Graphical abstract
Keywords: Prostate cancer, Bladder, Genitourinary toxicity, CBCT, DVH, Spatial
Abstract
Background and purpose
The risk of genitourinary (GU) toxicity is dose-limiting in radiotherapy (RT) for prostate cancer. This study investigated whether motion-inclusive spatial dose/volume metrics explain the GU toxicity manifesting after high-precision RT for prostate cancer.
Material and methods
A matched case-control was performed within a cohort of 258 prostate cancer patients treated with daily cone-beam CT (CBCT)-guided RT (prescription doses of 77.4–81.0 Gy). Twenty-seven patients (10.5%) presented late RTOG GU ≥ Grade 2 toxicity and those without symptoms of toxicity prior treatment (N = 7) were selected as cases. Each case was matched with three controls based on pre-treatment GU symptoms, age, Gleason score, follow-up time, and hormone therapy. Thirteen CBCTs per patient were rigidly registered to the planning CT using the recorded treatment shifts, and the bladder was manually contoured on each CBCT. Planned and actually delivered dose/volume metrics (the latter averaged across the CBCTs) were extracted from the bladder and its subsectors, and compared between cases and controls (two-way ANOVA test).
Results
There were no significant differences between planned and delivered dose/volume metrics; also, there were no significant differences between cases and controls at any dose level, neither for planned nor delivered doses. The cases tended to have larger bladder volumes during treatment than controls (221 ± 71 cm3 vs 166 ± 73 cm3; p = 0.09).
Conclusions
High-precision RT for prostate cancer eliminates differences between planned and delivered dose distributions. Neither planned nor delivered bladder dose/volume metrics were associated to the remaining low risk of developing GU toxicity after high-precision radiotherapy for prostate cancer.
1. Introduction
Modern high-precision external-beam radiotherapy (RT) for prostate cancer enables dose escalation to the prostate gland by using resource-intensive protocols, including daily image-guided RT (IGRT) [1], monitoring of bladder and rectum filling status [2], [3], and narrow margins [1], [4]. These protocols have improved clinical outcomes including overall survival [5], [6]. However, the risk of genitourinary (GU) toxicity, which compromises patient’s quality of life [7], [8], [9], still has to be balanced against the risk of local failure, owing to the close proximity between the bladder and the prostate. GU toxicities represent the dominating domain of late normal tissue effects (also gastro-intestinal toxicity affects patients, but at a much lower level), being the primary dose-limiting factor in conventional fractionated high-precision RT for prostate cancer [5].
Early pre-IGRT era studies reported that bladder volumes receiving intermediate to high doses as seen in the planning CT were only moderately associated with the risk of GU toxicity (AUC = 0.74–0.78) [10], [11]. It was therefore suggested that planned dose-volume histograms (DVHs) are not representative of the dose being delivered [12]. The introduction of IGRT, and in particular the use of daily cone beam CT (CBCT)-based set-up verification, confirmed large variations in bladder volume throughout the RT course and the consequential variations in dose distributions [13], [14], [15]. Additionally, differences in motion and deformation patterns among bladder subsectors were observed, with the inferior part being less affected by changes in bladder filling [16], [17]. In particular, the inferior sector is in close proximity to the prostate, and typically receives doses up to the prescription level [13]. Recently, it has been demonstrated that high doses delivered to the trigone/bladder neck may drive the development of late GU toxicity [18], [19], [20], suggesting spatial effects in GU dose-response relationships. These methods require however additional computations or delineations during the RT planning process compared to a full bladder DVH-based analysis. The aim of this study was therefore to explore whether delivered spatial bladder DVHs explain the occurrence of GU toxicity after RT for prostate cancer. The analysis was conducted within a matched case-control approach and the delivered DVHs were derived from daily CBCT-based IGRT.
2. Material and methods
2.1. Patient cohort and treatment
A total of 449 patients were treated with external-beam RT for prostate cancer at the University of California, San Diego, between 2008 and 2014. Of these patients, 258 patients were treated with daily CBCT guidance with the remainder being kV imaging to fiducial markers or some combination of kV and CBCT. Within this group 27 patients (10.5%) had ≥Grade 2 late GU toxicity according to the Radiation Therapy Oncology Group (RTOG) criteria [21]. For case selection additional inclusion/exclusion criteria were applied and only patients with clear new onset grade 2 GU toxicity post-RT without prior symptoms were included as cases, for example hematuria requiring bladder irrigation, new obstruction requiring dilation, etc. Patients with subjectively graded toxicities (e.g. mild for grade 1, moderate for grade 2) or patients with some level of urinary frequency prior to treatment or unclear baseline urinary function receiving alpha blockers were excluded from further analysis. Finally, there were eight patients with grade 2 toxicity that were without subjective assessment or any pre-treatment level of dysfunction in the area of interest were selected as cases. The remaining patients presenting with Grade 0 late GU toxicity and non pre-existing significant GU symptoms were considered potential candidates for controls. For each case three controls were matched according to age (± five years), Gleason score, pre-treatment GU status, follow-up time and use of neoadjuvant androgen deprivation therapy. For one of the cases it was not possible to find matched controls fulfilling the matching criteria, and seven cases were finally included in the study (total of 28 patients, cases and controls). Each case and the matched controls received the same treatment regimen, dose prescription and fractionation schedule; where three cases received pelvic irradiation and four cases local treatment. If a case presented more than three potential controls, the selected controls were those presenting the smallest difference in the follow-up time. The collection of the toxicity information and the classification of the patient status were performed by the responsible medical doctor (AH), who was present in all the visits of the patients related to problems following treatment. The follow-up time (mean ± SD) for the cases was 3.1 ± 1.3 years, whereas for the controls was 3.2 ± 1.3 years.
The patients were prescribed to total doses of 79.2–81.0 Gy (in 43–45 fractions), delivered to the intact prostate in two treatment options: either local treatment to the prostate and seminal vesicles or pelvic node irradiation, followed by a boost to the prostate and seminal vesicles. All patients underwent planning CT scanning and all daily treatments in supine position with the lower extremities immobilized in a VacLock device (Civco Radiotherapy, Coralville, IA). Planning target volumes (PTVs) were generated in the planning CT using margins of 3 mm posteriorly and 7 mm in all other directions from the clinical target volumes (CTVs). All treatment plans were performed in Eclipse v.8–10 (Varian Medical Systems, Palo Alto, CA, USA), with the dose to the bladder restricted to V80Gy < 15%, V75Gy < 25%, V70% < 35% and V65Gy < 50% according to the QUANTEC recommendation [13]. All patients included in the study received intensity-modulated radiation therapy (IMRT) or volumetric-modulated arc therapy (VMAT) in combination with a strict full bladder/empty rectum protocol. More information on the utilized treatments modalities can be found in Casares-Magaz et al. [22].
2.2. Registration and Contouring
For each patient, thirteen CBCTs (all daily scans from the first week of treatment, and then weekly) were rigidly registered to the planning CT and connected dose matrix using the clinically recorded 3D treatment shifts (only translations). Dose distributions at each CBCTs were a copy of the dose matrix at the planning CT, assuming that variations in dose distributions are negligible due to the interfractional changes in the patient’s anatomy under strict full bladder and empty rectum protocol. This assumption has been confirmed in a previous study from our group where dose distributions were recalculated on set of worst-case scenarios with respect to varying anatomies, where only differences up to 2% were observed [23]; similar findings were reported by Sharma et al. using a larger cohort of patients [24].
On each CBCT the bladder was manually contoured, and contours were reviewed and approved by the responsible radiation oncologist. The bladder shell was extracted for each of the registered CBCT using a 3 mm inner margin, and then bladder shell halves and quadrants were created using two orthogonal planes (axial and coronal) drawn through the center of mass of each bladder. A total of ten structure definitions were investigated: whole bladder, bladder shell, anterior, posterior, superior, inferior, anterior/superior, anterior/inferior, posterior/superior, posterior/inferior. Contouring, registration and extraction of bladder shells and substructures were performed in MIM Maestro v.6.5.4 (Mim Software Inc., Cleveland, OH, USA) following our previously used workflow [22].
2.3. Statistical analysis
For each patient, bladder volume and DVH metrics (absolute and relative V5%–V105% in 5% steps) were extracted for the planning CT, for each registered CBCT, and for all segmented structures. DVH metrics were compared between cases and controls using two-way ANOVA test accounting for the matching information. For the analysis of the delivered dose/volume metrics the weighted average was used, where the weight was equal to the number of fractions applied to each CBCT (one for daily CBCTs from the first week, and five for the weekly CBCTs from the following weeks). The statistical analysis was performed in Stata 13.1 (StataCorp, College Station, TX, USA) and in Matlab R2017b (The MathWorks Inc., Natick, MA, USA).
3. Results
3.1. Spatial DVH metrics for cases vs. controls
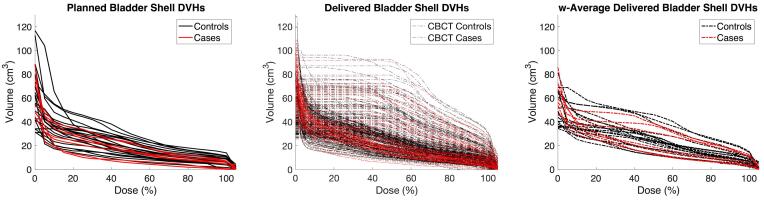
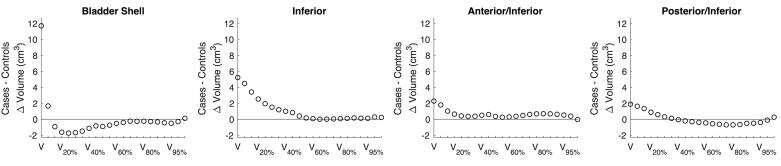
Absolute volume DVH metrics of the bladder and the bladder shell subsectors were similar between cases and controls (two-way ANOVA) for both the planned (p > 0.26) and the delivered (p > 0.57) dose distributions (Fig. 1). However, spatial DVH metrics captured differences between cases and controls in dose re-distribution patterns across the bladder sectors. Inferior and anterior/inferior sectors had slightly higher delivered metrics for cases (p-value >0.07), although overall, controls had slightly higher delivered DVH metrics for the bladder shell. Additionally, the V105% = V85Gy at the posterior-inferior sector was also higher for cases compared to controls (Fig. 2).
Fig. 1.
Planned (left), delivered fractions (middle) and weighted average delivered (right) DVHs in absolute volume for cases (red) and controls (black). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Mean differences in the weighted average DVH metrics (absolute volume) between cases and controls for the bladder shell and subsectors of the inferior part of the bladder shell. None of these differences were significant.
The delivered relative volume DVH metrics of the bladder shell were significantly higher for controls compared to cases, mostly at the intermediate dose region, due to the smaller bladder volume of the controls during the treatment. Significant differences were also observed at the inferior and posterior/inferior sectors with higher DVH metrics in the low to intermediate dose range (Suppl. Material A). There was a minor negative trend of lower doses delivered after the first week of treatment compared to the dose delivered between week 2 and 5, but this was not at a significant level (Suppl. Material B).
3.2. Spatial DVH metrics for the planning CT vs. on treatment
Differences between planned and average delivered in absolute dose/volume metrics were negligible for the whole group of patients and at all subsectors (paired t-test, p > 0.25, Suppl. Material B). Additionally, although the delivered relative volume DVH metrics were overall larger compared to planned, population average DVHs fulfilled dose/volume QUANTEC constraints (mean ± SD): 6 ± 5%, 11 ± 6%, 14 ± 8%, 19 ± 10%, for the V80Gy, V75Gy, V70Gy and V65Gy respectively [13]. Furthermore, similar metrics for the planning CT at the bladder and bladder shell were also found between cases and controls (two-side ANOVA test, p > 0.15).
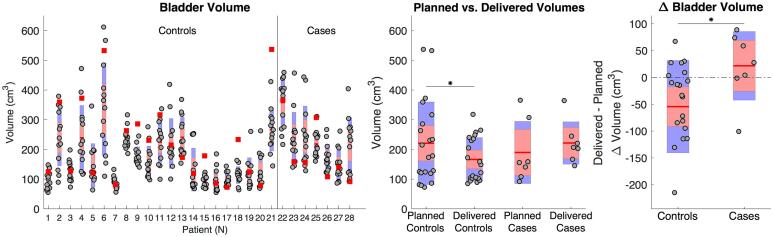
Similar bladder volumes were found at the planning CT for cases and controls (ΔV = −22 cm3, p = 0.72). However, during treatment bladder volumes were slightly larger for cases compared to controls although not statistically significant (ΔV = 55 cm3, p = 0.09). During treatment bladder volume were significantly lower compared to the planning CT for controls (p < 0.01, Fig. 3). Additionally, a slight negative trend in bladder volume was observed for both cases and controls during the treatment course, but not a significant level (Suppl. Material C).
Fig. 3.
Left panel: box plot for delivered bladder volume at each CBCT and bladder volumes at the planning CT (red squares) for both groups. Middle panel: box plot for planned and weighted average delivered bladder volumes in cases controls. Right panel: box plot for the difference between weighted average bladder volume and planned. * indicates significant differences, p-value <0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this case-control study we explored whether daily DVH metrics extracted from bladder shell and bladder shell subsectors may be related to the risk of developing ≥Grade 2 GU toxicity after high-precision RT for prostate cancer. Actually delivered bladder dose distributions were extracted using thirteen CBCTs per patient for a total of ten bladder (sub)structures. We observed that cases and controls presented similar delivered DVH metrics, based on two-way ANOVA test accounting for the matching information. However, we observed differences in delivered dose re-distribution patterns between cases and controls, and across bladder shell subsectors. To the best of our knowledge this is the first study comparing daily dose/volume metrics at multiple bladder subsectors between patients with and without GU toxicity following RT for prostate cancer.
In this study spatial DVH metrics were extracted from dose distributions at bladder shells of 3 mm inner margin to the bladder contour. This approach was motivated by Carillo et al. [25], which showed that DVHs from ≤5 mm margin bladder shells were equivalent to dose surface histograms (DSH). Also other studies have showed that absolute volume DVH metrics of the bladder shell presented the highest correlations with GU toxicity [10], [11], [26] compared to other dose/volume metrics. In the present study we observed a trend of higher delivered DVH metrics (absolute volume) at the inferior sector(s) for the cases, although not at a significant level. Also, the use of high-precision RT in the present study implies smaller areas of the bladder treated to high doses, which in turn may decrease the grade of association with GU toxicity, in concordance with previous studies [27], [28]. On the other hand, although spatial DVHs were used, there is still a loss of spatial information, which may blur associations between dose delivered and GU toxicity. Therefore, more developed methods than segmented DVHs, such as anatomical localization of the trigone [19], dose surface maps [20] or dose accumulation techniques, might improve predictive power of GU toxicity. Previous studies indeed showed the importance of dose delivered to different structures of the GU track such as urethra, trigone or bladder neck [29]. However, definition of these substructures of GU track was not feasible at the CBCTs due to the low image contrast. Overall, although number of patients included is small, the strict inclusion criteria for the cases (free from other co-factors increasing the risk or GU toxicity) and the design of the matched case-control study point out that that doses at the bladder shell do not solely explain the risk of developing GU toxicity.
Comparing actually delivered DVH metrics for cases vs. controls we identified considerable discrepancies using either absolute or relative volumes. DVH metrics in relative volume were indeed higher for the controls, but this was attributed to their smaller bladder volumes compared to cases. However, in the high-dose region we observed similar values at both groups for DVH metrics in relative volume despite the considerable bladder volume differences (Suppl. Material A). Hoogeman et al. [16] have already demonstrated that absolute DSH metrics were the most representative of the actually delivered dose. Also differences in stretching patterns between bladder subsectors [16], [30] might be considered in dose-response relationships. In fact, the group of patients included in this study received treatments fulfilling DVH-based QUANTEC recommendations [13], which are based on relative volume. These constraints were fulfilled in all patients except for one of the controls. This might further indicate a poor reliability of relative volume based bladder DVHs, which are not representative of the amount of functional tissue irradiated [25].
The strict image-based control in bladder filling led to a good overall agreement between planned and delivered dose distributions, however even small changes in bladder volume during the RT course may also imply variations in dose-volume metrics (up to 20%) conditioned by the treatment delivered, as we already observed in the previous work [22] (Suppl. Material B). On the other hand, changes in bladder filling, shape and position during the RT course have been extensively reported [12], [14], [16], [30], and are major reasons for the differences between planned and delivered DVH metrics [13]. In the previous study, we also demonstrated that the posterior/inferior bladder wall tended to move towards the high dose region when the bladder volume increased [22]. In the present study, we observed larger delivered bladder volumes compared to planned for cases, and smaller delivered compared to planned for controls (Fig. 3). Actually, the difference between planned and average delivered (ΔVolume = Vdelivered − Vplanned) bladder volume presented the highest association with GU toxicity (p = 0.04, two-way ANOVA test, Suppl. Material C). This observation suggests that differences between planning CT and delivered bladder volume might play a role in the dose received in the posterior-inferior sector of the bladder and the further manifestation of GU toxicity. Thor et al. [12] also observed generally larger bladder volumes during the treatment course in patients presenting with than patients without GU toxicity.
In conclusion, we found that neither planned nor delivered bladder DVH metrics were associated with the risk of developing GU toxicity after high-precision radiotherapy for prostate cancer, and other existing co-factors than dose might have a higher impact. Strict CBCT-based evaluation of full bladder and empty rectum protocol prior to daily dose delivery in treatment of prostate cancer assures small variation in delivered spatial DVHs with respect to planned. Current constrains applied over the planning CT appear adequate to warrant low prevalence in GU toxicity after RT for prostate cancer under these treatment conditions.
Conflict of interest
None.
Footnotes
Dr. Ludvig Muren, a co-author of this paper, is Editor-in-Chief of Physics & Imaging in Radiation Oncology. A member of the Editorial Board managed the editorial process for this manuscript independently from Dr. Muren and the manuscript was subject to the Journal’s usual peer-review process.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2018.09.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mayyas E., Chetty I.J., Chetvertkov M., Wen N., Neicu T., Nurushev T. Evaluation of multiple image-based modalities for image-guided radiation therapy (IGRT) of prostate carcinoma: a prospective study. Med Phys. 2013;40 doi: 10.1118/1.4794502. [DOI] [PubMed] [Google Scholar]
- 2.Moiseenko V., Liu M., Kristensen S., Gelowitz G., Berthelet E. Effect of bladder filling on doses to prostate and organs at risk: a treatment planning study. J Appl Clin Med Phys. 2006;8:55–68. doi: 10.1120/jacmp.v8i1.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stasi M., Munoz F., Fiorino C., Pasquino M., Baiotto B., Marini P. Emptying the rectum before treatment delivery limits the variations of rectal dose–volume parameters during 3DCRT of prostate cancer. Radiother Oncol. 2006;80:363–370. doi: 10.1016/j.radonc.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Ariyaratne H., Chesham H., Pettingell J., Alonzi R. Image-guided radiotherapy for prostate cancer with cone beam CT: dosimetric effects of imaging frequency and PTV margin. Radiother Oncol. 2016;121:103–108. doi: 10.1016/j.radonc.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Zelefsky M.J., Kollmeier M., Cox B., Fidaleo A., Sperling D., Pei X. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–129. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 6.Heemsbergen W.D., Al-Mamgani A., Slot A., Dielwart M.F.H., Lebesque J.V. Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014;110:104–109. doi: 10.1016/j.radonc.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky M.J., Levin E.J., Hunt M., Yamada Y., Shippy A.M., Jackson A. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Kole T.P., Tong M., Wu B., Lei S., Obayomi-Davies O., Chen L.N. Late urinary toxicity modeling after stereotactic body radiotherapy (SBRT) in the definitive treatment of localized prostate cancer. Acta Oncol. 2016;55:52–58. doi: 10.3109/0284186X.2015.1037011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Callaghan M.E., Raymond E., Campbell J.M., Vincent A.D., Beckmann K., Roder D. Patient-reported outcomes after radiation therapy in men with prostate cancer: a systematic review of prognostic tool accuracy and validity. Int J Radiat Oncol Biol Phys. 2017;98:318–337. doi: 10.1016/j.ijrobp.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Cheung R., Tucker S.L., Dong L., de Crevoisier R., Lee A.K., Frank S. Investigation of bladder dose and volume factors influencing late urinary toxicity after external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1059–1065. doi: 10.1016/j.ijrobp.2006.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carillo V., Cozzarini C., Rancati T., Avuzzi B., Botti A., Borca V.C. Relationships between bladder dose-volume/surface histograms and acute urinary toxicity after radiotherapy for prostate cancer. Radiother Oncol. 2014;111:100–105. doi: 10.1016/j.radonc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Thor M., Bentzen L., Hysing L.B., Ekanger C., Helle S.-I., Karlsdóttir Á. Prediction of rectum and bladder morbidity following radiotherapy of prostate cancer based on motion-inclusive dose distributions. Radiother Oncol. 2013;107:147–152. doi: 10.1016/j.radonc.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan A.N., Yorke E.D., Marks L.B., Eifel P.J., Shipley W.U. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76:S116–S122. doi: 10.1016/j.ijrobp.2009.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatanaka S., Kawada Y., Washizu K., Utsumi N., Yamano T., Nishimura K. The impact of variation in bladder volume on the doses of target and organ-at-risk in intensity-modulated radiation therapy for localized prostate cancer. J Cancer Ther. 2016;07:741–751. [Google Scholar]
- 15.Thor M., Andersen E.S., Petersen J.B.B., Sørensen T.S., Noe K.Ø., Tanderup K. Evaluation of an application for intensity-based deformable image registration and dose accumulation in radiotherapy. Acta Oncol. 2014;53:1329–1336. doi: 10.3109/0284186X.2014.928742. [DOI] [PubMed] [Google Scholar]
- 16.Hoogeman M.S., Peeters S.T.H., de Bois J., Lebesque J.V. Absolute and relative dose–surface and dose–volume histograms of the bladder: which one is the most representative for the actual treatment? Phys Med Biol. 2005;50:3589–3597. doi: 10.1088/0031-9155/50/15/007. [DOI] [PubMed] [Google Scholar]
- 17.Improta I., Palorini F., Cozzarini C., Rancati T., Avuzzi B., Franco P. Bladder spatial-dose descriptors correlate with acute urinary toxicity after radiation therapy for prostate cancer. Phys Medica. 2016;32:1681–1689. doi: 10.1016/j.ejmp.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Heemsbergen W.D., Al-Mamgani A., Witte M.G., van Herk M., Pos F.J., Lebesque J.V. Urinary obstruction in prostate cancer patients from the Dutch trial (68 Gy vs. 78 Gy): relationships with local dose, acute effects, and baseline characteristics. Int J Radiat Oncol Biol Phys. 2010;78:19–25. doi: 10.1016/j.ijrobp.2009.07.1680. [DOI] [PubMed] [Google Scholar]
- 19.Ghadjar P., Zelefsky M.J., Spratt D.E., Munck af Rosenschöld P., Oh J.H., Hunt M. Impact of dose to the bladder trigone on long-term urinary function after high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:339–344. doi: 10.1016/j.ijrobp.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palorini F., Cozzarini C., Gianolini S., Botti A., Carillo V., Iotti C. First application of a pixel-wise analysis on bladder dose-surface maps in prostate cancer radiotherapy. Radiother Oncol. 2016;119:123–128. doi: 10.1016/j.radonc.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Lawton C.A., Won M., Pilepich M.V., Asbell S.O., Shipley W.U., Hanks G.E. Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706. Int J Radiat Oncol Biol Phys. 1991;21:935–939. doi: 10.1016/0360-3016(91)90732-j. [DOI] [PubMed] [Google Scholar]
- 22.Casares-Magaz O., Moiseenko V., Hopper A., Pettersson N.J., Thor M., Knopp R. Associations between volume changes and spatial dose metrics for the urinary bladder during local versus pelvic irradiation for prostate cancer. Acta Oncol. 2017;56:884–890. doi: 10.1080/0284186X.2017.1312014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casares-Magaz O., Thor M., Muren L.P., Hopper A., Einck J., Cornell M. The need for dose re-calculation on cone-beam CTs in dose-response studies of pelvic normal tissues (abstr) Radiother Oncol. 2015;115:S508. [Google Scholar]
- 24.Sharma M., Weiss E., Siebers J.V. Dose deformation-invariance in adaptive prostate radiation therapy: implication for treatment simulations. Radiother Oncol. 2012;105:207–213. doi: 10.1016/j.radonc.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carillo V., Cozzarini C., Chietera A., Perna L., Gianolini S., Maggio A. Correlation between surrogates of bladder dosimetry and dose–volume histograms of the bladder wall defined on MRI in prostate cancer radiotherapy. Radiother Oncol. 2012;105:180–183. doi: 10.1016/j.radonc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Harsolia A., Vargas C., Yan D., Brabbins D., Lockman D., Liang J. Predictors for chronic urinary toxicity after the treatment of prostate cancer with adaptive three-dimensional conformal radiotherapy: dose-volume analysis of a Phase II dose-escalation study. Int J Radiat Oncol Biol Phys. 2007;69:1100–1109. doi: 10.1016/j.ijrobp.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 27.Georg P., Dimopoulos J., Kirisits C., Lang S., Berger D., Pötter R. The Predictive value of dose volume parameters in MRI based cervical cancer brachytherapy for late adverse side effects in rectum, sigmoid and bladder. Int J Radiat Oncol Biol Phys. 2006;66:S42–S43. [Google Scholar]
- 28.Georg P., Lang S., Dimopoulos J.C.A., Dörr W., Sturdza A.E., Berger D. Dose-volume histogram parameters and late side effects in magnetic resonance image-guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:356–362. doi: 10.1016/j.ijrobp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Hindson B.R., Millar J.L., Matheson B. Urethral strictures following high-dose-rate brachytherapy for prostate cancer: analysis of risk factors. Brachytherapy. 2013;12:50–55. doi: 10.1016/j.brachy.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Palorini F., Botti A., Carillo V., Gianolini S., Improta I., Iotti C. Bladder dose–surface maps and urinary toxicity: robustness with respect to motion in assessing local dose effects. Phys Medica. 2016;32:506–511. doi: 10.1016/j.ejmp.2016.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.