Abstract
Background and purpose
Applying needles in the first brachytherapy (BT) fraction for patients with locally advanced cervical cancer allows for more dose conformality and OAR sparing, but is more challenging than in subsequent fractions, as pre-implant imaging with applicator in situ is lacking. We investigate whether a needle simulation, a fixed needle configuration or a multidisciplinary discussion-based configuration can predict more accurately which applicator needle positions are best suited for use in the first BT fraction.
Materials and methods
For 20 patients we retrospectively determined the “reference” needle configuration (RC) for the first BT fraction using magnetic resonance imaging (MRI) scans with applicator in situ. We simulated a pre-MRI needle configuration (PC) using the MRI made in the fourth week of external beam radiotherapy (EBRT) without applicator in situ. We generated a fixed needle configuration (FC) from the most common RC needles. Using Dice’s similarity coefficient (DSC) we compared each of these needle configurations, including the clinically applied “multidisciplinary consensus” needle configuration (MC), with RC. We considered two scenarios: allowing up to ten needles (scenario 1), and limiting the needle number (scenario 2). The analysis was repeated omitting two mid-ventral needles previously determined as non-essential to treatment planning.
Results
For both scenarios, the median DSC for PC and FC was higher than for MC (scenario1:DSCPC = 0,78; DSCFC = 0,75; DSCMC = 0,57; scenario 2:DSCPC = 0,74; DSCFC = 0,73; DSCMC = 0,59), while omitting mid-ventral needles resulted in no statistically significant differences in DSC.
Conclusions
The PC or FC method are at least as accurate as the MC, with the FC preferred for efficiency.
Keywords: Cervical cancer, Brachytherapy, Intracavitary/interstitial applicator, Interstitial needle
1. Introduction
Magnetic resonance imaging (MRI)-compatible combined intracavitary/interstitial (IC/IS) applicators are commonly used in the image-guided adaptive brachytherapy (IGABT) treatment of locally advanced cervical cancer (LACC) [1], [2], [3], [4]. Such applicators are clearly visible on MR images, allowing for superior delineation of soft tissues [5], and have the possibility to include interstitial needles along with the standard intracavitary channels. The combined IC/IS approach has been shown to result in improvements in dose-volume histogram (DVH) parameters as compared to using more traditional IC applicators [6], [7], [8].
Based on dose effect evidence from the EMBRACE and retroEMBRACE studies (www.embracestudy.dk) [3], [6], [9], [10], [11], [12], [13], [14], [15], [16], the strict dose prescription protocol of the multi-center EMBRACE II study aims at improving local control and decreasing morbidity. However, reaching the EMBRACE II dose planning aims and constraints, and thereby achieving tumour dose-escalation and/or organ-at-risk (OAR) dose de-escalation, requires increased use of the combined IC/IS brachytherapy (BT) approach. The strategy in EMBRACE II is to aim for an IC/IS application in at least 20% of the patients in each participating center. Thus, optimally applying needles in the first BT fraction is becoming more important.
Various approaches are possible in choosing which needles to apply for BT. Many centers use an IC-only approach for the first fraction, and base the needle choice for the subsequent fractions on the shortcomings of the first [7], while some institutes apply all ten needles in order to ensure optimal planning degrees of freedom, although the latter approach is uncommon. Another less-commonly used approach involves an additional planning MRI with applicator in situ, taken a few days before the start of BT, in order to determine the needle configuration best suited to the first fraction. Trans-rectal or abdominal ultrasound may also be used during applicator implantation to determine the needle configuration to be used. In our institute we use a multidisciplinary approach in selecting needles, keeping in mind possible needle-related complications, time constraints and costs. The choice of needles and their insertion depth for each fraction is based on discussion amongst radiation oncologists, medical physicists and radiotherapy technologists (RTT). Patients receive three (or in certain instances four) weekly fractions of high dose rate (HDR) BT, with MR images for treatment planning made on the treatment day itself. This makes predicting the needle configuration for the second and subsequent fraction(s) easier than for the first. For instance, at the time of the second fraction we have at our disposal the first fraction treatment plan (and thus the relative position of the applicator and used needles with respect to the target), and are able to infer which needles we should subsequently choose for the second fraction. This is not the case for the first BT fraction. Instead, we make use of the MRI scan recorded in the fourth week of external beam radiotherapy (EBRT) (pre-MRI), at around fraction 16 of 23 fractions, one week before the first BT fraction. This pre-MRI scan is acquired without the applicator in situ, and targets and organs are not delineated, so deciding which needle configuration will be optimal for the first fraction based on this scan is often non-trivial. To our knowledge, to date no studies have been performed comparing methods for choosing needles for the first BT fraction.
The option to apply needles in all fractions, including the first, allows for more dose conformality, potentially increasing local control and decreasing treatment related morbidity [17]. In this study we investigated which of three alternative methods to determine the needle configuration in the first BT fraction is best, when compared to a ground truth “reference” needle configuration.
2. Materials and methods
2.1. Patients and treatment
The study included 20 subsequent cervical cancer patients treated with EBRT followed by HDR BT, between June 2014 and December 2015. The EBRT dose for 19 of the patients was 46 Gy in 23 fractions, and for one patient 45 Gy in 25 fractions, given over the course of five weeks. For nine patients EBRT included a lymph node boost. For 19 patients in the study, EBRT was followed by three fractions of weekly HDR BT, one patient received four fractions of BT. The first BT fraction took place in the fifth week of EBRT.
The Utrecht applicator (Elekta, Veenendaal, NL), consisting of an intra-uterine tube, two ovoids and the possibility to insert up to ten interstitial needles, was used for BT. The applicator components used for the patients in this study were the 15°, 30° or 45° intra-uterine tubes, and 20 and/or 25 mm ovoids. We labeled the needle positions in the ovoids from A to J, starting with the mid-ventral position in the right ovoid and moving anticlockwise to the mid-ventral position in the left ovoid (Fig. 1). Needle position and insertion depth for the first fraction were determined using the pre-MRI and multidisciplinary discussion, and for subsequent fractions according to the MRI and treatment plan of the previous fraction.
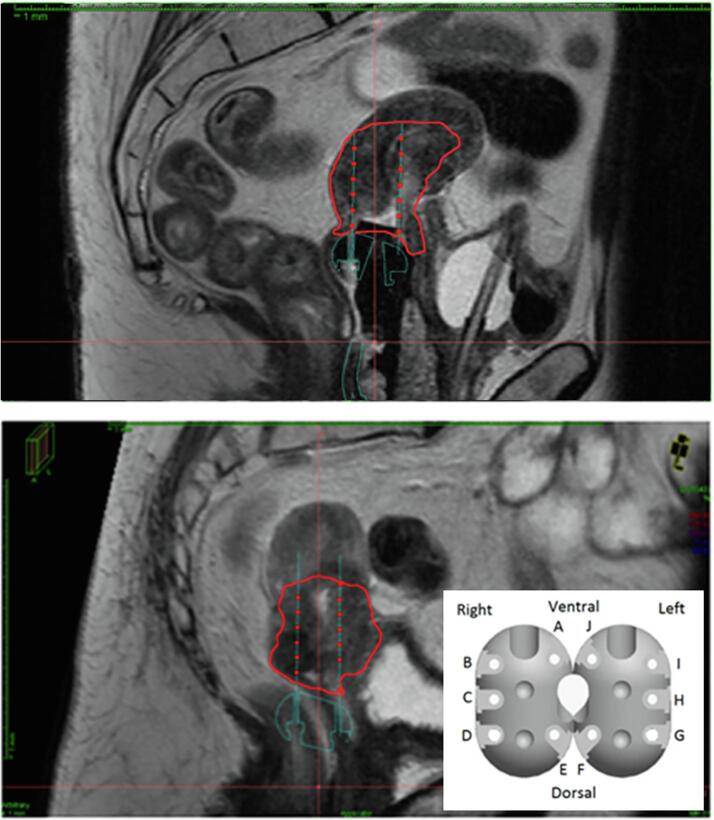
Fig. 1.
Sagittal view of applicator modeling and needle placement on delineated MRIs for the reference needle configuration (RC) (above) and pre-brachytherapy MRI needle configuration (PC) (below). The high-risk clinical target volume (CTVHR) is shown in red. The needle positions in the applicator ovoids, labeled A–J are shown (bottom right). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For each BT fraction, MR images of the patient with applicator in place were obtained using a 1.5-T Philips Achieva MRI scanner. Axial, sagittal and coronal T2-weighted turbo spin-echo (TSE) images with 3 mm slice thickness were acquired, as well as a fast field echo (FFE) 3D scan with 1.5 × 1.5 × 1.5 mm3 voxel resolution. The gross tumour volume (GTV) and high-risk clinical target volume (CTVHR) were contoured, as well as the OAR, namely bladder, rectum, sigmoid and small bowel. Applicator reconstruction and treatment planning were performed in Oncentra Brachytherapy Planning System (BPS) (Elekta, Veenendaal, NL). All channels were reconstructed manually without the use of the applicator modeling functionality of Oncentra BPS. Dose optimization involved activating all source positions within the CTVHR and ovoids, and then manually adjusting dwell times first in the intra-uterine and ovoid channels, and then in the needles.
Our planning aim was a total treatment (EBRT + BT) equieffective dose in 2-Gy fractions (EQD210 with α/β = 10 Gy) to 90% of the CTVHR (D90%) of 85 Gy. The limiting prescribed dose to the OARs was as follows: a total treatment dose to 2 cm3 () of the bladder of no more than 90 Gy EQD23 (with α/β = 3 Gy), as well as a rectum, sigmoid and small bowel of no more than 75 Gy EQD23, respectively.
2.2. Study
For all patients, the “reference configuration” (RC) of needles was retrospectively determined using the MRI scan of the first BT fraction with the applicator in place, and the CTVHR delineated. In contrast to the clinical workflow, the applicator was reconstructed using the applicator modeling functionality in Oncentra BPS. The needles which intersected the CTVHR were simulated, and only needles with more than one active source position within the CTVHR were included in the RC set. Note that the RC does not necessarily coincide with the clinically applied needle configuration of the first BT fraction.
To obtain the “pre-MRI configuration” (PC) of needles for each patient, the CTVHR was delineated on the pre-MRI. Using applicator modeling, the applicator was virtually placed on the pre-MRI. Our approach was to place the intra-uterine tube of the model into the visible uterine cavum via the ostium of the cervix, as it would be placed clinically, and then to place the ovoids so that they lie up against the caudal delineation of the CTVHR (which includes at least the entire cervix), on either side of the intra-uterine tube. Needles which obviously entered the CTVHR were then simulated by following the needle holes in the ovoids in a straight line all the way to the edge of the CTVHR delineation. Needles were included based on the same criteria as for the RC. Fig. 1 shows an example of the applicator modeling and needle placement for an RC and a PC. Note that the CTVHR delineated on the pre-MRI was different to the one delineated on the MRI scan of the first BT fraction.
The “fixed configuration” (FC) of needles consisted of the most common needles in the RC’s of the 20 patients in the study. The “multidisciplinary consensus” needle configuration (MC) made use of the pre-MRI without the applicator in place, and was based on a consensus reached by radiation oncologists, medical physicists and RTT’s. The MC was clinically applied, and limited to six needles.
The PC, FC and MC were then compared with the RC. The similarity of each of these three needle configurations to the RC was quantified using Dice’s similarity coefficient (DSC), which is defined as
where X = (PC, FC or MC) and NX is the number of needles in configuration X. In the above, NX ∩ RC is the number of needles which configuration X has in common with the RC. DCS = 1 corresponds to identical sets, while DCS = 0 corresponds to no overlap between sets. Significance was determined using the Wilcoxon signed-rank test, with a value of p < 0.05 considered significant.
Two separate needle scenarios were tested. In the first, the number of needles used for the first fraction was not limited, allowing up to ten needles. In the second, the needle number was limited. Since mid-ventral needles A and J previously were found to be non-essential to treatment planning [18], the analysis was repeated without these needles for both scenarios. When needles A and J were included in the analysis, scenario 2 involved limiting the number of needles to six, to mimic the conservative clinical approach used for the patients in this study while gaining experience in needle application. In this case the FC consisted of the six most common needles in the RCs. When needles A and J were excluded from the analysis, the number of needles in scenario 2 was limited to four. Here, the FC consisted of the four most commonly used needles in the RC. Currently in our clinic we do not limit the number of needles applied, making scenario 1 the more realistic scenario in a more experienced setting.
3. Results
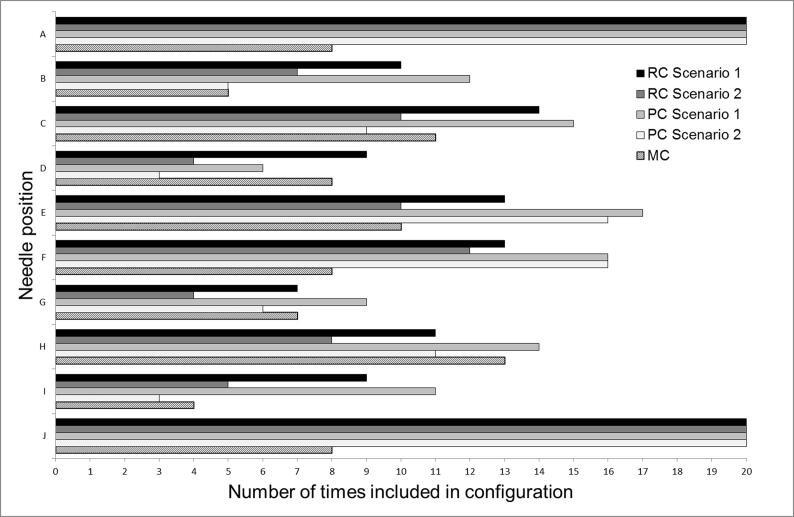
The mid-ventral needles A and J were present in all of the PC and FC, but in less than half of the clinically used MC (Fig. 2). The largest differences between the various needle configuration approaches within both scenarios involved needles A and J. The six most common needles in the RC’s were A, C, E, F, H and J, therefore, the FC consisted of these six needles, when including A and J in the analysis. Excluding needles A and J resulted in an FC consisting of C, E F and H.
Fig. 2.
Number of times each of the needle position A–J was included in the needle configurations RC, PC and MC, respectively, for the 20 patients in this study. The RC per patient was retrospectively determined using the MRI scan of the first BT fraction with the applicator in place. The MC was the clinical needle configuration applied after a consensus reached by radiation oncologists, medical physicists and RTT’s, using the (non-delineated) MRI scan from the fourth week of external beam radiotherapy (pre-MRI). The PC was determined per patient using this pre-MRI with the high-risk clinical target volume (CTVHR) delineated, and the applicator virtually simulated on the scan. Two separate scenarios were implemented: in scenario 1 the number of needles used for the first fraction was not limited, allowing up to ten needles, while in scenario 2 the needle number was limited.
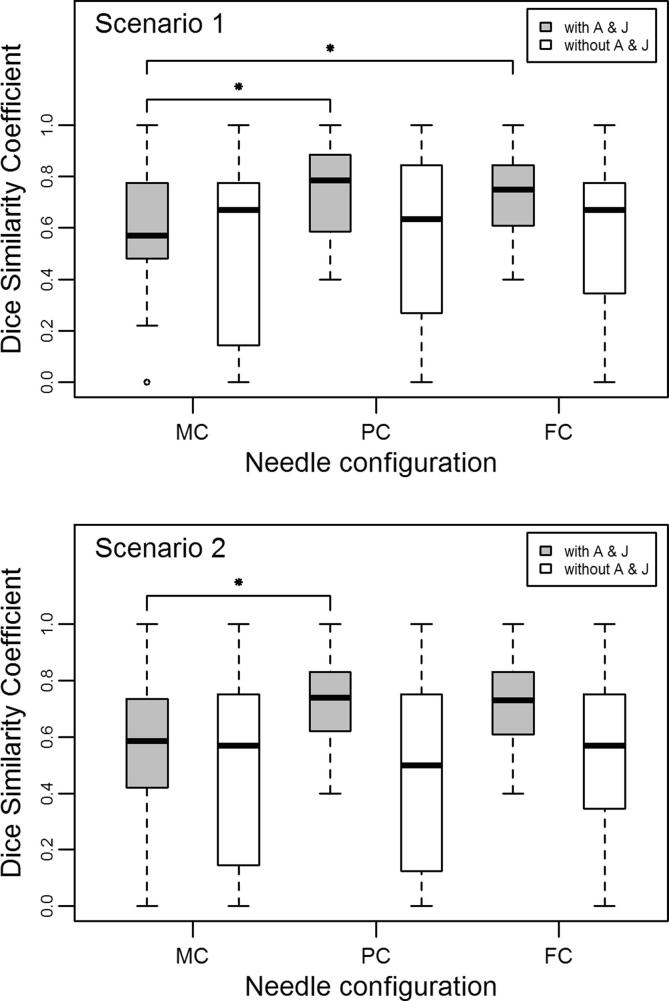
When needles A and J were included in the analysis, the median DSCX for scenario 1, where X = (PC, FC or MC), was respectively 0.78, 0.75 and 0.57, while the median DSCX for scenario 2 was respectively 0.74, 0.73 and 0.59 (Fig. 3). Thus, in both scenarios when A and J were not omitted, the PC and FC outperformed the MC in terms of similarity to the RC and only the difference in median DSC between MC and FC in scenario 2 was not statistically significant (scenario 1: pMC/PC = 0.009, pMC/FC = 0.036; scenario 2: pMC/PC = 0.028, pMC/FC = 0.053). When A and J were omitted from the analysis, the median DSCX for scenario 1, where X = (PC, FC or MC), was respectively 0.63, 0.67 and 0.67, while the median DSCX for scenario 2 was respectively 0.50, 0.57 and 0.57 (Fig. 3). In this case, there were no statistically significant differences in median DSC, and all methods performed equally well in terms of similarity to the RC (scenario 1: p MC/PC = 0.554, p MC/FC = 0.625; scenario 2: p MC/PC = 0.779, p MC/FC = 0.861).
Fig. 3.
Dice’s similarity coefficient for the three separate needle configurations MC, PC and FC as compared to the reference needle configuration (RC), for the 20 patients in this study. Two separate scenarios were implemented: in scenario 1 the number of needles used for the first fraction was not limited, while in scenario 2 the number was limited. The RC per patient was retrospectively determined using the MRI scan of the first BT fraction with the applicator in place, while the FC was made up of the most commonly used needles in the RC’s of the 20 patients in the study. The MC was the clinical needle configuration applied after a consensus reached by radiation oncologists, medical physicists and RTT’s, using the (non-delineated) MRI scan from the fourth week of external beam radiotherapy (pre-MRI). The PC was determined per patient using this pre-MRI with the high-risk clinical target volume (CTVHR) delineated, and the applicator virtually simulated on the scan. The analysis was repeated omitting mid-ventral needles A and J. The median is represented by the horizontal line, the box represents the first and third quartiles, and the whiskers extend to 1.5 times the interquartile range from the box. The dots represent the outliers, and the star shows statistically significant differences.
4. Discussion
The additional interstitial component in IC/IS BT applicators has been shown to be beneficial to treatment planning, resulting in DVH parameter improvements. Having the option to apply needles in all BT fractions provides many degrees of freedom for dose optimization. However, choosing which needles to apply in the first BT fraction can be non-trivial. In this study, we compared three different methods of determining the needle configuration.
In a study investigating the benefit of an IC/IS applicator for pulsed dose rate (PDR) BT of LACC, a correspondence was shown between the frequency of a needle’s application and the CTVHR location [7]. In our study, the RC needle configuration gave an indication of the CTVHR location for each patient at the time of the first BT fraction, when needle positions A and J were included in the analysis. In this case, the low median DSC for the MC method when compared to the RC from scenario 1 might originate from the fact that clinically we only allowed a maximum of six needles, while in scenario 1 the number of needles was not limited. This gave a negative bias for the MC in scenario 1. However, the low median DSC for the MC method when compared to the RC from scenario 2 (the MC method was always performed within the setting of scenario 2), indicated an insufficiency in the MC method for accurate needle application in corresponding CTVHR locations. When the mid-ventral needles A and J, previously found to be non-essential to treatment planning [18], were omitted from the analysis, the median DSC between MC and RC improved, but was still relatively low. However, the patients in this study were treated at a stage when we were gaining experience in needle application. Moreover, the MC was based on a non-delineated pre-MRI with no applicator in situ, while in a separate study of applicator needle use involving two PDR applications, the needle configuration for the second application (IC/IS) was based on the MRI scan of the first (IC only) [7]. An analysis of needle use performed with a more recent patient set showed that our current MC method (using scenario 1) was successful for achieving planning criteria, but on average we applied one more needle in the first fraction than was loaded in treatment planning [18]. Thus, in spite of the current success of this method, there is space for improvement.
Since the MC method relies on a multidisciplinary discussion and sufficient opportunity to gain experience, it may not be a realistic option for clinics with limited time or staffing resources, or those new to the IC/IS approach. Our aim was to test additional methods, in the hope that these might be viable and efficient alternatives to the MC method. The PC method required an additional delineation of the CTVHR and experience in simulating an applicator on a scan with no applicator in situ. It was thus more resource-intensive than the FC method, but also patient specific. The CTVHR differs among patients, both in shape and volume, making the FC approach to needle use in the first fraction of BT seem like an oversimplification. However, we showed that needles C and H have a particular reliability of use [18], while A and J were non-essential in treatment planning, unless in the vicinity of the GTV. The FC including only needles C, E, F and H may be used as a starting point which can be even further refined depending on patient examination and any other available scans. Our study showed that when A and J are omitted from the analysis, the FC approach equaled the MC and PC approaches in accuracy, but surpassed both in efficiency.
The FC was made up of the most common needles in the RC’s of the 20 patients in the study, a method which potentially biases results towards high DSC between FC and RC. However, in another independent study on a more recent patient cohort [18] we found that the CTVHR was located most often in the sectors corresponding to needle positions A, C, E, F, H and J, which coincided with the FC found in this study. Using DSC to compare the FC with RC was viable.
Differences in the PC and RC arose for a number of reasons. Firstly, the CTVHR delineated on the MRI scan from week four of EBRT, which was used to make the PC, differed from the CTVHR delineated for the first fraction of BT due to on-going tumour response to EBRT. Moreover, the PC method involved placing an applicator model on an MRI scan which was taken without the applicator in situ. An applicator in situ had an effect on the internal shape and positioning of the target. The ovoids of the applicator pressed tightly against the cervix and the rigid intra-uterine channel affected the positioning of the uterus when compared to scans taken without the applicator in place. Thus, the simulation of needles in the PC method might not precisely mimic what happens in reality when needles are clinically inserted. However, the PC method was a viable alternative to our current clinical approach.
The RC represented the “gold standard” needle configuration for the first fraction of BT, with which to compare the three separate methods of choosing needles dealt with in this study. The RC was based on the MRI scans from the first BT fraction, with applicator in situ and CTVHR delineated, allowing us to determine which needle configuration would have penetrated the CTVHR optimally. We used the same method to find RC needles as PC needles: all needles entering the CTVHR were considered potentially useful to treatment planning and included in the respective needle configuration, however we discarded those needles with only one active source position within the CTVHR. As mentioned, such needles might indeed be useful to obtain an optimal dose distribution. In order to test whether the RC needle configurations we obtained are indeed a “gold standard” we would need to re-optimize the treatment plans of the first BT fraction using these RC needles, and see if we can achieve an even more optimal treatment plan within the desired planning aims than what was achieved clinically using the MC needles. Such a dosimetric comparison is also non-trivial, however, since there are different ways of planning, different criteria per institute and clinical considerations per patient. This is an interesting follow-up study, but goes beyond the scope of this work. In this study, however, we aimed to provide and compare methods which may be used as a first step in determining the needle configuration for the first BT fraction.
In conclusion, when allowing up to ten needles to be applied for the first fraction of BT, and including the mid-ventral needle positions in the analysis, we found that the PC and FC needle configurations are more similar to the RC than the MC needle configurations. When the mid-ventral needles, which are non-essential to treatment planning, were omitted from the analysis, the PC, FC and MC needle configurations were all equally similar to the RC. Either the PC or FC method could thus provide an equally accurate and/or efficient way to predict the needles that should be used in the first BT fraction in the treatment of cervical cancer as compared to the MC method, especially in a non-experienced setting. In terms of workload, the FC needle configuration is preferred.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Tanderup K., Georg D., Pötter R., Kirisits C., Grau C., Lindegaard J.C. Adaptive management of cervical cancer radiotherapy. YSRAO. 2010;20:121–129. doi: 10.1016/j.semradonc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Tanderup K., Viswanathan A.N., Kirisits C., Frank S.J. Magnetic resonance image guided brachytherapy. Semin Radiat Oncol. 2016;24:181–191. doi: 10.1016/j.semradonc.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanderup K., Christian J., Kirisits C., Haie-meder C., Kirchheiner K., De Leeuw A. Image guided adaptive brachytherapy in cervix cancer: a new paradigm changing clinical practice and outcome. Radiother Oncol. 2016;120:365–369. doi: 10.1016/j.radonc.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Tanderup K., Kynde S., Nyvang G., Morre E., Røhl L., Aagaard T. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother Oncol. 2010;94:173–180. doi: 10.1016/j.radonc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan A.N., Dimopoulos J., Kirisits C., Berger D., Pötter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68:491–498. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Fokdal L., Sturdza A., Mazeron R., Haie-meder C., Tee L., Gillham C. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: analysis from the retroEMBRACE study. Radiother Oncol. 2016;120:434–440. doi: 10.1016/j.radonc.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Nomden C.N., de Leeuw A.A.C., Moerland M.A., Roesink J.M., Tersteeg R.J.H.A., Jürgenliemk-Schulz I.M. Clinical use of the Utrecht applicator for combined intracavitary/interstitial brachytherapy treatment in locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2012;82:1424–1430. doi: 10.1016/j.ijrobp.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Jürgenliemk-Schulz I.M., Tersteeg R.J.H.A., Roesink J.M., Bijmolt S., Nomden C.N., Moerland M.A. MRI-guided treatment-planning optimisation in intracavitary or combined intracavitary/interstitial PDR brachytherapy using tandem ovoid applicators in locally advanced cervical cancer. Radiother Oncol. 2009;93:322–330. doi: 10.1016/j.radonc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Sturdza A., Pötter R., Ulrik L., Haie-meder C., Tee L., Mazeron R. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120:428–433. doi: 10.1016/j.radonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Tanderup K., Ulrik L., Sturdza A., Haie-meder C., Mazeron R., Van Limbergen E. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol. 2016;120:441–446. doi: 10.1016/j.radonc.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Mazeron R., Fokdal L.U., Kirchheiner K., Georg P., Jastaniyah N., Šegedin B. Dose – volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: results from the prospective multicenter EMBRACE study q. Radiother Oncol. 2016;120:412–419. doi: 10.1016/j.radonc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Kirchheiner K., Nout R.A., Lindegaard J.C., Haie-meder C., Mahantshetty U., Segedin B. Dose – effect relationship and risk factors for vaginal stenosis after definitive radio (chemo) therapy with image-guided brachytherapy for locally advanced cervical cancer in the EMBRACE study. Radiother Oncol. 2016;118:160–166. doi: 10.1016/j.radonc.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Kirchheiner K, Nout RA, Tanderup K, Lindegaard JC, Westerveld H, Haie-meder C. Manifestation pattern of early-late vaginal morbidity after definitive radiation (chemo) therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: an analysis from the EMBRACE study 2014;89:88–95. doi: 10.1016/j.ijrobp.2014.01.032. [DOI] [PubMed]
- 14.Kirchheiner K, Po R, Tanderup K, Lindegaard JC, Haie-meder C, Rai B, et al. Health-related quality of life in locally advanced cervical cancer patients after definitive chemoradiation therapy including image guided adaptive brachytherapy: an analysis from the EMBRACE study n.d. doi: 10.1016/j.ijrobp.2015.12.363. [DOI] [PubMed]
- 15.Dimopoulos J.C.A., Pötter R., Lang S., Fidarova E., Georg P., Dörr W. Dose – effect relationship for local control of cervical cancer by magnetic resonance image-guided brachytherapy. Radiother Oncol. 2009;93:311–315. doi: 10.1016/j.radonc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro I., Janssen H., Brabandere M. De, Nulens A., Bal D. De, Vergote I. Long term experience with 3D image guided brachytherapy and clinical outcome in cervical cancer patients. Radiother Oncol. 2016;120:447–454. doi: 10.1016/j.radonc.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Pötter R., Dimopoulos J., Georg P., Lang S., Waldhäusl C., Wachter-Gerstner N. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83:148–155. doi: 10.1016/j.radonc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Smolic M., Sombroek C., Bloemers M.C.W.M., van Triest B., Nowee M.E., Mans A. Needle use and dosimetric evaluation in cervical cancer brachytherapy using the Utrecht applicator. Radiother Oncol. 2017 doi: 10.1016/j.radonc.2017.11.007. [DOI] [PubMed] [Google Scholar]