Abstract
Background and purpose
The amino-acid positron emission tomography (PET) tracer 3,4-dihydroxy-6-[18F] fluoro-l-phenylalanine (18F-DOPA) has increased sensitivity for detecting regions of biologically aggressive tumors compared to T1 contrast-enhanced (T1-CE) magnetic resonance imaging (MRI). We performed dosimetric evaluation of treatment plans prepared with and without inclusion of 18F-DOPA-based biological target volume (BTV) evaluating its role in guiding radiotherapy of grade III/IV gliomas.
Materials and methods
Eight patients (five T1-CE, three non-contrast-enhancing [NCE]) were included in our study. MRI only-guided anatomic plans and MRI+18FDOPA-PET-guided biologic plans were prepared for each patient, and dosimetric data for target volumes and organs at risk (OAR) were compared. High-dose BTV60Gy was defined as regions with tumor to normal brain (T/N) >2.0, while low-dose BTV51Gy was initially based on T/N >1.3, but refined per Nuclear Medicine expert.
Results
For T1-CE tumors, planning target volumes (PTV) were larger than MRI-only anatomic target volumes. Despite increases in size of both gross target volumes and PTV, with volumetric-modulated arc therapy planning, no increase of dose to OAR was observed while maintaining similar target dose coverage. For NCE tumors, MRI+18F-DOPA PET biologic imaging identified a sub-region of the large, T2-FLAIR abnormal signal which may allow a smaller volume to receive the high dose (60 Gy) radiation.
Conclusions
For T1-CE tumors, PTVs were larger than MRI-only anatomic target volumes with no increase of dose to OARs. Therefore, MRI+18F-DOPA PET-based biologic treatment planning appears feasible in patients with high-grade gliomas.
Keywords: 18F-DOPA PET, PET-guided radiation therapy, Planning study, Amino acid PET
1. Introduction
Evidence has emerged for a role of metabolic or biologic imaging in gliomas [1], [2], [3], [4]. The amino-acid positron emission tomography (PET) tracer 3,4-dihydroxy-6-[18F] fluoro-l-phenylalanine (18F-DOPA) was found to have considerably increased sensitivity for detecting regions of biologically aggressive tumors compared to T1 contrast-enhanced (T1-CE) magnetic resonance imaging (MRI). In addition, T1-CE substantially underestimated the volume of the highly aggressive disease components [4]. Utilizing a derived threshold (uptake ratio of tumor to contralateral normal brain >2.0), high uptake regions were identified outside of T1-CE in 8 of the 21 patients in our previous pilot study, including 3 non-contrast-enhanced (NCE) patients [4].
Ledezma et al. [5] also demonstrated that 18F-DOPA uptake was increased in tumors that were NCE on MRI. Lee et al. [3] evaluated the site of glioblastoma failure in relation to pre-treatment 11C-labeled methionine (11C-MET) PET uptake, which was not used for radiotherapy (RT) targeting. Inadequate coverage of the high-risk region defined by 11C-MET PET uptake was associated with an increased risk of regional recurrence, indicating that knowledge about high-risk regions outside the T1-CE region may be important in treatment planning.
These and other recent studies suggest that accurate delineation of brain tumors was improved by incorporating biologic imaging [6], [7], [8]. This is consistent with our previous work in which 18F-DOPA uptake regions which showed aggressive, high-grade disease components extending as distant as 3.5 cm beyond the T1-CE region [4]. Because aggressive disease was reported beyond regions of T1-CE, it was expected that target volumes would be larger if biologic-based imaging was incorporated into treatment planning. Increased volume of uninvolved brain receiving RT has been associated with an increased risk of acute and late toxicity including fatigue, endocrine, and neurocognitive effects [9], therefore in preparation for a prospective phase II trial evaluating the role of 18F-DOPA PET in guiding RT treatment in patients with high-grade gliomas, we sought to evaluate the effect of 18F-DOPA PET biologic information on RT treatment planning. This study also describes a methodology for robust dosimetric evaluation of treatment plans with and without the inclusion of 18F-DOPA PET biologic information.
2. Materials and methods
2.1. Patients and basic characteristics
This was a retrospective treatment planning study comparing treatment planning objectives in eight patients with high-grade glioma histology with and without the incorporation of 18F-DOPA PET imaging. Because 18F-DOPA is not FDA approved for clinical use, the patients had all previously enrolled in an 18F-DOPA surgical planning pilot study at our institution. A total of 21 patients were enrolled on the pilot study [4], but only eight high-grade histology patients were for included in this study. Five patients had T1-CE, and three with grade III tumors did not (NCE). The pilot study was open to both newly diagnosed and recurrent patients with gliomas who were able to undergo both MRI with contrast and 18F-DOPA PET scans. Basic patient characteristics have been summarized in Supplementary Table 1.
2.2. MRI and 18F-DOPA PET/CT acquisition
All patients underwent intra- or pre-operative brain MRI and 18F-DOPA PET/computed tomography (CT) (GE Healthcare Discovery 690, Waukesha, WI, USA) imaging. The pilot study and retrospective analysis of these results were both approved by the Institutional Review Board. Technical parameters of PET and MRI acquisition have been previously published [4]. The 18F-DOPA PET biologic data corresponded to the pre-operative imaging, thus the helical CT image (pixel size 0.59 mm, slice thickness 2.0 mm), which was performed for attenuation correction of the PET data, served as our RT treatment planning CT. Acquired images were transferred to MIM Maestro (MIM Software, Inc., Cleveland, OH, USA) for PET uptake analysis for BTV volumes and MRI GTV volume delineation, and to the Eclipse Treatment Planning System (Varian Medical Systems Inc., Palo Alto, CA, USA) for subsequent GTV and PTV delineation and RT planning.
2.3. Target volumes and organ-at-risk definition
18F-DOPA PET uptake was used for biological target volume (BTV) delineation. High-dose BTV60Gy was defined as regions with T/N >2.0, based on previously reported results of our pilot study with spatial-related histopathological correlations [4]. Low-dose BTV51Gy was first contoured based on T/N >1.3, but was modified in the clinical judgment of an experienced nuclear medicine physician.
T1-CE determined GTV60Gy_MR. GTV51Gy_MR was determined by T2-weighted-Fluid-Attenuated Inversion Recovery (T2-FLAIR) volume, with the exception of NCE tumors, in which GTV60_MR was the entire T2-FLAIR. The MRI criteria used to define target volumes was based on the historical standard of care at our institution and the North Central Cancer Treatment Group [10]. Definitions of all gross tumor volume (GTV), clinical target volume (CTV), planning target volume (PTV), and associated prescribed doses are summarized in Table 1.
Table 1.
Target definition based on 18F-DOPA PET and MRI images.
| Dose | GTV/CTV/PTV definition | GTV_MR definition | BTV definition |
|---|---|---|---|
| 51 Gy | GTV51Gy = GTV51Gy_MR + BTV51Gy CTV51Gy = GTV51Gy + 1 cm PTV51Gy = CTV51Gy + 0.3 cm |
GTV51Gy_MR = abnormal T2/FLAIR signal + GTV60Gy_MR |
BTV51Gy = PET gold standard volume |
| 60 Gy | GTV60Gy = GTV60Gy_MR + BTV60Gy CTV60Gy = GTV60Gy + 1 cm PTV60Gy = CTV60Gy + 0.3 cm |
GTV60Gy_MR = resection cavity + any T1 contrast-enhanced lesion (Grade IV) or Abnormal T2/FLAIR if no contrast enhancement. (Grade III)* |
BTV60Gy = PET volume with threshold T/N > 2.0 within the BTV51Gy |
Targets based solely on MRI were created similarly, with exclusion of BTV51Gy and BTV60Gy. BTV = biological target volume based on 18F-DOPA PET, GTV = gross tumor volume, CTV = clinical target volume, PTV = planning target volume, FLAIR = Fluid attenuation inversion recovery.
*Grade III patients presenting without contrast enhancement received 60 Gy boost to entire FLAIR abnormality.
We analyzed the discordant region between boost volume PTVs created utilizing MRI only, and MRI+18F-DOPA PET [11]. The brainstem, optic nerves, and chiasm were defined as high priority organs at risk (OAR), while the eyes, retinas, and both hippocampi were defined as low priority OAR [12]. Hippocampi were delineated based on the Radiation Therapy Oncology Group online contouring atlas.
2.4. Radiation treatment planning and dosimetric evaluation
Three patients with grade III tumors had NCE gliomas. For these patients, inclusion of PET biologic imaging and limiting the 60 Gy volume to the areas of highly aggressive disease components as determined by 18F-DOPA PET, led to a substantial reduction of volume intended for boost doses of 60 Gy (Fig. 1). As shown in Fig. 2, for patients with T1-CE, including the 18F-DOPA PET can lead to an increase in the 60 Gy volumes.
Fig. 1.
Example of differences between 60 Gy target volumes between MRI and MRI + PET for a NCE patient. In the upper row are FLAIR MRI (A), corresponding 18F-DOPA PET (B) images for patient FDOPA 05, with a NCE 2016 WHO grade III anaplastic astrocytoma in the middle row, and (C) MRI + PET fusion in the lower row, illustrating the potential reduction in 60 Gy target region coverage with the inclusion of PET for planning. For illustration of difference, PET-based contours were overlapped on the MRI image and vice versa. Legend: Blue = BTV60Gy_PET; Yellow = GTV60Gy_MRI; Magenta = PTV60Gy (expansion of BTV60Gy_PET for MRI + PET planning); and Green = PTV60Gy MRI (expansion of GTV60Gy_MRI for MRI only planning). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
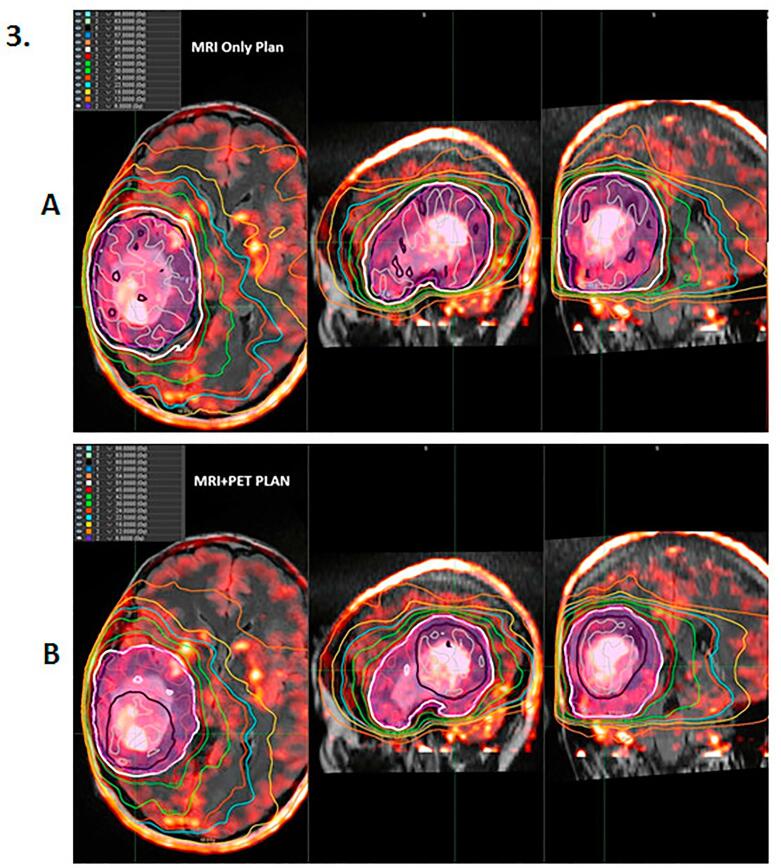
Fig. 2.
Example of differences between 60 Gy target volumes between MRI and MRI + PET for a T1-CE patient. In the upper row are the T1-CE MRI (A), corresponding 18F-DOPA PET (B) images for patient for patient FDOPA 03 with a T1-CE 2016 WHO grade IV tumor (glioblastoma) in the middle row, and (C) MRI + PET fusion in the lower row, illustrating the increase in 60 Gy target region coverage with PET uptake beyond MRI T1-CE. Legend: Blue = BTV60Gy_PET; Yellow = GTV60Gy_MRI; Magenta = PTV60Gy (expansion of BTV60Gy_PET+GTV60Gy_MRI for MRI + PET planning); and Green = PTV60Gy MRI (expansion of GTV60Gy_MRI for MRI only planning). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For all patients, two experimental treatment plans were created: one based solely on MRI only information and another which included both MRI and 18FDOPA-PET biological target volume (BTV). Volumetric-modulated arc therapy (VMAT) planning was performed in all cases for the TrueBeam system (Varian Medical Systems Inc.). Dose prescription was specified as 51 Gy (1.7 Gy per fraction) to the PTV51Gy and 60 Gy (2.0 Gy per fraction) in 30 fractions to the PTV60Gy with a simultaneous integrated boost technique. For all plans, 95% of PTV60Gy volume received 100% of the prescribed dose. All plans were also limited to <0.5 cc received <110% of the prescribed dose (66 Gy).
Two full 360° coplanar arcs and one sagittal non-coplanar half arc were arranged for all patients. Because of shape, size, and location of target volume, an additional sagittal half arc was added in one patient to meet all PTV dose coverage constraints. The same arrangement was used for both MRI only and MRI + PET biologic plans in this particular patient. Supplementary Table 2 shows the OAR dose constraints which were used to evaluate the plan quality for each patient, with and without the incorporation of 18F-DOPA. Corresponding parameters from MRI only and MRI + PET biologic plans were compared for each patient.
Basic statistics were used to calculate percentages and averages. Treatment plans for each patient were compared separately. Because the T1-CE region is always included in high-dose target volume in treatment planning, we did not compare T1-CE to the high 18F-DOPA uptake region (represented by T/N ratio >2.0), but instead compared T1-CE to the union of T1-CE and the high 18F-DOPA uptake region, which enabled direct incorporation into the evolution of corresponding CTVs and PTVs for creation of treatment plans. Because it is standard practice to include a margin for clinical and planning target volumes, we also evaluated the differences after volume expansions.
3. Results
3.1. Target volume comparisons
For all T1-CE patients, all volumetric changes in target volumes were less marked when expressed as PTV compared to GTV. BTV60Gy volume ranged from being less than to 4.4 times larger compared to GTV60Gy, whereas PTV60Gy including MRI + PET ranged from being the same to 1.8 times larger compared to PTV60Gy using MRI only (represented in Fig. 2, patient FDOPA 03). Between 6% and 36% of the BTV60Gy and GTV60Gy_MR volumes were overlapping, where FDOPA uptake was present (patient FDOPA01 did not have FDOPA uptake). Corresponding values for NCE patients were as follows: BTV60Gy ranged from 48 to 202 times smaller than the GTV60Gy_MR (comprised of the FLAIR MRI volume), while the resulting PTV60Gy ranged from 3.2 to 72 times smaller (represented in Fig. 1 for patient FDOPA 05). Statistically speaking, all of the BTV60Gy volume was contained within the GTV60Gy_MR FLAIR signal abnormality. In the NCE subgroup of patients, no 51 Gy volumes were defined for MRI anatomic plans. Comparison of all GTVs, PTVs, and the intersection volumes between PET and MRI have been summarized in Table 2 (the three NCE patients were grouped separately).
Table 2.
Volumetric analysis for GTVs and PTVs defined for MRI only and MRI + PET treatment plans.
| Tumors with T1-CE |
NCE |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 01 | 03 | 07 | 14 | 21 | 05 | 13 | 15 | ||
| GTV60Gy_MR (cc) BTV60Gy (cc) |
3.1 | 14.0 | 27.6 | 21.2 | 2.1 | 61.4 | 40.5 | 198.2 | |
| 0.0 | 55.2 | 4.0 | 18.0 | 9.2 | 4.5 | 0.2 | 4.1 | ||
| Intersection (cc) | 0.0 | 13.3 | 1.9 | 14.0 | 1.5 | 4.5 | 0.2 | 4.1 | |
| GTV51Gy_MR (cc) BTV51Gy (cc) Intersection (cc) |
46.2 | 250.0 | 53.4 | 51.4 | 24.5 | – | – | – | |
| 7.5 6.8 |
57.4 57.3 |
4.1 2.4 |
26.6 23.4 |
16.3 14.7 |
8.2 – |
1.4 – |
4.2 – |
||
| PTV60Gy Intersection (cc) |
MRI only(cc) | 42.2 | 115.5 | 139.6 | 132.0 | 61.2 | 231.8 | 157.9 | 577.3 |
| +PET(cc) | 42.2 42.2 |
213.2 115.5 |
143.7 139.5 |
146.4 131.9 |
82.5 61.2 |
71.5 71.5 |
2.2 2.2 |
55.8 55.7 |
|
| PTV51Gy Intersection (cc) |
MRI only(cc) | 183.5 | 682.9 | 205.1 | 233.4 | 131.7 | – | – | – |
| +PET(cc) | 185.2 | 682.9 | 206.8 | 238.0 | 132.5 | 231.9 | 158.0 | 577.7 | |
| 183.4 | 682.9 | 205.0 | 233.3 | 131.6 | – | – | – | ||
3.2. Target coverage
All priority 1 dose volume constraints for PTV60Gy (V100% ≥95% and V110% <0.5 cc) were met in both treatment plans for all patients. After inclusion of 18F-DOPA PET biologic imaging, the average 60 Gy isodose volumes for the five patients with contrast enhancement increased by 1.3 times and decreased by 2.5 times in the three patients without contrast enhancement, which was consistent with PTV analysis data (data not shown).
3.3. Discordant region analysis
To evaluate the coverage of unrecognized biologic target volumes in the setting of the standard expansions used in glioma radiotherapy for CTV(1 cm) and PTV(5 mm), evaluation of dose coverage of the discordant region between PTV60Gy MRI only planning and PTV60Gy MRI + PET planning has been summarized in Table 3. For T1-CE patients, for all but one patient, MRI-only based plans resulted in under-dosing of regions of disease detected by 18F-DOPA PET biologic imaging. For example, in patients FDOPA03 and FDOPA07, only 5% of the discordant target region was covered in the MRI-only based plan. In patient FDOPA 05, Fig. 3 illustrates an example of isodose coverage in the treatment plan, where 18F-DOPA PET revealed a high-risk region within the T2-FLAIR signal abnormality. Because these structures were cropped to remain within the brain parenchyma, the relative differences were diminished when comparing PTV volumes. As expected, this region has typically been undertreated when treatment plans for patients with T1-CE are based on MRI only imaging data, while the region has typically been over-treated in NCE patients.
Table 3.
Percentage of discordant volume covered by MRI-based PTV 51 and 60 Gy, respectively.
| V51Gy [%] | V60Gy [%] | |
|---|---|---|
| FDOPA 01 | – | – |
| FDOPA 03 | 52 | 5 |
| FDOPA 07 | 26 | 5 |
| FDOPA 14 | 62 | 13 |
| FDOPA 21 | 62 | 13 |
| FDOPA 05 | 100 | 94 |
| FDOPA 13 | 100 | 80 |
| FDOPA 15 | 98 | 95 |
Fig. 3.
Isodose MRI (A) and MRI + PET (B) plan for patient FDOPA 05. The black isodose represents the prescribed dose (60 Gy). The magenta segment represents PTV60Gy. The discordant region was covered by 51 Gy (white isodose) in the MRI + PET plan (B). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Irradiation of organs at risk
Dose coverage of OAR in MRI + PET biologic plans did not lead to a substantial increase in radiation dose to critical structures. No clinically significant differences in OAR irradiation between MRI only and MRI + PET biologic plans were noted, and all plans met critical OAR constraints.
4. Discussion
This dosimetric analysis was performed to evaluate the effect of 18F-DOPA PET biologic information on RT treatment planning. Based on the results of our pilot study demonstrating the additional utility of 18F-DOPA PET in target delineation [4], we developed a prospective, phase II study utilizing 18F-DOPA PET biologic with conventional MRI anatomic imaging for RT planning in high-grade gliomas (NCT02104310). We hypothesized that the target volumes would be considerably larger in cases with T1-CE lesions.
To evaluate the effect of the increase in target volume on radiation dosimetry, we created two representative treatment plans, one based solely on MRI anatomic information and one based on information from both MRI and 18F-DOPA PET biologic imaging. Of note, the median volume of 60 Gy isodose on PET + MRI biologic plans for patients with T1-CE was 1.12 times larger, with a range of 1.0–1.9, than the 60 Gy volume for MRI only plans. From a clinical toxicity standpoint, reporting of differences in PTV instead of GTV is a more clinically meaningful interpretation of observed differences. This was consistent with prior reports of similar studies utilizing 18F-Fluoro-ethyl-tyrosine (FET) based RT contouring [13], [14], [15].
Although target volumes increased as expected, which could increase the risk of radionecrosis, the relative increase appeared to be a reasonable volume and, based on our prior work, represented areas with aggressive disease. In addition, by using volumetric-modulated arc therapy with simultaneous integrated boost planned with similar planning constraints and arc arrangements, we were able to demonstrate that treatment of the additional target volume suggested by 18F-DOPA PET biologic imaging did not result in increased doses to OAR. This was consistent with a recent study evaluating VMAT plans, standard intensity-modulated RT, and spot-scanning proton RT [15]. In this report, all techniques spared OAR with FET-PET guided dose-escalation, although protons resulted in the greatest sparing. Analysis of the discordant region was the primary purpose of comparing planning studies when evaluating the impact of biologic information on conventional treatment planning. For patients without contrast enhancement, this region represents brain that can be saved from boost dosing and covered by low-dose irradiation (51 Gy in our study). In addition, in this cohort of patients, 25% of the volume of PTV60Gy was discordant between PTV60Gy_MRI and PTV60Gy_PET, which suggests a substantial risk of marginal and/or geographic miss in the area of highest-risk disease. No obvious differences were observed in discordant volumes intended to be covered by 51 Gy, which was consistent with our hypothesis that the main advantage of 18F-DOPA PET biologic imaging is in definition of aggressive disease. Although more patients are needed to better analyze subgroups of patients with and without contrast enhancement, there were obvious trends in changes of volume of GTV60Gy MRI + PET as well as PTV60Gy MRI + PET.
There were several limitations to our current study. First, because our previous study examined the correlation between histopathology and 18F-DOPA uptake, the patient population was small and included a range of diagnoses. For the current study, we selected patients with high-grade gliomas as the largest subgroup, which led to the inclusion of eight patients. Although the results from this study suggested that 18F-DOPA PET biologic-based treatment planning is feasible, future studies should strive to incorporate a larger sample with more homogeneity among diagnoses.
Second, although MRI anatomic imaging is the current gold standard for glioma RT treatment planning, a consensus in target delineation strategy has not yet been reached. While there has been increasing evidence of the usefulness of identifying high-risk portions of tumor outside the T1-CE region, there is no consensus in regards to the best imaging modality to identify these high-risk areas, especially in regards to inclusion or exclusion of T2-FLAIR signal abnormality [16], [17]. Because of the radio-resistance of these tumors, some favor treatment of the T1-CE region with a limited margin (1.0 cm) only, and suggest that a low dose of radiation to T2-FLAIR abnormality may cause toxicity without a meaningful chance of controlling microscopic disease [16].
Third, 18F-DOPA PET biologic-based imaging is not widely available, limiting applicability to many centers. In addition, because of normal dopamine uptake in the basal ganglia tumors immediately adjacent to this region can be difficult to differentiate from normal uptake. Patients with a history of Parkinson’s disease and those who are taking anti-dopaminergic or other interfering medications were excluded in this study due to concerns of reliability of 18F-DOPA PET in the setting of dopamine metabolism. However, a relative increase in non-central recurrences has been reported recently in patients treated in the temozolomide era [18]. Although this could be related to the biologic effect of temozolomide, during this time period, considerable advances in RT have led to increased conformity and dose fall-off outside the target, as well as a trend to limit the volumes to contrast-enhanced regions, which may also be contributing to the increase in marginal failures. Thus, ongoing research of new imaging modalities to improve high-grade glioma RT targeting is essential, and the dosimetric analysis proposed in our present study may provide insight into how new imaging techniques influence RT delivery.
In conclusion, our report demonstrated that in NCE, high-grade gliomas, incorporating 18F-DOPA PET biologic imaging decreased the volume targeted to high dose, while in patients presenting with T1-CE, 18F-DOPA PET biologic imaging increased the volume of areas requiring high-dose irradiation. Based on these results, 18F-DOPA PET biologic-based treatment planning appears feasible in patients with high-grade gliomas. Appropriate dose coverage (V60Gy>95%) was feasible to the PTV60Gy PET + MRI volume, and OAR constraints were achieved in all treatment plans.
Acknowledgments
Acknowledgements
Supported by the Mayo Clinic Brain Cancer Specialized Program of Research Excellence (SPORE) (National Institutes of Health-5P50CA108961-08). Supported by the European Regional Development Fund – Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123). Supported by MH CZ – DRO (MMCI, 00209805). Supported by Brains Together For a Cure (BTFC-01, NCT01165632). Supported by National Institutes of Health R01CA178200-06.
Conflict of interest statement
The authors of this manuscript certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.phro.2018.06.004.
Appendix A. Supplementary data
References
- 1.Villani V., Carapella C.M., Chiaravalloti A., Terrenato I., Piludu F., Vidiri A. The role of PET [18F]FDOPA in evaluating low-grade glioma. Anticancer Res. 2015;35:5117–5122. [PubMed] [Google Scholar]
- 2.Bell C., Dowson N., Puttick S., Gal Y., Thomas P., Fay M. Increasing feasibility and utility of (18)F-FDOPA PET for the management of glioma. Nucl Med Biol. 2015;42:788–795. doi: 10.1016/j.nucmedbio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Lee I.H., Piert M., Gomez-Hassan D., Junck L., Rogers L., Hayman J. Association of 11C-methionine PET uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73:479–485. doi: 10.1016/j.ijrobp.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pafundi D.H., Laack N.N., Youland R.S., Parney I.F., Lowe V.J., Giannini C. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 2013;15:1058–1067. doi: 10.1093/neuonc/not002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledezma C.J., Chen W., Sai V., Freitas B., Cloughesy T., Czernin J. 18F-FDOPA PET/MRI fusion in patients with primary/recurrent gliomas: initial experience. Eur J Radiol. 2009;71:242–248. doi: 10.1016/j.ejrad.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Chiti A., Kirienko M., Gregoire V. Clinical use of PET-CT data for radiotherapy planning: what are we looking for? Radiother Oncol. 2010;96:277–279. doi: 10.1016/j.radonc.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Grosu A.L., Weber W.A. PET for radiation treatment planning of brain tumours. Radiother Oncol. 2010;96:325–327. doi: 10.1016/j.radonc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Marvaso G., Barone A., Amodio N., Cascini G.L., Scotti V., Bianco C. The current status of novel PET radio-pharmaceuticals in radiotherapy treatment planning of glioma. Curr Pharm Biotechnol. 2014;14:1099–1104. doi: 10.2174/1389201015666140408122318. [DOI] [PubMed] [Google Scholar]
- 9.Laack N.N., Brown P.D. Cognitive sequelae of brain radiation in adults. Semin Oncol. 2004;31:702–713. doi: 10.1053/j.seminoncol.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Brown P.D., Krishnan S., Sarkaria J.N., Wu W., Jaeckle K.A., Uhm J.H. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26:5603–5609. doi: 10.1200/JCO.2008.18.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knoos T., Kristensen I., Nilsson P. Volumetric and dosimetric evaluation of radiation treatment plans: radiation conformity index. Int J Radiat Oncol Biol Phys. 1998;42:1169–1176. doi: 10.1016/s0360-3016(98)00239-9. [DOI] [PubMed] [Google Scholar]
- 12.Shaw E., Kline R., Gillin M., Souhami L., Hirschfeld A., Dinapoli R. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27:1231–1239. doi: 10.1016/0360-3016(93)90548-a. [DOI] [PubMed] [Google Scholar]
- 13.Terezakis S.A., Schoder H., Kowalski A., McCann P., Lim R., Turlakov A. A prospective study of (1)(8)FDG-PET with CT coregistration for radiation treatment planning of lymphomas and other hematologic malignancies. Int J Radiat Oncol Biol Phys. 2014;89:376–383. doi: 10.1016/j.ijrobp.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieken S., Habermehl D., Giesel F.L., Hoffmann C., Burger U., Rief H. Analysis of FET-PET imaging for target volume definition in patients with gliomas treated with conformal radiotherapy. Radiother Oncol. 2013;109:487–492. doi: 10.1016/j.radonc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Holm A.I.S., Petersen J.B.B., Muren L.P., Seiersen K., Borghammer P., Lukacova S. Functional image-guided dose escalation in gliomas using of state-of-the-art photon vs. proton therapy. Acta Oncol. 2017;56:826–831. doi: 10.1080/0284186X.2017.1285498. [DOI] [PubMed] [Google Scholar]
- 16.Chang E.L., Akyurek S., Avalos T., Rebueno N., Spicer C., Garcia J. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007;68:144–150. doi: 10.1016/j.ijrobp.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Whitfield G.A., Kennedy S.R., Djoukhadar I.K., Jackson A. Imaging and target volume delineation in glioma. Clin Oncol (R Coll Radiol) 2014;26:364–376. doi: 10.1016/j.clon.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Dobelbower M.C., Burnett Iii O.L., Nordal R.A., Nabors L.B., Markert J.M., Hyatt M.D. Patterns of failure for glioblastoma multiforme following concurrent radiation and temozolomide. J Med Imaging Radiat Oncol. 2011;55:77–81. doi: 10.1111/j.1754-9485.2010.02232.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.