotherapy for spinal and paraspinal tumours using cone-beam CT and positional correction in six degrees of freedom. J Med Imaging Radiat Oncol. 2016;60:112–118. doi: 10.1111/1754-9485.12353. [DOI] [PubMed] [Google Scholar]
- 17.Gerszten P.C., Monaco E.A., 3rd, Quader M., Novotny J., Jr., Kim J.O., Flickinger J.C. Setup accuracy of spine radiosurgery using cone beam computed tomography image guidance in patients with spinal implants. J Neurosurg Spine. 2010;12:413–420. doi: 10.3171/2009.10.SPINE09249. [DOI] [PubMed] [Google Scholar]
- 18.Han Z., Bondeson J.C., Lewis J.H., Mannarino E.G., Friesen S.A., Wagar M.M. Evaluation of initial setup accuracy and intrafraction motion for spine stereotactic body radiation therapy using stereotactic body frames. Pract Radiat Oncol. 2016;6:e17–e24. doi: 10.1016/j.prro.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Hyde D., Lochray F., Korol R., Davidson M., Wong C.S., Ma L. Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: intrafraction motion analysis accounting for all six degrees of freedom. Int J Radiat Oncol Biol Phys. 2012;82:e555–e562. doi: 10.1016/j.ijrobp.2011.06.1980. [DOI] [PubMed] [Google Scholar]
- 20.Li W., Sahgal A., Foote M., Millar B.A., Jaffray D.A., Letourneau D. Impact of immobilization on intrafraction motion for spine stereotactic body radiotherapy using cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2012;84:520–526. doi: 10.1016/j.ijrobp.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Hoogeman M.S., Nuyttens J.J., Levendag P.C., Heijmen B.J. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys. 2008;70:609–618. doi: 10.1016/j.ijrobp.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 22.Annede P., Darreon J., Benkemouche A., Valdenaire S., Tyran M., Kaeppelin B. Flattening filter free vs. flattened beams for lung stereotactic body radiation therapy. Anticancer Res. 2017;37:5133–5139. doi: 10.21873/anticanres.11933. [DOI] [PubMed] [Google Scholar]
- 23.Dang T.M., Peters M.J., Hickey B., Semciw A. Efficacy of flattening-filter-free beam in stereotactic body radiation therapy planning and treatment: a systematic review with meta-analysis. J Med Imaging Radiat Oncol. 2017;61:379–387. doi: 10.1111/1754-9485.12583. [DOI] [PubMed] [Google Scholar]
- 24.Nalichowski A., Kaufman I., Gallo J., Bossenberger T., Solberg T., Ramirez E. Single fraction radiosurgery/stereotactic body radiation therapy (SBRT) for spine metastasis: a dosimetric comparison of multiple delivery platforms. J Appl Clin Med Phys. 2017;18:164–169. doi: 10.1002/acm2.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L., Djemil T., Zhuang T., Andrews M., Chao S.T., Suh J.H. Treatment plan quality and delivery accuracy assessments on 3 IMRT delivery methods of stereotactic body radiotherapy for spine tumors. Med Dosim. 2019;44:11–14. doi: 10.1016/j.meddos.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q.J., Yoo S., Kirkpatrick J.P., Thongphiew D., Yin F.F. Volumetric arc intensity-modulated therapy for spine body radiotherapy: comparison with static intensity-modulated treatment. Int J Radiat Oncol Biol Phys. 2009;75:1596–1604. doi: 10.1016/j.ijrobp.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Zach L., Tsvang L., Alezra D., Ben Ayun M., Harel R. Volumetric modulated arc therapy for spine radiosurgery: superior treatment planning and delivery compared to static beam intensity modulated radiotherapy. Biomed Res Int. 2016;2016:6805979. doi: 10.1155/2016/6805979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallory M., Pokhrel D., Badkul R., Jiang H., Lominska C., Wang F. Volumetric modulated arc therapy treatment planning of thoracic vertebral metastases using stereotactic body radiotherapy. J Appl Clin Med Phys. 2018;19:54–61. doi: 10.1002/acm2.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang E.L., Shiu A.S., Mendel E., Mathews L.A., Mahajan A., Allen P.K. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen Q.N., Shiu A.S., Rhines L.D., Wang H., Allen P.K., Wang X.S. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1185–1192. doi: 10.1016/j.ijrobp.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 31.Sahgal A., Ames C., Chou D., Ma L., Huang K., Xu W. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol Biol Phys. 2009;74:723–731. doi: 10.1016/j.ijrobp.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Sahgal A., Bilsky M., Chang E.L., Ma L., Yamada Y., Rhines L.D. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine. 2011;14:151–166. doi: 10.3171/2010.9.SPINE091005. [DOI] [PubMed] [Google Scholar]
- 33.Sahgal A., Larson D.A., Chang E.L. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys. 2008;71:652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 34.Tseng C.L., Soliman H., Myrehaug S., Lee Y.K., Ruschin M., Atenafu E.G. Imaging-based outcomes for 24 Gy in 2 daily fractions for patients with de novo spinal metastases treated with spine stereotactic body radiation therapy (SBRT) Int J Radiat Oncol Biol Phys. 2018;102:499–507. doi: 10.1016/j.ijrobp.2018.06.047. [DOI] [PubMed] [Google Scholar]