Abstract
Background and purpose
Organ motion is a challenge during high-precision external beam radiotherapy in cervical cancer, and improved strategies for treatment adaptation and monitoring of target dose coverage are needed. This study evaluates a cone beam computed tomography (CBCT)-based approach.
Materials and methods
In twenty-three patients, individualized internal target volumes (ITVs) were generated from pre-treatment MRI and CT scans with full and empty bladders. The target volumes encompassed high-risk clinical target volume (CTV-T HR) (gross tumor volume + remaining cervix) and low risk (LR) CTV-T (CTV-T HR + uterus + parametriae + upper vagina). Volumetric Modulated Arc Therapy (VMAT) was used to deliver a dose of 45 Gy in 25 fractions. CBCTs were used for setup and for radiation therapists (RTTs) to evaluate the target coverage (inside/outside the planning target volume). CBCTs were reviewed offline. Estimates of the dose delivered with minimum (point) doses across all fractions to CTV-T HR (aim 42.75 Gy) and CTV-T LR (aim 40 Gy) were assessed. In patients with insufficient dose coverage, re-plans were generated based on previous imaging.
Results
Median (range) of the ITV-margins (mean of anterior-posterior margins) related to uterus and cervix was 1.2 (0.5–2.2 and 1.0–2.1) cm. RTTs were able to assess the target coverage in 90% of all CBCTs (505/563). With re-planning, one patient had considerable benefit (12.7 Gy increase of minimum dose) to CTV-T LR_vagina, four patients had improved dose to the CTV-T LR_uterus (1.2–1.8 Gy), and 3 patients did not benefit from re-planning.
Conclusions
Daily CBCT-based monitoring of target coverage by the RTTs has proven safe with limited workload. It allows for reduction in the treated volumes without compromising the target dose coverage.
Keywords: Image guidance, External beam radiotherapy, Adaptive radiotherapy, Cervical cancer, Cone-beam computed tomography, Interfraction motion
1. Introduction
In patients with locally advanced cervical cancer (LACC) interfraction target and organ-related motion is a challenge in high-precision radiotherapy. Intensity Modulated radiation Therapy/Volumetric Modulated Arc Therapy (IMRT/VMAT) has shown a high dose conformality and healthy tissue sparing for pelvic tumors [1]. In LACC patients, large population-based margins ranging from 15 to 40 mm have been applied to secure target coverage [2]. Large margins may however, reduce the clinical advantages of IMRT/VMAT. Different methods to deal with interfraction target- and organ motion have been explored, and image-guided adaptive strategies (IGART) such as library plan selection most often based on differences in bladder filling throughout the treatment have emerged [3], [4], [5], [6], [7], [8], [9]. Treating LACC patients with a full bladder is currently recommended to stabilize the target and further improve bowel sparing during external beam radiotherapy (EBRT). It is however challenging to maintain the same bladder filling throughout the whole course of EBRT [10].
In LACC patients, mild and intermediate vaginal [11], rectal- [12], urinary- [13] and gastrointestinal [14] morbidity that potentially impacts the patient’s quality of life is prevalent. Non-organ-related morbidity such as fatigue, insomnia and hot flashes as well as pelvic insufficiency fractures and limb edema has also been reported [15], [16], [17]. In previous studies, these morbidities have been found to be associated with treatment-related factors [12], [16], [18], and future reduction in treatment-related toxicity may be reached through more conformal RT techniques. The EMBRACE II study on advanced image-guided EBRT and MRI-guided adaptive brachytherapy (MR-IGABT) [19], hypothesises that reduction in morbidity can be achieved by providing EBRT with increased conformality that in term decreases the treated volumes [20]. The introduction of radiotherapy with high conformality emphasizes the need for strategies that monitor target as well as organ motion during treatment. EMBRACE II recommends that individualised internal target volume margins are used, and evaluates clinical outcome in a prospective setting [20].
This study evaluates a cone-beam computed tomography (CBCT)-based monitoring strategy of target coverage and the influence of organ filling in LACC patients during the course of VMAT. It is based on individualised ITV margins, daily CBCT monitoring, patient feedback and re-planning, conceptually introduced during the progression from EMBRACE I to EMBRACE II practice. It serves as an institutional quality assurance of accumulated dose.
2. Materials and methods
In our department, an adaptive workflow for CBCT-based monitoring and bladder filling during EBRT was introduced in October 2016. The workflow was constructed by the radiation oncologists and the medical physicists involved in LACC treatment. The first 23 consecutive patients with FIGO stage IB-IVA treated within the first year after the introduction were included in this study. Treatment consisted of radiochemotherapy and MR-IGABT with concomitant weekly cisplatin administered in up to 5 weekly cycles. Median age was 51 years (range 28–80 years), and FIGO-stage distribution was 4% IB, 50% IIB, 4% IIIA, 34% IIIB and 8% IVA.
2.1. Treatment planning
MRI and Positron Emission Tomography-Computed Tomography (PET/CT) scans were performed with the patient in supine position. A bladder filling protocol was applied before MRI and each treatment fraction, with instructions to drink 450 ml of water at fixed intervals. These were 30 min post micturition half an hour before MRI, and during EBRT it was 15 min post micturition one hour before each fraction. Two CTs were acquired at the PET/CT scanner: 1) before PET with an empty bladder and 2) after PET with a bladder filling according to the urinary inflow during PET. Further preparation included emptying of stool/gas, if the rectum diameter on the planning CT exceeded 4 cm in the anterior-posterior direction at the cervix level. EBRT contouring was according to EMBRACE II guidelines [19]. CTVs related to the primary tumor were: 1) high risk CTV-tumor (CTV-T HR) containing GTV and any remaining cervix not infiltrated by tumor and 2) low risk CTV-tumor (CTV-T LR) containing CTV-T HR, parametriae, uninvolved vagina with a 20 mm margin inferiorly, paravaginal tissue and the uninvolved uterine corpus. A 5 mm margin towards bladder and rectum at the level of cervix was included in the CTV-T LR, respecting the mesorectal fascia and bladder wall unless infiltrated.
The low risk internal target volume (ITV-T LR) related to CTV-T LR was generated using an individualised approach. This was based on the range of target- and organ motion visually assessed between the pre-treatment imaging (CT and MRI) with different filling of the bladder and rectum fused to the planning CT with comfortably filled bladder (supplementary Fig. S1). It was further adapted according to the physician’s expectations of motion during treatment, e.g. additional ITV margin was added in the posterior direction in case rectum was not empty on the planning scan, as it may appear empty during EBRT. Furthermore it did not include the whole uterus range (from full to empty bladder), as a certain bladder volume was expected. The elective nodal CTV (CTV-E) included involved lymph nodes and relevant draining node groups according to risk of nodal spread [19]. A simultaneous integrated boost with 55.0–57.5 Gy in 25 fractions was planned according to the lymph node coverage probability approach [21] in patients with pathological lymph nodes on imaging. The CTV-E and ITV-T LR were expanded by an isotropic 5 mm margin into the planning target volume (PTV45). VMAT was used to deliver a dose of 45 Gy in 25 fractions, with at least 95% of the PTV covered by 95% of the prescribed dose. MR-IGABT was performed with two fractions of pulsed-dose rate (PDR) technique, aiming to deliver a total EBRT + BT EQD2 D90 of >85–90 Gyα/β=10 for the high risk clinical target volume (CTV-T HR).
2.2. IGRT monitoring and dose coverage criteria
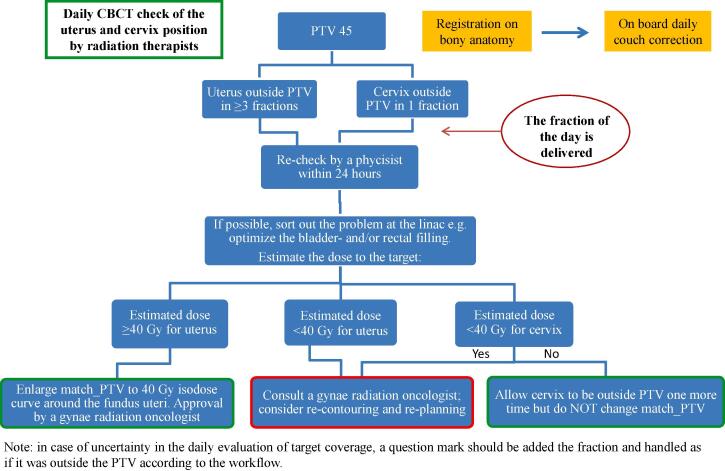
Daily CBCTs (Varian, Truebeam v. 2.0 or 2.5) were performed for rigid fusion and couch alignment according to bony structures, and used for target coverage- and organ filling monitoring. Prior to each treatment fraction, the radiation therapists (RTTs) assessed whether uterus (CTV-T LR) and/or cervix (CTV-T HR) including upper vagina (CTV-T LR) was inside or outside (binary) the planning target volume (PTV), by evaluating the transferred PTV structure to each CBCT. The coverage of cervix and upper vagina was evaluated as one target region, because of the difficulty to accurately distinguish between anatomical borders of cervix and vagina on the CBCTs. The RTTs followed the constructed workflow (Fig. 1), and thresholds for action were 1) if uterus was outside the PTV at least three times or 2) if cervix/upper vagina was outside the PTV once. In this case (or if the RTTs were not able to evaluate the CBCTs), a medical physicist did an off-line review to make a dose estimation carried out by crude addition of point doses across the CBCTs, and decided jointly with a radiation oncologist, whether a new treatment plan with enlarged ITV based on previous CBCTs was necessary.
Fig. 1.
Clinical adaptive workflow with thresholds for action according to target coverage and minimum point dose estimates. Abbreviations: PTV 45 = planning target volume treated to 45 Gy; Match_PTV = a structure applied by the physicists to verify target dose coverage.
The quality of the daily RTT assessments was evaluated independently by a medical physicist supported by a physician. Minimum point doses to uterus, cervix and upper vagina were estimated across all fractions for the original plans and re-plans. Points were placed in the region of minimum dose, and represent a worst-case scenario as compared to full 3D dose addition using deformable registration. According to EMBRACE II guidelines, a total EBRT + BT dose of >45 Gy EQD2 is considered appropriate for targeting risk of microscopic disease in the non-involved uterus, which is part of the CTV-T LR. The typical BT dose contribution to the non-involved uterus is >5 Gy [22], and therefore a delivered EBRT dose of 40 Gy is considered acceptable. For the CTV-T HR it is considered crucial that at least 95% of the prescribed dose is delivered [23].
In order to achieve as optimal bladder- and rectum filling as possible and to avoid extreme cervix/uterus positions, the RTTs also assessed the bladder and rectum as either “small”, “medium” or “large” on the CBCTs compared to corresponding pre-treatment status. This was visually assessed for the bladder by adding on the pre-treatment structure of full and empty bladders. The “size” of rectum was defined according to the influence on the cervix position, as “large” if cervix was pushed anteriorly or “small” if cervix was located more posterior as compared to the planning CT scan. The information was used for daily feedback to the patients. It was possible to modify the bladder filling instructions when needed, and the RTTs gave general information regarding hydration status advising a regular fluid intake of at least 1.5 L/day. Furthermore, the RTTs had the option to apply the institutional guidelines for management of diarrhea and constipation.
2.3. Education of RTTs prior to CBCT-based monitoring
Fourteen RTTs were educated to evaluate the relevant structures on the CBCTs. A learning programme with online self-education (2½ h) as well as class teaching and “hands on training” (7 h) was conducted, similar to previous experience with ART education [24]. Two general online modules introduced adaptive RT and CBCT image quality. Three modules addressed disease and treatment of LACC, female pelvic anatomy and interfractional challenges during EBRT. After clinical implementation, the daily monitoring was performed by two RTTs having a medical physicist as back-up.
2.4. ITV margins and target motion
To quantify the individualised ITV margins, the distances from the anterior (A) and posterior (P) border of mid-uterus and cervix to the ITV margin were manually measured on the planning CT scan (supplementary Fig. S2). These anchor points were chosen as they were considered to be the most critical part of the CTV-T LR and CTV-T HR with respect to target dose coverage. This is in agreement with Eminowicz et al., who found the lowest dose coverage at the anterior mid-uterus point [25]. In our study, the uterus ITV margin (mean of the A-P distance to ITV) was categorised as “large” if ≥1.5 cm, “intermediate” if between 1 and 1.5 cm and “small” if <1 cm, respectively. Similarly the cervix ITV margin (mean of the A-P distance to ITV) was categorised as “large” if >1.5 cm and “small” if ≤1.5 cm. A patient was characterized as having “large target motion” at treatment planning if uterus and/or cervix moved >1.5 cm visually assessed between the fused MRI and CT scan with full bladder, or between the fused CT scans with full and empty bladders. This distance was manually measured with a point at the anterior border of the uterus perpendicular to the mid-uterus axis in one imaging modality to the corresponding point in the other imaging. Similar a point at the anterior border of the cervix-uterus angle was chosen to measure the motion of cervix between the imaging modalities (supplementary Fig. S2).
2.5. Correlation of bladder and bowel volume
A subgroup analysis on bladder and bowel correlation was performed in ten consecutively selected patients, in whom the bladder filling protocol was applied. Every third CBCT (n = 80) was used to contour the bladder and the outer extension of bowel loops with the cranial border 2 cm above the PTV. Linear regression analysis was used to correlate bladder volume and bowel V30Gy and V43Gy, and the model was checked by using graphical inspection of the residuals.
3. Results
From the total of 575 CBCTs, six were not available in the database, and six CBCTs in one patient were not registered by the RTTs. This resulted in 563 CBCTs available for the evaluation of target coverage and 569 for the evaluation of the influence of organ filling on target coverage. The bladder filling protocol was abandoned in three patients due to bilateral nephrostomies or poor compliance. During the 3 months introduction, time consumption at the linac increased from 15 to 25 min slot time per patient. After 3 months the slot time was reduced to 20 min per patient and included two additional post-treatment CBCTs (3–5 min) in patients treated with paraaortic irradiation.
“Large”, “intermediate” and “small” ITV margins for uterus were applied in seven, eight and eight patients, respectively (Table 1). For cervix, “large” margins were applied in four patients, and “small” ITV margins with at least 1.0 cm were applied in 19 patients. Median (range) A-P ITV margins were 1.2 cm for both uterus (0.5–2.2 cm) and cervix (1.0–2.1 cm). Pre-treatment “large target motion” was seen in seven of the 23 patients, and three of these were re-planned during treatment, as uterus motion was more pronounced then expected from the pre-treatment scans. Another four patients were re-planned as target motion during treatment was not predictable from the pre-treatment scans.
Table 1.
Individualised internal target volume (ITV) margins related to target (uterus and cervix) measured at time of treatment planning as the mean between the anterior and posterior ITV margin. Target motion measurements are shown in the last columns as the distances from the borders of the anterior part of the uterus and cervix to the corresponding borders in the fused pre-treatment imaging modalities. All measurements are visually assessed.
| Patients | Mean of the anterior-posterior ITV margin (cm) |
Distance between “full” bladder CT and MRI (cm) |
Distance between “full” and “empty” bladder CTs (cm) |
|||
|---|---|---|---|---|---|---|
| Uterus | Cervix | Uterus | Cervix | Uterus | Cervix | |
| 1 | 0.9 | 1.2 | 0.4 | 0.0 | 0.0 | 0.0 |
| 2 | 1.9 | 1.6 | 0.2 | 0.0 | 2.3† | 0.1 |
| 3 | 0.9 | 1.1 | 0.2 | 0.2 | 0.4 | 0.0 |
| 4* | 1.2 | 1.2 | 2.0† | 0.9 | 1.5 | 0.8 |
| 5 | 1.2 | 1.0 | 0.3 | 0.3 | 0.5 | 0.3 |
| 6 | 0.9 | 1.5 | 0.5 | 0.4 | 0.0 | 0.9 |
| 7 | 0.8 | 1.1 | 0.8 | 0.4 | 0.0 | 0.0 |
| 8 | 0.5 | 1.0 | 0.1 | 0.3 | 0.0 | 0.0 |
| 9* | 0.8 | 1.4 | 0.3 | 0.4 | 0.0 | 0.0 |
| 10* | 1.5 | 1.0 | 0.6 | 0.3 | 0.5 | 0.2 |
| 11 | 0.9 | 1.0 | 0.4 | 0.0 | 0.0 | 0.0 |
| 12 | 2.2 | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 13* | 1.3 | 1.1 | 0.2 | 0.3 | 0.8 | 0.4 |
| 14 | 1.0 | 1.0 | 0.0 | 0.6 | 0.6 | 0.3 |
| 15 | 0.9 | 1.2 | 0.0 | 0.2 | N/A | N/A |
| 16 | 1.3 | 1.0 | 0.3 | 0.3 | 2.5† | 1.4 |
| 17 | 1.3 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 18* | 2.0 | 2.1 | 0.7 | 0.3 | 2.9† | 0.8 |
| 19 | 1.6 | 1.0 | 1.4 | 0.4 | 2.1† | 0.9 |
| 20* | 1.9 | 1.5 | 3.0† | 0.6 | 2.2† | 0.3 |
| 21* | 1.1 | 1.4 | 0.5 | 0.0 | 0.0 | 0.0 |
| 22 | 1.6 | 1.7 | 2.3† | 0.3 | 1.6† | 1.0 |
| 23 | 1.3 | 1.2 | 0.3 | 0.3 | 0.0 | 0.0 |
Abbreviations: N/A = not available (empty bladder scan missing).
Re-planned patients (n = 7).
“Large target motion” defined as uterus and/or cervix distances >1.5 cm measured between the pre-treatment imaging modalities.
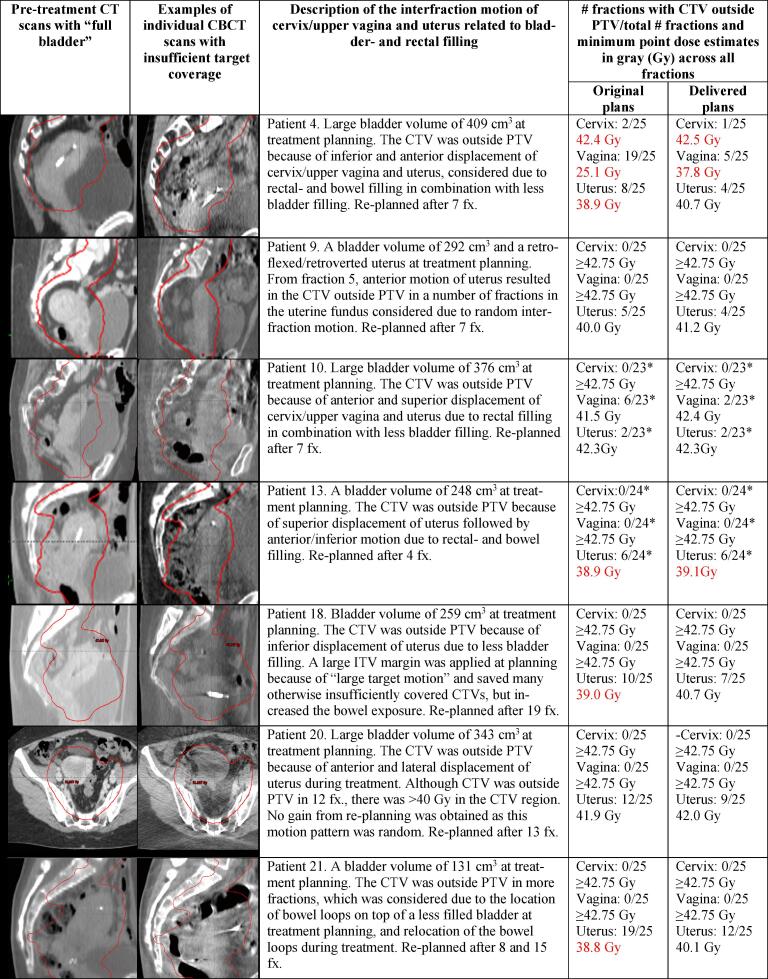
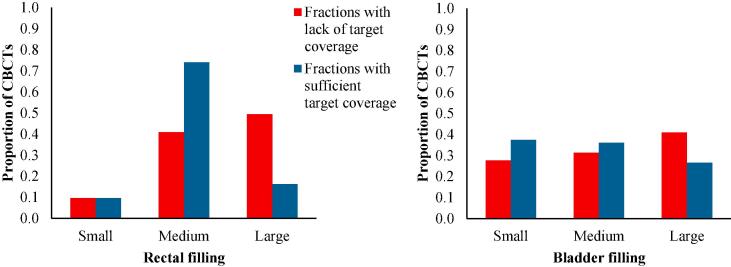
Re-planning was done due to organ motion of uterus alone (five patients) or both cervix/upper vagina and uterus (two patients) (Fig. 2). In three of the seven re-planned patients, the target doses would have been within our general aims (>40 Gy for CTV-T LR and >42.75 Gy for CTV-T HR) without re-planning (Fig. 2). In four patients CTV-T LR uterus and/or vagina would have received less than 40 Gy without re-planning (range 25.1–39.0 Gy). CTV-T HR (cervix) would have been fully covered without re-planning in all except from one patient with marginal under-dosage (42.4 Gy) (Fig. 2). With re-planning, four patients gained 1.2–1.8 Gy to CTV-T LR uterus, one patient gained 12.7 Gy to CTV-T LR vagina, and three patients gained less than 1 Gy. Two patients had limited under-dosage after re-planning with 37.8 and 39.1 Gy to CTV-T LR, respectively, and marginally insufficient dose to CTV-T HR (42.5 Gy) in one patient. Furthermore it was found, that it was the “large” rectal fillings assessed by the RTTs, that were most often associated with lack of target coverage during treatment (Fig. 3). This was not as obvious for the bladder fillings, as “small” bladders were not more frequently associated with lack of target coverage during treatment compared to “large” bladders (Fig. 3).
Fig. 2.
Target- and organ-related motion illustrated with the pre-treatment CT and a cone beam CT scan (CBCT) in re-planned patients. Number of fractions (fx.) with CTV outside PTV for cervix, upper vagina and uterus are provided with minimum point dose estimates across all fractions for original- and delivered plans (dose estimates from the original plan up to re-planning + dose estimates from the re-plan). “Large planning bladder volumes” are defined as volumes exceeding 300 cm3. Abbreviations: PTV = planning target volume (red line); CTV = clinical target volume; ITV = internal target volume. *Three CBCTs are not available in the database. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
The influence of rectum and bladder fillings on target coverage in 23 patients with a total of 569 cone beam CTs (CBCTs) (75 CBCTs were not included in the right panel because the bladder filling protocol was not applied in three patients). Blue columns represent pooled fractions with sufficient target coverage (cervix, upper vagina and uterus) with a total of 486 CBCTs in the left panel and 411 CBCTs in the right panel. Red columns represent pooled fractions with lack of target coverage (total of 83 CBCTs). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In 54 of the 563 CBCTs (9.6%), the RTTs were not able to evaluate target coverage because of poor image quality. These specific CBCTs were evaluated by a medical physicist with expertise in cervical cancer EBRT. In 505 of the 563 CBCTs (89.7%), the RTTs assessed the target correctly and in four of the 563 CBCTs (0.7%), the target was assessed as being inside the PTV although it was marginally outside. In nine patients, the RTT’s changed the bladder filling protocol by increasing or decreasing the waiting time 1–1½ hour (3 patients) and/or the amount of fluid 300–650 ml (6 patients).
The mean bowel V30Gy and V43Gy at planning were 572 cm3 (±215 cm3) and 208 cm3 (±90 cm3). Linear regression analysis in 10 patients demonstrated a correlation between bowel V30Gy (Fig. 4) and V43Gy and bladder volumes. An increase in bladder volume of 100 cm3 resulted in a decrease in bowel V30Gy of 51 (95%CI [22; 80]) cm3 and V43Gy of 12 (95%CI [2; 26]) cm3. Two patients were significantly different from the others. Patient 6 demonstrated a steep decrease in V30Gy for small bladder volumes (<300 ml), and patient 9 did not have any change in bowel V30Gy despite large bladder variations.
Fig. 4.
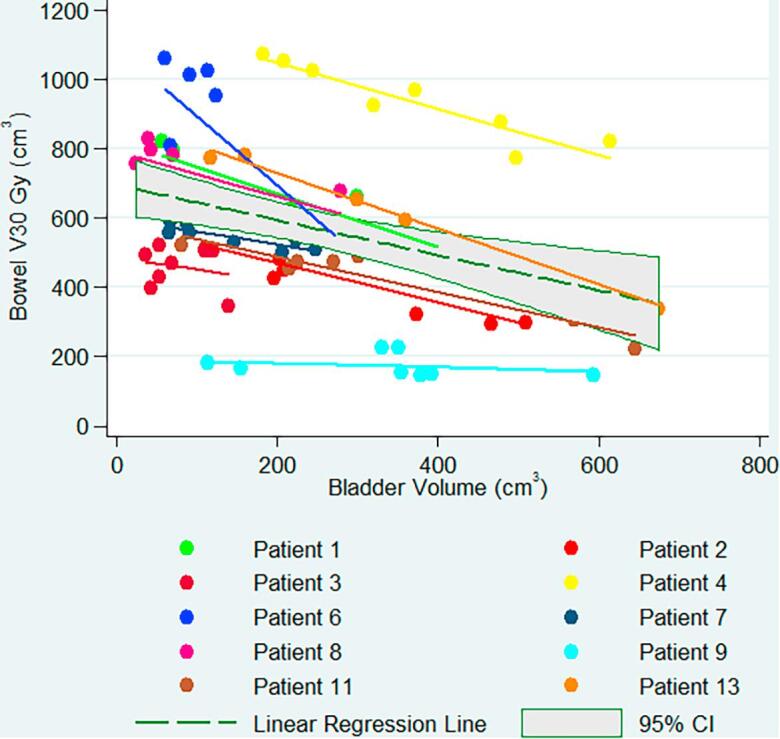
Volume of bowel receiving 30 Gy as a function of bladder volume in 10 patients.
4. Discussion
This study demonstrated a practical way of treating LACC patients in the era of high-precision EBRT and reductions in treated volumes. By daily CBCT-based monitoring, the interfractional target and organ motion was evaluated with high precision by RTTs, and the dose to target was secured in all patients with limited resources for planning. Pre-treatment target motion was accounted for at treatment planning by adding patient specific ITVs. This was in contrary to unexpected target motion during treatment which was accounted for through daily dose monitoring and re-planning (seven patients). After the retrospective assessment of all CBCTs, three patients would have been sufficiently covered without re-planning. Three patients had some benefit in the CTV-T LR region and one patient had major benefit of re-planning. The CTV-T HR criterion with at least 95% of prescribed dose delivered was fulfilled for all patients even without re-planning, except from one with only marginally under-dosage. The findings underlined the need for sufficient ITV margins in particular in the cervix/upper vagina region, as unpredictable motion patterns were seen, e.g. inferior displacement where the dose gradient is steep. Lateral movement of cervix has previous been demonstrated to be less pronounced [2].
The benefit of bladder filling was demonstrated with the reduction of the volume of bowel in the treatment field, as shown with the bladder and bowel V30Gy and V43Gy correlation. The correlation varied between individuals, which could be explained by the patient anatomy e.g. the location of the uterus, the sigmoid and elective lymph nodes, the amount of fatty tissue between the bowel loops etc. Some institutions deliver EBRT with patient instructions to void before treatment to benefit from a more reproducible anatomy with empty bladder [26]. However, as it is known that bowel dose (e.g. V15Gy and V45Gy) has shown to significantly correlate with GI toxicity [26], [27], [28], treatment with a bladder filling protocol is recommendable. Furthermore, daily monitoring and patient feedback has potential to improve the management of the bladder- and rectal filling in order to optimize the treatment, and especially to avoid extreme positions of the target. To explore this further, a larger study is needed. Another finding of the study to consider is the planning condition, as e.g. in one of the re-planned patients (patient 4) we saw that a large bladder at planning led to significant insufficient target dose coverage for both cervix/upper vagina and uterus. Similar finding was done by Eminowicz et al. [25], proposing to strictly maintain bladder volumes with an upper limit of 300 cm3.
The present study shows that LACC patients can benefit from highly conformal RT with IMRT/VMAT techniques without compromising target dose coverage. The strategy is now being disseminated through ESTRO School and the annual EMBRACE meetings for other institutions to consider. The strategy can be effectively implemented through specific RTT educational programs. This is due to the fact that the RTTs demonstrated a steep learning curve, where it was possible to reduce the time slots at the accelerator after three months. It is important however, to have a certain number of patients and/or methods to maintain the competencies in a large group of RTTs [24].
Current IGART strategies are mainly guided by the influence of the bladder filling on target coverage, and motion patterns are identified at time of treatment planning [5], [6], [8], [29]. We found that target coverage was dependent on a combination of various factors, e.g. rectal/bowel- and bladder fillings, which was patient specific and not always with a systematic pattern during treatment. Furthermore target motion was not always predictable from the pre-treatment scans, which makes the use of plan selection less effective and CBCT-based monitoring or other IGART methods necessary. On the other hand, patients with up front “large target motion” and no repeated motion during treatment could have benefitted from a plan with smaller ITV margins.
The relatively small patient cohort is a limitation in present study. However, the patient number is sufficient to demonstrate that CBCT-based monitoring can add in high-precision radiotherapy to secure target dose coverage, and works well in clinical practice. Tumor regression during treatment was not evaluated in this study, but may also impact the actual dose delivered to the target and OARs. A previous study has shown limited advantage through adaptation of the EBRT target to the tumor shrinkage [30]. An explanation of this finding may be that the macroscopic tumour occupies only a small fraction of the entire target volume irradiated to 45 Gy [30]. Therefore, the organ motion (and in particular systematic relocation of organs) is by far the most challenging aspect in EBRT for LACC.
Reporting of dose accumulation of adaptive EBRT with administration of several treatment plans on varying anatomy is challenging. Dosimetric calculation based on 3D target contouring taking the non-rigid motion and deformation of target and OARs into account would in principle be preferable. Due to limitations in soft tissue contrast on CBCTs, deformable registration has not proven successful for registration of CBCTs yet. As a minimum, in case of administration of different treatment plans (e.g. plan selection or re-planning) the DVH parameters for each plan should be reported together with the frequency of administration of each plan. In this study, the assessment of minimum doses was therefore performed through point dose estimates, although this method is prone to uncertainties due to challenges in precise identification of especially the cervix borders on CBCTs, and therefore a “worst case” point dose estimation was chosen.
While possibilities for daily re-planning may solve the challenge of interfraction motion, the image quality of the CBCTs compromises the monitoring accuracy and is a challenge towards daily re-planning. However, the image quality of 90% of the available CBCTs were good enough for the evaluation by the RTTs in this study. The success of DIR algorithms and auto-contouring would significantly benefit from improved image quality, which may also facilitate improved monitoring of the upper part of the lymph node target for paraaortic irradiation.
In conclusion, this institutional adaptive monitoring strategy secured the dose coverage of the CTV related to the primary tumor, and supports the new practice of individualised ITV margins as introduced with the EMBRACE II study. Future adaptation of our monitoring strategy will focus on the inferior margin for cervix/upper vagina and more precise assessment of the accumulated dose, with avoidance of unnecessary re-planning. An overall benchmark of morbidity after treatment with reduced EBRT treated volumes and image guided brachytherapy will become available through EMBRACE II.
Acknowledgments
Acknowledgments
We acknowledge support from The Danish Cancer Society (grant number R108-A6854-14-S31) and the Danish Cancer Research Foundation through research grants. Varian Medical Systems supported this work through unrestricted research grants.
Conflict of interest statement
NBKJ reports grants from the Danish Cancer Society, Varian Medical Systems and the Danish Cancer Research Foundation during the conduct of the study as well as non-financial support from Varian Medical Systems outside the submitted work. MSA reports grants from the Danish cancer Society and Varian Medical Systems during the conduct of the study. AV reports grants from Varian Medical Systems outside the submitted work. KT reports grants from the Danish Cancer Society and Varian Medical Systems during the conduct of the study.
All other authors have no declarations of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2018.12.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hymel R., Jones G.C., Simone C.B., 2nd Whole pelvic intensity-modulated radiotherapy for gynecological malignancies: a review of the literature. Crit Rev Oncol Hematol. 2015;94:371–379. doi: 10.1016/j.critrevonc.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jadon R., Pembroke C.A., Hanna C.L., Palaniappan N., Evans M., Cleves A.E. A systematic review of organ motion and image-guided strategies in external beam radiotherapy for cervical cancer. Clin Oncol. 2014;26:185–196. doi: 10.1016/j.clon.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Bondar L., Hoogeman M., Mens J.W., Dhawtal G., de Pree I., Ahmad R. Toward an individualized target motion management for IMRT of cervical cancer based on model-predicted cervix-uterus shape and position. Radiother Oncol. 2011;99:240–245. doi: 10.1016/j.radonc.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Tyagi N., Lewis J.H., Yashar C.M., Vo D., Jiang S.B., Mundt A.J. Daily online cone beam computed tomography to assess interfractional motion in patients with intact cervical cancer. Int J Radiat Oncol Biol Phys. 2011;80:273–280. doi: 10.1016/j.ijrobp.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Bondar M.L., Hoogeman M.S., Mens J.W., Quint S., Ahmad R., Dhawtal G. Individualized nonadaptive and online-adaptive intensity-modulated radiotherapy treatment strategies for cervical cancer patients based on pretreatment acquired variable bladder filling computed tomography scans. Int J Radiat Oncol Biol Phys. 2012;83:1617–1623. doi: 10.1016/j.ijrobp.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad R., Hoogeman M.S., Bondar M., Dhawtal V., Quint S., De Pree I. Increasing treatment accuracy for cervical cancer patients using correlations between bladder-filling change and cervix-uterus displacements: Proof of principle. Radiother Oncol. 2011;98:340–346. doi: 10.1016/j.radonc.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad R., Bondar L., Voet P., Mens J.W., Quint S., Dhawtal G. A margin-of-the-day online adaptive intensity-modulated radiotherapy strategy for cervical cancer provides superior treatment accuracy compared to clinically recommended margins: a dosimetric evaluation. Acta Oncol. 2013;52:1430–1436. doi: 10.3109/0284186X.2013.813640. [DOI] [PubMed] [Google Scholar]
- 8.Heijkoop S.T., Langerak T.R., Quint S., Bondar L., Mens J.W., Heijmen B.J. Clinical implementation of an online adaptive plan-of-the-day protocol for nonrigid motion management in locally advanced cervical cancer IMRT. Int J Radiat Oncol Biol Phys. 2014;90:673–679. doi: 10.1016/j.ijrobp.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 9.Buschmann M., Majercakova K., Sturdza A., Smet S., Najjari D., Daniel M. Image guided adaptive external beam radiation therapy for cervix cancer: evaluation of a clinically implemented plan-of-the-day technique. Z Med Phys. 2018;28:184–195. doi: 10.1016/j.zemedi.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad R., Hoogeman M.S., Quint S., Mens J.W., de Pree I., Heijmen B.J. Inter-fraction bladder filling variations and time trends for cervical cancer patients assessed with a portable 3-dimensional ultrasound bladder scanner. Radiother Oncol. 2008;89:172–179. doi: 10.1016/j.radonc.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Kirchheiner K., Nout R.A., Tanderup K., Lindegaard J.C., Westerveld H., Haie-Meder C. Manifestation pattern of early-late vaginal morbidity after definitive radiation (Chemo)therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: an analysis from the embrace study. Int J Radiat Oncol Biol Phys. 2014;89:88–95. doi: 10.1016/j.ijrobp.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Mazeron R., Fokdal L.U., Kirchheiner K., Georg P., Jastaniyah N., Segedin B. Dose-volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: results from the prospective multicentre EMBRACE study. Radiother Oncol. 2016;120:412–419. doi: 10.1016/j.radonc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Fokdal L., Pötter R., Kirchheiner K., Lindegaard J.C., Jensen N.B.K., Kirisits C. Physician assessed and patient reported urinary morbidity after radio-chemotherapy and image guided adaptive brachytherapy for locally advanced cervical cancer. Radiother Oncol. 2018;127:423–430. doi: 10.1016/J.RADONC.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Jensen N.B.K., Pötter R., Kirchheiner K., Fokdal L., Lindegaard J.C., Kirisits C. Bowel morbidity following radiochemotherapy and image-guided adaptive brachytherapy for cervical cancer: physician- and patient reported outcome from the EMBRACE study. Radiother Oncol. 2018;127:431–439. doi: 10.1016/j.radonc.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Smet S., Pötter R., Haie-Meder C., Lindegaard J.C., Schulz-Juergenliemk I., Mahantshetty U. Fatigue, insomnia and hot flashes after definitive radiochemotherapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: an analysis from the EMBRACE study. Radiother Oncol. 2018;127:440–448. doi: 10.1016/j.radonc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Ramlov A., Pedersen E.M., Røhl L., Worm E., Fokdal L., Lindegaard J.C. Risk factors for pelvic insufficiency fractures in locally advanced cervical cancer following intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97:1032–1039. doi: 10.1016/j.ijrobp.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Najjari Jamal D., Pötter R., Haie-Meder C., Lindegaard J.C., Juergenliemk-Schulz I.M., Mahantshetty U. Physician assessed and patient reported lower limb edema after definitive radio(chemo)therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: a report from the EMBRACE study. Radiother Oncol. 2018;127:449–455. doi: 10.1016/j.radonc.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Kirchheiner K., Nout R.A., Lindegaard J.C., Haie-Meder C., Mahantshetty U., Segedin B. Dose-effect relationship and risk factors for vaginal stenosis after definitive radio(chemo)therapy with image-guided brachytherapy for locally advanced cervical cancer in the EMBRACE study. Radiother Oncol. 2016;118:160–166. doi: 10.1016/j.radonc.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 19.EMBRACE (An international study on MRI-guided brachytherapy in locally advanced cervical cancer) www.embracestudy.dk [accessed 25 June 2018].
- 20.Pötter R., Tanderup K., Kirisits C., de Leeuw A., Kirchheiner K., Nout R. The EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018;9:48–60. doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindegaard J.C., Assenholt M., Ramlov A., Fokdal L.U., Alber M., Tanderup K. Early clinical outcome of coverage probability based treatment planning for simultaneous integrated boost of nodes in locally advanced cervical cancer. Acta Oncol. 2017;56:1479–1486. doi: 10.1080/0284186X.2017.1349335. [DOI] [PubMed] [Google Scholar]
- 22.Sapru S., Mohamed S., Fokdal L., Nkiwane K., Swamidas J., Mahantshetty U. Dose to the non-involved uterine corpus with MRI guided brachytherapy in locally advanced cervical cancer. Radiother Oncol. 2013;107:93–98. doi: 10.1016/j.radonc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Tanderup K., Fokdal L.U., Sturdza A., Haie-Meder C., Mazeron R., van Limbergen E. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol. 2016;120:441–446. doi: 10.1016/J.RADONC.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Boejen A., Vestergaard A., Hoffmann L., Ellegaard M.B., Rasmussen A.M., Moeller D. A learning programme qualifying radiation therapists to manage daily online adaptive radiotherapy. Acta Oncol. 2015;54:1697–1701. doi: 10.3109/0284186X.2015.1062914. [DOI] [PubMed] [Google Scholar]
- 25.Eminowicz G., Rompokos V., Stacey C., Hall L., McCormack M. Understanding the impact of pelvic organ motion on dose delivered to target volumes during IMRT for cervical cancer. Radiother Oncol. 2017;122:116–121. doi: 10.1016/j.radonc.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Chen V.E., Gillespie E.F., Manger R.P., Skerritt L.A., Tran J.H., Proudfoot J.A. The impact of daily bladder filling on small bowel dose for intensity modulated radiation therapy for cervical cancer. Med Dosim. 2018 doi: 10.1016/j.meddos.2018.02.010. [in press] [DOI] [PubMed] [Google Scholar]
- 27.Chopra S., Dora T., Chinnachamy A.N., Thomas B., Kannan S., Engineer R. Predictors of grade 3 or higher late bowel toxicity in patients undergoing pelvic radiation for cervical cancer: results from a prospective study. Int J Radiat Oncol Biol Phys. 2014;88:630–635. doi: 10.1016/j.ijrobp.2013.11.214. [DOI] [PubMed] [Google Scholar]
- 28.Kavanagh B.D., Pan C.C., Dawson L.A., Das S.K., Li X.A., Ten Haken R.K. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:101–107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 29.van de Schoot A.J.A.J., de Boer P., Visser J., Stalpers L.J.A., Rasch C.R.N., Bel A. Dosimetric advantages of a clinical daily adaptive plan selection strategy compared with a non-adaptive strategy in cervical cancer radiation therapy. Acta Oncol. 2017;56:667–674. doi: 10.1080/0284186X.2017.1287949. [DOI] [PubMed] [Google Scholar]
- 30.Van De Bunt L., Van Der Heide U.A., Ketelaars M., De Kort G.A., Jürgenliemk-Schulz I.M. Conventional, conformal, and intensity-modulated radiation therapy treatment planning of external beam radiotherapy for cervical cancer: the impact of tumor regression. Int J Radiat Oncol Biol Phys. 2006;64:189–196. doi: 10.1016/j.ijrobp.2005.04.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.