The use of imaging data in radiation oncology has a long tradition. Since the first idea of ‘dose painting’ [1], anatomical and functional imaging modalities have been reported to improve and personalize radiotherapy (RT) (Fig. 1) [2], [3], [4]. Computed Tomography (CT) has been used for more than three decades for three dimensional (3D) target volume and organ at risk delineation as well as for accurate dose calculation, due to the inherent representation of mass or electron density values of the underlying tissue. Moreover, cone-beam CT imaging has found its way into clinical usage for position verification before and during delivery of fractionated RT [5], [6]. More recently, magnetic resonance imaging (MRI) has been proposed for online image-guided RT in hybrid MRI linear accelerators (MR-Linacs) [7], [8], [9], [10], [11]. Functional MRI and positron emission tomography (PET) have been shown to be able to guide personalized RT applications in terms of dose painting where the first studies are currently on their way [12], [13], [14], [15], [16]. Similarly, different imaging biomarkers are currently being investigated for their potential to predict tumor outcome and side effects after RT [15], [16], [17]. To realize these various applications of imaging in RT, dedicated methods and tools for image acquisition, reconstruction, post-processing, registration and analysis are required. These methods include research areas such as radiomics, artefact reduction strategies or analysis methods for time-resolved imaging data, with a special focus on the needs of RT applications [18], [19], [20].
Fig. 1.
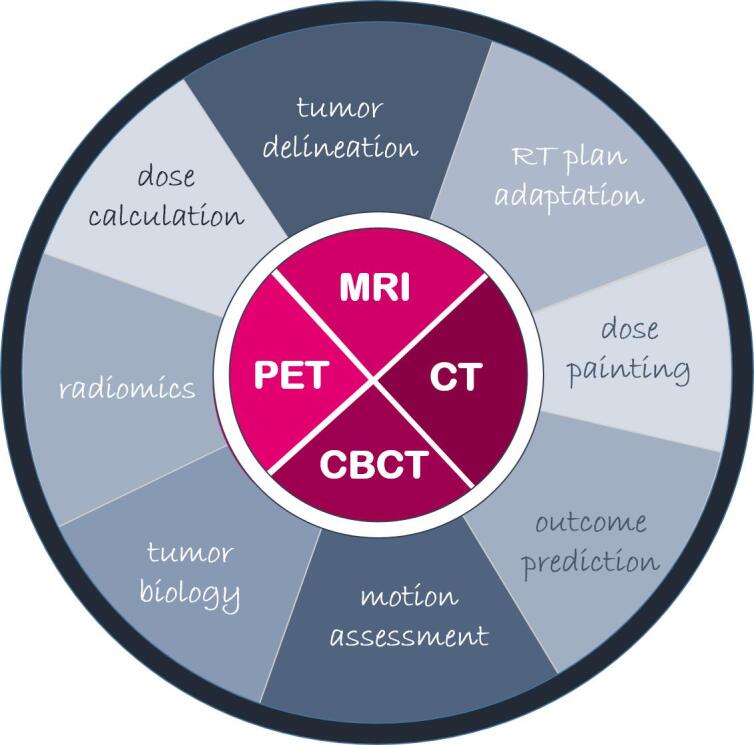
Different imaging modalities and strategies for using anatomical and functional imaging data in radiation oncology.
Almost three years ago, the first issue of physics and imaging in Radiation Oncology (phiRO) was launched. The journal aims at publishing studies reporting investigations in the field of medical physics and imaging sciences devoted to improving RT planning and delivery. Since early 2017, more than 50 articles were published in phiRO reporting on different aspects of imaging for radiation oncology, corresponding to around half of the published papers. These imaging papers focus on different aspects of imaging in radiation oncology and show thus nicely the spectrum of imaging applications for RT planning, delivery and outcome prediction.
1. CT imaging for RT
Since the invention of CT imaging as a method to visualize 3D maps of mass or electron density, CT-based RT planning is standard of care for the vast majority of RT patients. Nevertheless, research in the field of CT imaging is still ongoing to improve image quality and reduce artefacts for more accurate dose calculation by using e.g. novel reconstruction algorithms [21], [22], [23], [24], [25]. With the clinical availability of proton therapy, the requirements for CT-based density estimation changed enormously as stopping power determination and dose calculation depend strongly on the tissue composition [26], [27]. Consequently, dual energy CT (DECT) was proposed as an alternative CT-technique for proton therapy planning [28], [29], [30], [31], [32], [33], [34]. Furthermore, time-resolved CT imaging, so-called 4D-CT, has been shown to inform about tumor and organ motion and thus provide valuable information to be integrated into RT planning and delivery [24], [35], [36].
2. CBCT for RT position verification and adaptation
Onboard CBCT imaging has proven its clinical value for inter-fraction patient positioning for high-precision RT. Furthermore, new strategies to base RT dose calculations on CBCT data are currently explored [37], [38]. In recent research projects, new methods were investigated to improve 4D-CBCT functionality in order to assess intra-fraction motion of moving targets and to suggest strategies for treatment adaptation [39], [40], [41]. Furthermore, accurate simulation of radiation dose contributions resulting from repeated CBCT examinations have been investigated in detail by several groups [42], [43], [44], [45].
3. MRI-guided RT
Due to its high geometrical resolution in addition to an excellent soft tissue contrast, MRI is a powerful imaging technique for image-guided RT. Thus it has potential for improving target volume delineation, offline and online plan adaptation and therefore provides the basis for personalization of RT [46], [47]. In the last years, a variety of research projects investigated the value of using additional MRI data for target definition. Consequently, an important technical focus was the characterization and improvement of MR image quality and artefact reduction [48], [49], [50], [51], [52]. MRI offers higher soft tissue contrast for tumor and organ definition but lacks information about electron density, which is a prerequisite for accurate dose calculation. To overcome this, several groups have proposed strategies to generate synthetic CT data sets derived solely from MRI data [53], [54], [55], [56]. First dosimetric analysis of such MR-only workflow showed quite promising results for tumors in the pelvic region [57]. Recently, hybrid MR-Linacs are clinically available and offer online MR-guided RT. Physical challenges of MR-guided adaptive RT concern treatment planning and dose calculation in magnetic fields [58], [59], [60], [61], [62], [63] and strategies for MR-based RT plan adaptation with minimal latency time [64], [65]. In the future, imaging biomarkers assessed from functional MRI may be useful for the prediction of therapy outcome or side effects after RT [66], [67], [68].
4. Functional imaging using PET and MRI for personalization of RT
Several recent studies have investigated the role of multimodal functional imaging to stratify patient groups and individualize RT dose prescriptions and treatment planning / application strategies according to imaging information [69], [70], [71], [72]. PET data has been shown to have great prognostic potential in different tumor entities [73], [74] opening new possibilities for target volume definition [75]. Furthermore, the value of functional MRI, such as diffusion weighted imaging (DWI) [76], [77], [78] and perfusion imaging using dynamic contrast enhanced (DCE) MRI [79], [80] for outcome prognosis of functional image guided RT planning has been investigated. Further studies have analyzed the combined information from multi-modal PET and MRI [75], [81] for biologically adapted, personalized RT [82].
5. Image data processing, analysis and radiomics
With increasing availability of large amounts of imaging data, robust and reproducible ways for image analysis, integration and exploration are needed. Recent studies have investigated the potential of radiomics for precision RT [19], [83], [84], [85]. Of note, for high quality usage of functional and anatomic imaging data, dedicated robust strategies for image registration [86], [87], [88], [89] and data analysis [90] are needed. Only then, reliable new segmentation algorithms [91], [92] and prediction models can be trained [16], [93].
In conclusion, imaging in radiation oncology has many facets ranging from dose calculation to outcome modeling. With increasing availability of anatomical and functional imaging modalities, the benefit and potential of using these technologies and the respective imaging data dedicatedly in radiation oncology to further improve the effectiveness of cancer treatment with RT are eminent. Nevertheless, integration of imaging data into planning and application of precision RT will be a key factor for future developments in personalized RT. Acknowledging the important role of imaging in radiation oncology, phiRO is now broadening its editorial composition with the appointment of a 2nd co-Editor-in-Chief dedicated to imaging (Daniela Thorwarth). With this in place, phiRO is fully prepared to receive your submissions from all areas in this expanding and vital area of radiation oncology, and to make sure that your research findings are structured and presented for publication in the best possible way.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ling C.C., Humm J., Larson S., Amols H., Fuks Z., Leibel S. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47:551–560. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 2.Baumann M., Krause M., Overgaard J., Debus J., Bentzen S.M., Daartz J. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234–249. doi: 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- 3.Lim-Reinders S., Keller B.M., Al-Ward S., Sahgal A., Kim A. Online Adaptive Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;99:994–1003. doi: 10.1016/j.ijrobp.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Thorwarth D. Biologically adapted radiation therapy. Z Med Phys. 2018;28:177–183. doi: 10.1016/j.zemedi.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Hvid C.A., Elstrom U.V., Jensen K., Grau C. Cone-beam computed tomography (CBCT) for adaptive image guided head and neck radiation therapy. Acta Oncol. 2018;57:552–556. doi: 10.1080/0284186X.2017.1398414. [DOI] [PubMed] [Google Scholar]
- 6.Posiewnik M., Piotrowski T. A review of cone-beam CT applications for adaptive radiotherapy of prostate cancer. Phys Med. 2019;59:13–21. doi: 10.1016/j.ejmp.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Pollard J.M., Wen Z., Sadagopan R., Wang J., Ibbott G.S. The future of image-guided radiotherapy will be MR guided. Br J Radiol. 2017;90:20160667. doi: 10.1259/bjr.20160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagendijk J.J., Raaymakers B.W., Van den Berg C.A., Moerland M.A., Philippens M.E., van Vulpen M. MR guidance in radiotherapy. Phys Med Biol. 2014;59:R349–R369. doi: 10.1088/0031-9155/59/21/R349. [DOI] [PubMed] [Google Scholar]
- 9.Slotman B., Gani C. Online MR-guided radiotherapy - A new era in radiotherapy. Clin Transl Radiat Oncol. 2019;18:102–103. doi: 10.1016/j.ctro.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl. Radiat Oncol. 2019;18:98–101. doi: 10.1016/j.ctro.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkel D., Bol G.H., Kroon P.S., van Asselen B., Hackett S.S., Werensteijn-Honingh A.M. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Elmpt W., De Ruysscher D., van der Salm A., Lakeman A., van der Stoep J., Emans D. The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer. Radiother Oncol. 2012;104:67–71. doi: 10.1016/j.radonc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Welz S., Monnich D., Pfannenberg C., Nikolaou K., Reimold M., La Fougere C. Prognostic value of dynamic hypoxia PET in head and neck cancer: Results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother Oncol. 2017;124:526–532. doi: 10.1016/j.radonc.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Monninkhof E.M., van Loon J.W.L., van Vulpen M., Kerkmeijer L.G.W., Pos F.J., Haustermans K. Standard whole prostate gland radiotherapy with and without lesion boost in prostate cancer: Toxicity in the FLAME randomized controlled trial. Radiother Oncol. 2018;127:74–80. doi: 10.1016/j.radonc.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Hong J.C., Cui Y., Patel B.N., Rushing C.N., Faught A.M., Eng J.S. Association of Interim FDG-PET Imaging During Chemoradiation for Squamous Anal Canal Carcinoma With Recurrence. Int J Radiat Oncol Biol Phys. 2018;102:1046–1051. doi: 10.1016/j.ijrobp.2018.04.062. [DOI] [PubMed] [Google Scholar]
- 16.Thorwarth D., Welz S., Monnich D., Pfannenberg C., Nikolaou K., Reimold M. Prospective evaluation of a tumor control probability model based on dynamic (18)F-FMISO PET for head-and-neck cancer radiotherapy. J Nucl Med. 2019 doi: 10.2967/jnumed.119.227744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hompland T., Hole K.H., Ragnum H.B., Aarnes E.K., Vlatkovic L., Lie A.K. Combined MR Imaging of Oxygen Consumption and Supply Reveals Tumor Hypoxia and Aggressiveness in Prostate Cancer Patients. Cancer Res. 2018;78:4774–4785. doi: 10.1158/0008-5472.CAN-17-3806. [DOI] [PubMed] [Google Scholar]
- 18.Aerts H.J., Velazquez E.R., Leijenaar R.T., Parmar C., Grossmann P., Carvalho S. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogowicz M., Tanadini-Lang S., Guckenberger M., Riesterer O. Combined CT radiomics of primary tumor and metastatic lymph nodes improves prediction of loco-regional control in head and neck cancer. Sci Rep. 2019;9:15198. doi: 10.1038/s41598-019-51599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grkovski M., Lee N.Y., Schoder H., Carlin S.D., Beattie B.J., Riaz N. Monitoring early response to chemoradiotherapy with (18)F-FMISO dynamic PET in head and neck cancer. Eur J Nucl Med Mol Imaging. 2017;44:1682–1691. doi: 10.1007/s00259-017-3720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Heyden B., Öllers M., Ritter A., Verhaegen F., van Elmpt W. Clinical evaluation of a novel CT image reconstruction algorithm for direct dose calculations. Phys Imag Radiat Oncol. 2017;2:11–16. [Google Scholar]
- 22.Chen G.-P., Noid G., Tai A., Liu F., Lawton C., Erickson B. Improving CT quality with optimized image parameters for radiation treatment planning and delivery guidance. Phys Imag Radiat Oncol. 2017;4:6–11. [Google Scholar]
- 23.Fang R., Mazur T., Mutic S., Khan R. The impact of mass density variations on an electron Monte Carlo algorithm for radiotherapy dose calculations. Phys Imag Radiat Oncol. 2018;8:1–7. doi: 10.1016/j.phro.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pettersson E., Bäck A., Björk-Eriksson T., Lindencrona U., Petruson K., Thilander-Klang A. Structure delineation in the presence of metal – A comparative phantom study using single and dual-energy computed tomography with and without metal artefact reduction. Phys Imag Radiat Oncol. 2019;9:43–49. doi: 10.1016/j.phro.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J., Bai T., Gu J., Sun Z., Wei Y., Li B. Effects of megavoltage computed tomographic scan methodology on setup verification and adaptive dose calculation in helical TomoTherapy. Radiat Oncol. 2018;13:80. doi: 10.1186/s13014-018-0989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Elmpt W., Landry G. Quantitative computed tomography in radiation therapy: A mature technology with a bright future. Phys Imag Radiat Oncol. 2018;6:12–13. doi: 10.1016/j.phro.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohlfahrt P., Richter C. Status and innovations in pre-treatment CT imaging for proton therapy. Br J Radiol. 2019;20190590 doi: 10.1259/bjr.20190590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilches-Freixas G., Taasti V.T., Muren L.P., Petersen J.B.B., Létang J.M., Hansen D.C. Comparison of projection- and image-based methods for proton stopping power estimation using dual energy CT. Phys Imag Radiat Oncol. 2017;3:28–36. [Google Scholar]
- 29.Möhler C., Wohlfahrt P., Richter C., Greilich S. On the equivalence of image-based dual-energy CT methods for the determination of electron density and effective atomic number in radiotherapy. Phys Imag Radiat Oncol. 2018;5:108–110. doi: 10.1016/j.phro.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vilches-Freixas G., Quiñones C.T., Létang J.M., Rit S. Deriving the mean excitation energy map from dual-energy and proton computed tomography. Phys Imag Radiat Oncol. 2018;6:20–24. doi: 10.1016/j.phro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taasti V.T., Muren L.P., Jensen K., Petersen J.B.B., Thygesen J., Tietze A. Comparison of single and dual energy CT for stopping power determination in proton therapy of head and neck cancer. Phys Imag Radiat Oncol. 2018;6:14–19. doi: 10.1016/j.phro.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Heyden B., Schyns L.E.J.R., Podesta M., Vaniqui A., Almeida I.P., Landry G. <em>VOXSI:</em> A voxelized single- and dual-energy CT scenario generator for quantitative imaging. Phys Imag Radiat Oncol. 2018;6:47–52. doi: 10.1016/j.phro.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wohlfahrt P., Mohler C., Troost E.G.C., Greilich S., Richter C. Dual-Energy Computed Tomography to Assess Intra- and Inter-Patient Tissue Variability for Proton Treatment Planning of Patients With Brain Tumor. Int J Radiat Oncol Biol Phys. 2019;105:504–513. doi: 10.1016/j.ijrobp.2019.06.2529. [DOI] [PubMed] [Google Scholar]
- 34.Dedes G., Dickmann J., Niepel K., Wesp P., Johnson R.P., Pankuch M. Experimental comparison of proton CT and dual energy x-ray CT for relative stopping power estimation in proton therapy. Phys Med Biol. 2019;64 doi: 10.1088/1361-6560/ab2b72. [DOI] [PubMed] [Google Scholar]
- 35.Oar A., Liney G., Rai R., Deshpande S., Pan L., Johnston M. Comparison of four dimensional computed tomography and magnetic resonance imaging in abdominal radiotherapy planning. Phys Imag Radiat Oncol. 2018;7:70–75. doi: 10.1016/j.phro.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambrecht M., Sonke J.-J., Nestle U., Peulen H., Weber D.C., Verheij M. Quality assurance of four-dimensional computed tomography in a multicentre trial of stereotactic body radiotherapy of centrally located lung tumours. Phys Imag Radiat Oncol. 2018;8:57–62. doi: 10.1016/j.phro.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landry G., Hansen D., Kamp F., Li M., Hoyle B., Weller J. Comparing Unet training with three different datasets to correct CBCT images for prostate radiotherapy dose calculations. Phys Med Biol. 2019;64 doi: 10.1088/1361-6560/aaf496. [DOI] [PubMed] [Google Scholar]
- 38.Hansen D.C., Landry G., Kamp F., Li M., Belka C., Parodi K. ScatterNet: A convolutional neural network for cone-beam CT intensity correction. Med Phys. 2018;45:4916–4926. doi: 10.1002/mp.13175. [DOI] [PubMed] [Google Scholar]
- 39.van den Bosch M., Öllers M., Reymen B., van Elmpt W. Automatic selection of lung cancer patients for adaptive radiotherapy using cone-beam CT imaging. Phys Imag Radiat Oncol. 2017;1:21–27. [Google Scholar]
- 40.Jensen N.B.K., Assenholt M.S., Fokdal L.U., Vestergaard A., Schouboe A., Kjaersgaard E.B. Cone beam computed tomography-based monitoring and management of target and organ motion during external beam radiotherapy in cervical cancer. Phys Imag Radiat Oncol. 2019;9:14–20. doi: 10.1016/j.phro.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graadal Svestad J., Ramberg C., Skar B., Paulsen Hellebust T. Intrafractional motion in stereotactic body radiotherapy of spinal metastases utilizing cone beam computed tomography image guidance. Phys Imag Radiat Oncol. 2019;12:1–6. doi: 10.1016/j.phro.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thing R.S., Bernchou U., Hansen O., Brink C. Accuracy of dose calculation based on artefact corrected Cone Beam CT images of lung cancer patients. Phys Imag Radiat Oncol. 2017;1:6–11. [Google Scholar]
- 43.Zöllner C., Rit S., Kurz C., Vilches-Freixas G., Kamp F., Dedes G. Decomposing a prior-CT-based cone-beam CT projection correction algorithm into scatter and beam hardening components. Phys Imag Radiat Oncol. 2017;3:49–52. [Google Scholar]
- 44.Kaplan L.P., Elstrøm U.V., Møller D.S., Hoffmann L. Cone beam CT based dose calculation in the thorax region. Phys Imag Radiat Oncol. 2018;7:45–50. doi: 10.1016/j.phro.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schröder L., Stankovic U., Remeijer P., Sonke J.-J. Evaluating the impact of cone-beam computed tomography scatter mitigation strategies on radiotherapy dose calculation accuracy. Phys Imag Radiat Oncol. 2019;10:35–40. doi: 10.1016/j.phro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grosu A.-L., van der Heide U.A. Imaging for radiation treatment planning and monitoring in prostate Cancer: Precision, personalization, individualization of therapy. Phys Imag Radiat Oncol. 2019;11:61–62. doi: 10.1016/j.phro.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bohoudi O., Bruynzeel A.M.E., Senan S., Cuijpers J.P., Slotman B.J., Lagerwaard F.J. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017;125:439–444. doi: 10.1016/j.radonc.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 48.Dinis Fernandes C., Dinh C.V., Steggerda M.J., ter Beek L.C., Smolic M., van Buuren L.D. Prostate fiducial marker detection with the use of multi-parametric magnetic resonance imaging. Phys Imag Radiat Oncol. 2017;1:14–20. [Google Scholar]
- 49.Lundman J.A., Bylund M., Garpebring A., Thellenberg Karlsson C., Nyholm T. Patient-induced susceptibility effects simulation in magnetic resonance imaging. Phys Imag Radiat Oncol. 2017;1:41–45. [Google Scholar]
- 50.Leger S., Löck S., Hietschold V., Haase R., Böhme H.J., Abolmaali N. Physical correction model for automatic correction of intensity non-uniformity in magnetic resonance imaging. Phys Imag Radiat Oncol. 2017;4:32–38. [Google Scholar]
- 51.Peerlings J., Compter I., Janssen F., Wiggins C.J., Postma A.A., Mottaghy F.M. Characterizing geometrical accuracy in clinically optimised 7T and 3T magnetic resonance images for high-precision radiation treatment of brain tumours. Phys Imag Radiat Oncol. 2019;9:35–42. doi: 10.1016/j.phro.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pathmanathan A.U., McNair H.A., Schmidt M.A., Brand D.H., Delacroix L., Eccles C.L. Comparison of prostate delineation on multimodality imaging for MR-guided radiotherapy. Br J Radiol. 2019;92:20180948. doi: 10.1259/bjr.20180948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyatt J., Hedley S., Johnstone E., Speight R., Kelly C., Henry A. Evaluating the repeatability and set-up sensitivity of a large field of view distortion phantom and software for magnetic resonance-only radiotherapy. Phys Imag Radiat Oncol. 2018;6:31–38. doi: 10.1016/j.phro.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maspero M., Tyyger M.D., Tijssen R.H.N., Seevinck P.R., Intven M.P.W., van den Berg C.A.T. Feasibility of magnetic resonance imaging-only rectum radiotherapy with a commercial synthetic computed tomography generation solution. Phys Imag Radiat Oncol. 2018;7:58–64. doi: 10.1016/j.phro.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunnlaugsson A., Persson E., Gustafsson C., Kjellén E., Ambolt P., Engelholm S. Target definition in radiotherapy of prostate cancer using magnetic resonance imaging only workflow. Phys Imag Radiat Oncol. 2019;9:89–91. doi: 10.1016/j.phro.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jonsson J., Nyholm T., Soderkvist K. The rationale for MR-only treatment planning for external radiotherapy. Clin Transl Radiat Oncol. 2019;18:60–65. doi: 10.1016/j.ctro.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kemppainen R., Suilamo S., Ranta I., Pesola M., Halkola A., Eufemio A. Assessment of dosimetric and positioning accuracy of a magnetic resonance imaging-only solution for external beam radiotherapy of pelvic anatomy. Phys Imag Radiat Oncol. 2019;11:1–8. doi: 10.1016/j.phro.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schrenk O., Spindeldreier C.K., Schmitt D., Roeder F., Bangert M., Burigo L.N. The effect of density overrides on magnetic resonance-guided radiation therapy planning for lung cancer. Phys Imag Radiat Oncol. 2018;8:23–27. doi: 10.1016/j.phro.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruiz B., Feng Y. Clinical and radiobiological evaluation of a method for planning target volume generation dependent on organ-at-risk exclusions in magnetic resonance imaging-based prostate radiotherapy. Phys Imag Radiat Oncol. 2018;8:51–56. doi: 10.1016/j.phro.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van de Schoot A.J.A.J., van den Wollenberg W., Carbaat C., de Ruiter P., Nowee M.E., Pos F. Evaluation of plan quality in radiotherapy planning with an MR-linac. Phys Imag Radiat Oncol. 2019;10:19–24. doi: 10.1016/j.phro.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.den Hartogh M.D., de Boer H.C.J., de Groot-van Breugel E.N., van der Voort van Zyp J.R.N., Hes J., van der Heide U.A. Planning feasibility of extremely hypofractionated prostate radiotherapy on a 1.5 T magnetic resonance imaging guided linear accelerator. Phys Imag Radiat Oncol. 2019;11:16–20. doi: 10.1016/j.phro.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedel M., Nachbar M., Monnich D., Dohm O., Thorwarth D. Development and validation of a 1.5 T MR-Linac full accelerator head and cryostat model for Monte Carlo dose simulations. Med Phys. 2019;46:5304–5313. doi: 10.1002/mp.13829. [DOI] [PubMed] [Google Scholar]
- 63.Pojtinger S., Kapsch R.P., Dohm O.S., Thorwarth D. A finite element method for the determination of the relative response of ionization chambers in MR-linacs: simulation and experimental validation up to 1.5 T. Phys Med Biol. 2019;64 doi: 10.1088/1361-6560/ab2837. [DOI] [PubMed] [Google Scholar]
- 64.Tetar S.U., Bruynzeel A.M.E., Lagerwaard F.J., Slotman B.J., Bohoudi O., Palacios M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys Imag Radiat Oncol. 2019;9:69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winkel D., Bol G.H., Werensteijn-Honingh A.M., Kiekebosch I.H., van Asselen B., Intven M.P.W. Evaluation of plan adaptation strategies for stereotactic radiotherapy of lymph node oligometastases using online magnetic resonance image guidance. Phys Imag Radiat Oncol. 2019;9:58–64. doi: 10.1016/j.phro.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thor M., Tyagi N., Hatzoglou V., Apte A., Saleh Z., Riaz N. A magnetic resonance imaging-based approach to quantify radiation-induced normal tissue injuries applied to trismus in head and neck cancer. Phys Imag Radiat Oncol. 2017;1:34–40. doi: 10.1016/j.phro.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olsson L.E., Johansson M., Zackrisson B., Blomqvist L.K. Basic concepts and applications of functional magnetic resonance imaging for radiotherapy of prostate cancer. Phys Imag Radiat Oncol. 2019;9:50–57. doi: 10.1016/j.phro.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kooreman E.S., van Houdt P.J., Nowee M.E., van Pelt V.W.J., Tijssen R.H.N., Paulson E.S. Feasibility and accuracy of quantitative imaging on a 1.5 T MR-linear accelerator. Radiother Oncol. 2019;133:156–162. doi: 10.1016/j.radonc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Vallières M., Serban M., Benzyane I., Ahmed Z., Xing S., El Naqa I. Investigating the role of functional imaging in the management of soft-tissue sarcomas of the extremities. Phys Imag Radiat Oncol. 2018;6:53–60. doi: 10.1016/j.phro.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zamboglou C., Eiber M., Fassbender T.R., Eder M., Kirste S., Bock M. Multimodal imaging for radiation therapy planning in patients with primary prostate cancer. Phys Imag Radiat Oncol. 2018;8:8–16. doi: 10.1016/j.phro.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Even A.J.G., Reymen B., La Fontaine M.D., Das M., Mottaghy F.M., Belderbos J.S.A. Clustering of multi-parametric functional imaging to identify high-risk subvolumes in non-small cell lung cancer. Radiother Oncol. 2017;125:379–384. doi: 10.1016/j.radonc.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 72.Leibfarth S., Simoncic U., Monnich D., Welz S., Schmidt H., Schwenzer N. Analysis of pairwise correlations in multi-parametric PET/MR data for biological tumor characterization and treatment individualization strategies. Eur J Nucl Med Mol Imaging. 2016;43:1199–1208. doi: 10.1007/s00259-016-3307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meijer T.W.H., Wijsman R., Usmanij E.A., Schuurbiers O.C.J., Pv Kollenburg, Bouwmans L. Stereotactic radiotherapy boost after definite chemoradiation for non-responding locally advanced NSCLC based on early response monitoring <sup>18</sup>F-FDG-PET/CT. Phys Imag. Radiat Oncol. 2018;7:16–22. doi: 10.1016/j.phro.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valstar M.H., Owers E.C., Al-Mamgani A., Smeele L.E., van de Kamer J.B., Sonke J.-J. Prostate-specific membrane antigen positron emission tomography/computed tomography as a potential tool to assess and guide salivary gland irradiation. Phys Imag Radiat Oncol. 2019;9:65–68. doi: 10.1016/j.phro.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schakel T., Peltenburg B., Dankbaar J.-W., Cardenas C.E., Aristophanous M., Terhaard C.H.J. Evaluation of diffusion weighted imaging for tumor delineation in head-and-neck radiotherapy by comparison with automatically segmented <sup>18</sup>F-fluorodeoxyglucose positron emission tomography. Phys Imag Radiat Oncol. 2018;5:13–18. doi: 10.1016/j.phro.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li A., Andersen E., Lervåg C., Julin C.H., Lyng H., Hellebust T.P. Dynamic contrast enhanced magnetic resonance imaging for hypoxia mapping and potential for brachytherapy targeting. Phys Imag Radiat Oncol. 2017;2:1–6. [Google Scholar]
- 77.Lee J., Dean C., Patel R., Webster G., Eaton D.J. Multi-center evaluation of dose conformity in stereotactic body radiotherapy. Phys Imag Radiat Oncol. 2019;11:41–46. doi: 10.1016/j.phro.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Boer P., Mandija S., Werensteijn-Honingh A.M., van den Berg C.A.T., de Leeuw A.A.C., Jürgenliemk-Schulz I.M. Cervical cancer apparent diffusion coefficient values during external beam radiotherapy. Phys Imag Radiat Oncol. 2019;9:77–82. doi: 10.1016/j.phro.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Detsky J.S., Milot L., Ko Y.-J., Munoz-Schuffenegger P., Chu W., Czarnota G. Perfusion imaging of colorectal liver metastases treated with bevacizumab and stereotactic body radiotherapy. Phys Imag Radiat Oncol. 2018;5:9–12. doi: 10.1016/j.phro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farr K.P., West K., Yeghiaian-Alvandi R., Farlow D., Stensmyr R., Chicco A. Functional perfusion image guided radiation treatment planning for locally advanced lung cancer. Phys Imag Radiat Oncol. 2019;11:76–81. doi: 10.1016/j.phro.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tulipan A.J., Vlatkovic L., Malinen E., Brennhovd B., Hole K.H., Lie A.K. Comparison of time curves from dynamic <sup>18</sup>F-fluciclovine positron emission tomography and dynamic contrast-enhanced magnetic resonance imaging for primary prostate carcinomas. Phys Imag Radiat Oncol. 2018;7:51–57. doi: 10.1016/j.phro.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gronlund E., Johansson S., Montelius A., Ahnesjo A. Dose painting by numbers based on retrospectively determined recurrence probabilities. Radiother Oncol. 2017;122:236–241. doi: 10.1016/j.radonc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q., Zhou S., Court L.E., Verma V., Koay E.J., Zhang L. Radiomics predicts clinical outcome in primary gastroesophageal junction adenocarcinoma treated by chemo/radiotherapy and surgery. Phys Imag Radiat Oncol. 2017;3:37–42. [Google Scholar]
- 84.Dinis Fernandes C., Dinh C.V., Walraven I., Heijmink S.W., Smolic M., van Griethuysen J.J.M. Biochemical recurrence prediction after radiotherapy for prostate cancer with T2w magnetic resonance imaging radiomic features. Phys Imag Radiat Oncol. 2018;7:9–15. doi: 10.1016/j.phro.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei L., Rosen B., Vallières M., Chotchutipan T., Mierzwa M., Eisbruch A. Automatic recognition and analysis of metal streak artifacts in head and neck computed tomography for radiomics modeling. Phys Imag Radiat Oncol. 2019;10:49–54. doi: 10.1016/j.phro.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Woolcot T., Kousi E., Wells E., Aitken K., Taylor H., Schmidt M.A. An evaluation of systematic errors on marker-based registration of computed tomography and magnetic resonance images of the liver. Phys Imag Radiat Oncol. 2018;7:27–31. doi: 10.1016/j.phro.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.White I., McQuaid D., McNair H., Dunlop A., Court S., Hopkins N. Geometric and dosimetric evaluation of the differences between rigid and deformable registration to assess interfraction motion during pelvic radiotherapy. Phys Imag Radiat Oncol. 2019;9:97–102. doi: 10.1016/j.phro.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zachiu C., Denis de Senneville B., Raaymakers B.W., Ries M.G. Biomechanical quality assurance criteria for deformable image registration algorithms used in radiotherapy guidance. Phys Med Biol. 2019 doi: 10.1088/1361-6560/ab501d. [DOI] [PubMed] [Google Scholar]
- 89.Leibfarth S., Monnich D., Welz S., Siegel C., Schwenzer N., Schmidt H. A strategy for multimodal deformable image registration to integrate PET/MR into radiotherapy treatment planning. Acta Oncol. 2013;52:1353–1359. doi: 10.3109/0284186X.2013.813964. [DOI] [PubMed] [Google Scholar]
- 90.Lao Y., David J., Torosian A., Placencio V., Wang Y., Hendifar A. Combined morphologic and metabolic pipeline for Positron emission tomography/computed tomography based radiotherapy response evaluation in locally advanced pancreatic adenocarcinoma. Phys Imag Radiat Oncol. 2019;9:28–34. doi: 10.1016/j.phro.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Astaraki M., Severgnini M., Milan V., Schiattarella A., Ciriello F., de Denaro M. Evaluation of localized region-based segmentation algorithms for CT-based delineation of organs at risk in radiotherapy. Phys Imag Radiat Oncol. 2018;5:52–57. doi: 10.1016/j.phro.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Houri J., Karunamuni R., Connor M., Pettersson N., McDonald C., Farid N. Analyses of regional radiosensitivity of white matter structures along tract axes using novel white matter segmentation and diffusion imaging biomarkers. Phys Imag Radiat Oncol. 2018;6:39–46. doi: 10.1016/j.phro.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Casares-Magaz O., Raidou R.G., Rørvik J., Vilanova A., Muren L.P. Uncertainty evaluation of image-based tumour control probability models in radiotherapy of prostate cancer using a visual analytic tool. Phys Imag Radiat Oncol. 2018;5:5–8. doi: 10.1016/j.phro.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


