Abstract
Recently, the interest to integrate magnetic resonance imaging (MRI) in radiotherapy for prostate cancer has increased considerably. MRI can contribute in all steps of the radiotherapy workflow from diagnosis, staging, and target definition to treatment follow-up. Of particular interest is the ability of MRI to provide a wide range of functional measures. The complexity of MRI as an imaging modality combined with the growing interest of the application to prostate cancer radiotherapy, emphasize the need for dedicated education within the radiation oncology community. In this context, an overview of the most common as well as a few upcoming functional MR imaging techniques is presented: the basic methodology and measurement is described, the link between the functional measures and the underlying biology is established, and finally relevant applications of functional MRI useful for prostate cancer radiotherapy are given.
1. Introduction
Due to its superior ability to define soft tissue structures, magnetic resonance imaging (MRI) is today the preferred imaging modality for several anatomical locations such as the brain, the vertebral column, the abdomen and the pelvis. This inherent ability makes MRI of interest for direct implementation in radiotherapy. Detailed anatomical description using a uniform standard nomenclature and content such as provided by The Prostate Imaging – Reporting and Data System Version 2 (PI-RADS v.2) [1] in prostate cancer improves consistency in pretreatment staging of patients and thereby patient selection [2]. MRI for target definition and organ at risk identification also has clear advantages over computed tomography (CT) in many cases, especially for the prostate [3], [4], [5]. These advantages has led to the development of MRI-only treatment planning, where the CT-simulation is excluded and the attenuation data are extracted from MR images [6]. Additionally, MRI offers many possibilities for treatment follow-up including adaptive adjustments during the course of the treatment [7].
Beside the tissue contrast and image resolution, of special interest is the ability of MRI to enable more or less quantitative functional information, such as diffusion, perfusion, and MR spectroscopy acting as imaging biomarkers for relevant tissue changes related to cancer. In this review, “functional MRI” is used for all functional MR imaging methods. Historically, the concept of functional MRI was introduced as a description for a special method sensitive to brain activity during stimulation with different paradigms and abbreviated fMRI. In this report functional MRI is used in a broader sense meaning all functional MR methods, abbreviated FMRI (with an uppercase F). The use of FMRI offers possible advantages in the clinical setting. FMRI may be used for identification of intraprostatic lesions for delineation of boost volumes which have been tested in a randomized clinical trial [8]. The advantages of FMRI may also be of use for identification of malignant lymph nodes and possibly for identification of local relapses after prostatectomy.
FMRI techniques address several important tumor specific characteristics, such as cell density, microvessel structure, tissue perfusion, and oxygenation. This information can be utilized in the diagnostic process, for treatment selection, treatment planning and follow-up. Thereby, FMRI may serve as a tool towards individualized radiotherapy [9]. In line with this increased interest and the growing need for FMRI studies, we present a tutorial review for applications of FMRI to radiotherapy of prostate cancer. It includes the major established FMRI techniques, but also some upcoming methods, which may gain clinical interest in the near future.
2. Method
Each functional method is characterized by a description of the basic measurement methodology and the link between the functional parameter and underlying biology is established. At the end of each section relevant applications of the functional method to prostate radiotherapy are given. The expectations are that the present review will serve as a base of knowledge for the discussions between the radiation oncologist, the radiologist, and the radiotherapy- and MR-physicists.
A search in the database PubMed (Medline) was performed to capture the most relevant functional MRI techniques for radiotherapy. The following search criteria was used:
(prostate AND cancer AND mri AND method) AND ((radiotherapy) OR (radiation therapy)
method was in each search replaced with the functional MRI method of interest.
For each FMRI-method relevant description of the biological interpretation and the applications for radiotherapy have been searched for in the literature. In this case, the search has not been limited to PubMed, but also EMBASE and “gray search” with GOOGLE has been used. From the identified papers or textbooks, the authors have selected the most appropriate sources for the present purpose, i.e. tutorial review.
3. Results
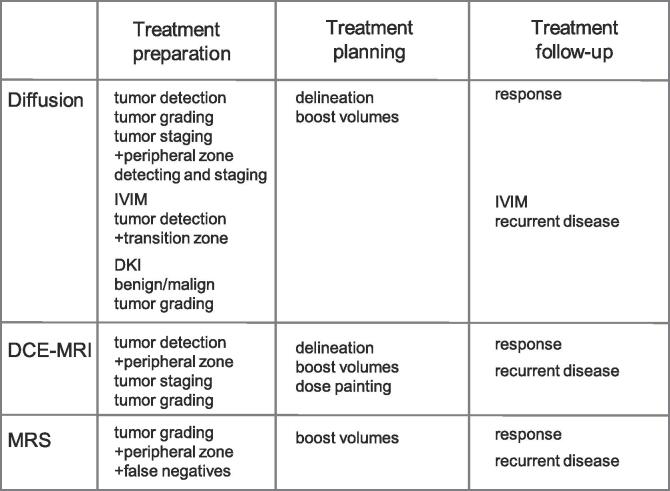
There was three FMRI methods that dominated the papers published (n = 159), i.e. diffusion weighted imaging, dynamic contrast enhanced MRI and magnetic resonance spectroscopy. The remaining methods had only 25 hits together. There are several limitations of this search strategy, but it serves to point out the three most relevant FMRI methods for radiotherapy applications to prostate cancer. These three methods will therefore have their own chapters, while the less common methods will be handled in one chapter. The major findings are summarized in Table 1 and Fig. 1.
Table 1.
The link between the functional measures and the underlying biology.
| DiffusionMRI | Tissue oxygenation MRI |
| cellularity | hypoxia |
| extracellular volume | |
| membrane permeability | Magnetization transfer MRI |
| tortuosity | cellularity |
| microcirculation in capillary network (IVIM) | fibrosis |
| hypoxia (IVIM) | HP 13C MRI |
| microstructural organisation of tissue (DTI) | pyruvate glycolysis metabolites |
| DCE-MRI | lactate |
| tumor vasculature | alanine |
| blood vessel permeability | bicarbonate |
| extravascular extracellular volume | |
| plasma volume | |
| 1H MRS | |
| metabolites | |
| citrate | |
| choline | |
| creatine | |
| polyamines |
Fig. 1.
A graphical illustration on how the three major functional MRI methods can be used in radiotherapy. A plus sign (+) indicate that the method has an advantage for the particular application.
3.1. Diffusion MRI
The measured MR signal is sensitive to spatial motion, including both flow and diffusion of water molecules. Diffusion is random free motion due to thermal energy, and the diffusion rate can be described by the diffusion coefficient. In tissue the diffusion of the water molecules are affected by macromolecules, cell membranes and other tissue microstructures that hinder diffusion. Therefore, since the phenomenon is not described by free diffusion molecules, it is denoted apparent diffusion. Several relevant properties of tumor tissue are known to affect the diffusion, e.g. cellularity, extracellular volume fraction, membrane permeability and tortuosity. Compared to normal tissues, tumors have lower diffusion rate due to the increased cellularity and decreased extracellular volume fraction. Diffusion weighted MRI (DWI) is a technique in which the signal intensity in the image is dependent on the degree of diffusion.
The sensitivity of the pulse sequence to diffusion can be regulated by using linear gradients, which encode the spins spatially to diffusion. The spins will precess at different rates depending on location, resulting in dispersion of the phase and signal loss. The same gradient with opposite direction will refocus the spins assuming they have not moved during the time interval between the pulses. Spins that have moved due to diffusion will not rephase completely, since they have experienced a slightly different gradient strength during the two gradient pulses. As a result, regions with low diffusion show high signal in the diffusion weighted MR-image.
The diffusion can also be quantitatively measured. If at least two diffusion weighted images are obtained with different diffusion weighting, often referred to as b-values, the apparent diffusion coefficient (ADC) can be calculated from a monoexponential fit and presented as an ADC-map. It is important to understand that the choice of b-values will result in ADCs, which reflect different aspects of the diffusion (see below) [10].
DWI has evolved to the most important functional method to be added to T2-weighted (T2w) imaging in an examination of the prostate. The sensitivity and specificity for tumor detection increases significantly when T2w imaging is used in combination with DWI [11]. In addition, ADC can also be used for characterization, tumor grading and local tumor staging [12], [13]. In the peripheral zone of the prostate, DWI is the single most important pulse sequence for detection and staging of prostate cancer, and to a large extent determine the PI-RADS v.2 grade together with T2w imaging [1]. Once diagnosed, DWI can be used to improve the definition of the prostate cancer in supplement to T2w images [14]. Since there is some evidence that the degree of restriction of diffusion of prostate cancer is related to Gleason grade, information from ADC could potentially be used in more detail for treatment planning [15]. For treatment techniques, aiming for higher doses (boost) within the prostate, DWI or ADC in a multiparametric setting, can be of guidance to identify the gross target volume for treatment planning [16]. Potentially, the ADC could steer the dose level within the target. Since detailed delineation would be performed using the data on a voxel level, the quality of the ADC measurement needs to be very high, the images geometrically accurate and image artifacts should be insignificant.
MRI diffusion has been used to study treatment response during radiotherapy of prostate cancer [17], [18], [19]. The ADC within tumor tissue has been shown to increase already during the initial phase of the treatment and continues to rise until the completion of therapy months later. These ADC changes seem to occur before changes in levels of PSA [19]. An early increase in ADC reflects increased water mobility through the loss of membrane integrity or an increase in the proportion of total extracellular fluid due to a decrease in cell size or number. The significant difference in ADC between tumor and benign tissue before radiotherapy disappear after completion of the therapy [17]. Diffusion MRI has great potential as biomarker for monitoring treatment response.
The ADC calculated from a monoexponential fit as described above assumes a normal distributed free diffusion. This is a simplification. The diffusion signal deviates from the monoexponential behavior and it becomes apparent for high b-values (>1000 mm2/s). The diffusion kurtosis model comprises the signal behavior by introducing a dedicated kurtosis parameter. It has been hypothesized that the kurtosis parameter represent the interaction of water molecules with cell membranes and intracellular compounds. More irregular and heterogeneous environments would increase the kurtosis [20]. Diffusion kurtosis imaging (DKI) provides an image representing the kurtosis parameter. DKI has been found to differentiate between benign and malignant prostate tissue [21] and also between low grade and high grade tumors [22]. However, a recent systematic review concluded that DKI did not add any value compared to DWI for clinical assessment of prostate cancer [23].The special contrast mechanism may be of interest for monitor treatment response [20]. This was recently indicated for rectal cancer but is yet to be shown for prostate cancer [24].
For low b-values (<200 s/mm2) there is substantial part of the diffusion signal which is affected the by microcirculation of blood in the capillary network. The phenomenon is referred to as intravoxel incoherent motion (IVIM) [10], [25]. Normally, this component is minimized by selecting b-values > 200 s/mm2, or in fact neglected when ADC is measured. However, it is possible to assess both IVIM and ADC, if measurements with a number of lower b-values are performed. There are now indications that the perfusion fraction calculated from the IVIM measurement corresponds to the parameter Ktrans measured by Dynamic Contrast Enhanced MRI (DCE-MRI), specifically when detecting prostate cancer in the transitional zone and locally recurrent disease [26]. In the future IVIM based analyses may provide further insight in diseases and treatment response. In addition, it has recently been found that IVIM in combination with ADC may have a role for hypoxia assessment (see below) [27].
Another special feature of MRI diffusion is diffusion tensor imaging (DTI). The effect from the diffusion on the signal is specific for the direction of the applied diffusion gradient. The ADC map may look very different dependent upon the orientation of the gradient, a result of anisotropic diffusion, i.e. that the diffusion is higher in certain directions. For the prostate, DTI corresponds to the microstructural organization of the tissue and the cancer may change this structure [13]. DTI may also be used to visualize nerve bundles, which may be a tool although not yet clinically used to plan nerve sparing prostatic treatment and monitor post treatment nerve injury and reinnervation [28]. In the future, DTI may contribute to tumor detection or treatment follow-up.
3.2. Dynamic contrast enhanced MRI
There are three different techniques to assess perfusion related parameters with MRI. One method, arterial spin labelling (ASL), is based on endogenous contrast from the blood in combination with a special scheme of radiofrequency pulses. The other two methods relies on an i.v. injection of an exogenous contrast agent (CA). Dynamic susceptibility contrast (DSC) MRI is technique that measures the paramagnetic effects in the vessels of the CA during the first pass, while the CA is still enclosed in the intravascular space. ASL and DSC are almost exclusively used for studies of perfusion in the brain, and are not generally used for characterization of tumors in the prostate. The third method, dynamic contrast enhanced MRI (DCE-MRI), is the main method to characterize the vascular properties of tumors in the body including the prostate, and will be described below.
Assessing certain vascular physiological properties is of special interest in cancer since tumor growth creates an environment, which often differs from normal soft tissue with respect to the vascular structure. During tumor growth new vessels are needed, i.e. angiogenesis, but the new vessels will be of different, often poor structure, having a tortuous topology and express a certain leakiness. In short, DCE-MRI can assess tumor vasculature, blood vessel permeability, and extravascular/extracellular volume fraction. Obviously, all these parameters will be of high interest to characterize tumor tissue, as for example with respect to staging and grading [29].
As indicated above, DCE-MRI relies on an i.v. injection of a contrast agent. The standard techniques use clinically available paramagnetic gadolinium (Gd) chelates. The contrast agent affects the relaxation times T1, T2 and T2*. DCE-MRI is entirely built on the T1 shortening effect of the CA. In order to follow the distribution of the CA, i.e. to characterize its pharmacokinetics, a dynamic T1-weighted acquisition is performed.
The most common model for quantitative data analyzing of the time intensity curve is the Tofts model [30]. The analysis results in the parameter Ktrans, which represents the transfer constant from plasma space to tissue space, often referred to as permeability. The analysis also results in the parameters extravascular-extracellular space fraction volume (ve), plasma volume (vp) and the rate constant kep between the ve and vp. The parameter Ktrans is often the parameter of interest for tumor characterization. The rationale behind using this parameter in oncological examinations is that the endothelium in neovascularized cancerous tissue is more prone to leak fluid into the extravascular space than normal vascular structures. The assumption that neovascularization is a key element in the formation and growth of many cancers including prostate cancer has been the basis for DCE-MRI. Although DCE-MRI was introduced more than two decades ago, there is today much more knowledge about the limitations in differentiating prostatitis from cancer in the peripheral zone and benign prostatic hyperplasia from cancer in the transitional zone [31].
The Tofts and similar quantitative models require information such as T1-map of the tissue of interest and an arterial input function (AIF). The T1-map is needed to convert changes of signal to changes of CA concentration and AIF describes the initial concentration of the CA transferred to the tissue of interest and the model. The need for T1-map and AIF make the acquisition protocol complicated. Additionally, the analysis of the data requires substantial computing efforts. Therefore, several semi-quantitative methods have emerged. The most applied method is the initial area under the CA uptake vs time curve (AUC), which basically reflects the initial dynamics of the contrast agent. The AUC often correlates to Ktrans, but the physiologic interpretation is unclear. Still, AUC and similar semi-quantitative techniques are used since the acquisition protocol and the analysis are simplified.
T2w images have similar signal intensities, i.e. low signal, for the malignant tumors as for benign prostatic hyperplasia, prostatitis and post-biopsy hemorrhage. DCE-MRI can therefore contribute to the diagnosis of prostate cancer by increasing the specificity for tumor detection especially for cancer in the peripheral zone of the prostate. T2w imaging with DCE-MRI has shown to be useful for detection recurrent prostate cancers after biochemical failure [32].
DCE-MRI has also a potential role as a method to improve target definition [33]. One benefit of DCE-MRI is that the measurement can often be performed in 3D with relatively high spatial resolution, which enables detailed anatomical information from the prostate. In multiparametric MR imaging (see below) of the prostate, the application of DCE-MRI add information regarding intra-prostatic extension of prostate cancer [34]. The functional information may hypothetically be used to define sub-volumes with different biological properties within the gross tumor volume. The sub-volume may have inferior permeability, with a potential need for different dose strategy based on a threshold, i.e dose sculpting by contours [35]. Biological information on a voxel level may also be used to steer the dose within the target volume, i.e. dose sculpting by numbers or dose painting [36]. Trials with dose painting based on MR imaging is presently scarce, but one example of this concept is represented by the Dutch FLAME study where the intraprostatic boost is delivered based on multiparametric MRI information [37].
DCE-MRI results in new information useful in prognosis as well as assessment of tumor and normal tissue responses to radiation. Use of DCE-MRI in multiparametric MRI of the prostate may play a role in treatment modality selection, target definition, and therapy individualization, although further validation studies are needed [38].
3.3. Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) takes advantage of that spins may resonate at slightly different frequencies, if their molecular environment – chemical bonding – differ. The term chemical shift comes from the difference in resonance frequency relative to a reference compound. A well-known example is the 3.5-ppm chemical shift of fat relative to water in the proton spectrum. The different compounds give rise to different peaks that together result in the MR spectrum. The area under the peak is proportional to the number of nuclei, i.e. the metabolite concentration.
The spectral information does not include spatial localization per se. The spatial localization can be obtained either by using surface coils or by the use of the gradients in the MR-scanner. The latter technique may range from single voxel spectroscopy to chemical shift imaging (CSI). CSI results in 2D/3D arrays of spectra, from which maps of the individual metabolites can be constructed. CSI has been considered a rather time-consuming technique, but new methods are evolving. There is now evidence that acquisition times even with sub ml volume resolution can be in the order of a conventional anatomical imaging sequence [39].
In 1H (proton) MRS, signals from citrate (Cit), choline-containing compounds (Cho), creatine (Cr) and polyamines (PA) are in general detectable in vivo at 1.5 T and higher field strengths as well as mobile lipids. The dominating signals come from water and lipid triglycerides, which therefore need to be suppressed in order to make the metabolites distinguishable. The citrate level are reduced in prostate cancer and can be used to differentiate between tumor and normal prostate tissue [40]. Similarly, the total choline increases in prostate cancer tissue. Generally, there is no difference in total creatine between tumor or normal tissue [40]. The ratio between the metabolites are often used as a readout, since absolute measurements of the metabolites is very difficult. For prostate cancer localization and characterization, the metabolite ratio (Cho + PA + Cr)/Cit has been used as biomarker for malignancies [40].
When 1H MRS is added to the MR imaging protocol the false negatives can be reduced and the specificity can be improved [41]. CSI is both more technically challenging and demanding than DWI and DCE-MRI and the spatial resolution is inherently lower with this technique. Furthermore, most of the reported knowledge around CSI dates several years back during which time other functional imaging techniques, in particular DWI has significantly improved and further developed and being implemented in clinical routine [31], [42]. Due to its complex nature, CSI is not listed as a mandatory functional technique according PI-RADS v.2 guidelines for multiparametric MRI of prostate cancer [1].
The information from CSI can be used to improve the definition of the target [43]. Additionally, spectroscopic data can be used to define regions within the prostate for boost dosing and targets for image guided radiotherapy [44], [45].
MRS has also a role in recurrent cancers. In patients, which have received radiotherapy the radiation induces changes in the tissue properties. It can therefore be difficult to use MRI to distinguish healthy from malignant tissue. The elevated ratio choline to creatine from MRS can still be used to identify residual or recurrent prostate cancer [46]. However, the most important methods for detecting recurrence in irradiated prostate are DWI and DCE-MRI [47].
3.4. New and upcoming MRI technologies
3.4.1. Tissue oxygenation MRI
In many tumors, there is an imbalance between oxygen delivery and consumption, leading to hypoxia. The hypoxic environment is known to promote angiogenesis, malignancy, metastases and genetic instability, and to reduce effectiveness of radiation and chemotherapy, and hypoxia is associated with poor prognosis of several cancers [48]. The oxygenation or degree of hypoxia in the tumor is therefore an important factor, both for designing the therapy, as well as an indicator of the progress. However, non-invasive MRI methods to assess the oxygenation level in vivo are scarce.
One way to assess the oxygenation is to use the endogenous contrast mechanism of oxygen on blood. The hemoglobin and deoxyhemoglobin have different magnetic properties and deoxyhemoglobin is strongly paramagnetic. This difference in magnetic susceptibility can be assessed by the T2* relaxation time, a parameter, which reflects susceptibility differences. This phenomenon is known as blood-oxygen-level-dependent (BOLD) contrast. The difference in T2* due to oxygen exposure reflects the deoxyhemoglobin concentration of blood, i.e. the oxygenation. However, the change in T2* will be affected by the blood oxygen saturation, blood volume and hematocrit since these parameters influence the concentration of deoxyhemoglobin of the blood [48]. Therefore, the results may be hard to interpret, unless T2* and T1 is measured simultaneously [49]. There has been indications that the T2* measured in prostate tumors, which were identified on T2w images, correlated to needle oxygen measurements and could be used to identify hypoxic regions [50]. In addition, exposure to 100% oxygen via inhalation during the measurement may facility hypoxia assessment. The change of T1 or T2* due to the oxygen exposure will be related to the initial oxygenation [51]. In prostate tumors, areas with large oxygen enhancement correlate with high perfusion, while no or little enhancement can be attributed to hypoxia, which thereby was possible to identify [52].
A different method to assess hypoxia is based on diffusion. DWI is used to generate separate images of ADC and IVIM, which correlate to the cell density and fractional blood volume (fBV), respectively [27]. The method has recently been validated in prostate tumors, for which areas with low ADC and low fBV represented areas of hypoxia, while oxygenated areas had high ADC and high fBV [27].
3.4.2. Magnetization transfer
In tissues, it is only the protons in the free water pool, which contribute to the MR signal. Protons in macromolecules (proteins, collagen etc) are tightly bound and have very short T2 and are therefore invisible. However, via magnetization transfer, the bound protons can affect the signal of the free protons. Dedicated RF-pulses are used to saturate the bound proton pool, which will exchange magnetization with the free water. This causes a reduced signal from the free water in tissues in which the magnetization transfer (MT) mechanism is prevalent. Since the extent of signal-decay depends on the exchange rate between free and hydration water, MT can be used to provide an alternative tissue contrast in addition to T1, T2, and proton density. The effect, i.e. the measure of the probed macromolecules, is often referred to as the magnetization transfer ratio (MTR), which is the signal without and with the applied RF-pulses.
MT of prostate cancer has not been the subject for many studies. In one study, suspected tumors in the prostate were identified by T2w imaging and the MTR of these regions were measured [53]. Of the patients in which the malignancy of the tumors verified by histopathology, there was a significant higher MTR compared to the patients with negative biopsies. The MTR from negative biopsies also matched values from healthy controls. The increased MTR in tumor is an indication of increased cellularity and an increased amount of high molecular weight material, which is known to correlate with malignity. Recently, it was also found that MTR was significant higher in tumors compared to normal prostate tissue [54]. However, there was no incremental diagnostic value of MTR over conventional DWI.
3.4.3. Hyperpolarized MRI
Normally the signal from tissues is created by the polarization of the nuclei, caused by the high magnetic field in the MR-scanner. However, polarization can also be created by other physical and chemical processes and to levels which are more than 10 000 times higher than what can be achieved by the magnetic field of clinical MR-scanners [55]. This phenomenon, called hyperpolarization (HP) has been used to enhance the signal from gases such as helium-3 or from low concentrations of carbon-13 (13C). The HP is created outside the MR-scanner and outside the body in a dedicated polarizer. Once the hyperpolarized substance leaves the polarizer and is injected in the body, the signal rapidly decays (∼1 min). In many molecules of interest, a carbon atom can be replaced by a 13C isotope, which subsequently is hyperpolarized. After injection of the hyperpolarized substance, it can be followed by MR-imaging or MR-spectroscopy including the metabolites of the molecule. It is worth to note a significant difference between functional studies performed with positron emission tomography (PET) and HP 13C MRI. In PET only the uptake of the injected tracer can be tracked. Using HP 13C MRI, the injected molecule as well as its metabolites can be tracked separately.
It is difficult to find a hyperpolarized molecule for which the decay rate in-vivo is slow enough to allow metabolic studies. However, one such molecule is 13C-pyruvate, a key component in glycolysis. Thereby it enables HP 13C MRI for interesting application in oncology. From injected HP 13C-puruvate, dynamic MR acquisition of the metabolites lactate, alanine and bicarbonate can be performed. Compared to normal cells, tumors have been proven to have a substantially higher uptake as well as higher metabolic activity of pyruvate [55]. The first clinical study of prostate cancer in man using HP 13C-pyruvate was completed recently [56]. The tumor metabolism in 31 patients with prostate cancer was assessed. The level of 13C-lactate/13C-pyruvate was elevated in regions of biopsy-proven cancer. The technique was also able to detect cancer in regions of the prostate that were previously considered tumor–free after examinations with other imaging modalities. However, these type of findings by HP 13C MRI have not yet been proven by histology of resected prostate glands. HP 13C MRI is a new technique, which offers many possibilities. At present there are twelve on-going clinical trials on HP 13C MRI for prostate cancer (December 2018, www.clinicaltrials.gov).
3.4.4. MR-elastography
MR-elastography (MRE) is a method for measurement of the mechanical properties of tissue. This technique requires dedicated hardware, which is commercially available. The mechanical properties can change dramatically due to pathologically processes, such as cancer, fibrosis or inflammation. MR-elastography can assess the tissue properties in large volumes and the measurement results in quantitative information on stiffness.
Mechanical waves, referred to as shear waves, are generated by an external acoustic driver, actuator, for low frequency vibrations. The shear wave displacements in the tissue can be monitored by motion encoded phase contrast MRI. The temporal and spatial characteristics of the wave-field form the basis for an algorithm that transforms the obtained data to a map of the tissue mechanical properties.
The prostate is challenging for MRE. The organ is centrally located in the body and waves from an external actuator are attenuated in the surrounding tissue. An alternative approach is an intra-cavitary endorectal actuator [57]. MRE of prostate has been validated against histopathology with moderate success [58]. One observation was that cancerous tissue was not always stiffer than normal tissue. However, in stiff regions or nodules the localization matched findings by the reference MR images [59]. There are also indications from MRE measurements that radiotherapy makes the gland stiffer [60]. MRE has only been tried out on a limited number of patients with prostate cancer. At present, it seems that MRE of the prostate is inferior to other MR imaging methods, such as T2w, ADC or DCE-MRI.
3.5. Multiparametric MRI
Multiparametric MRI (mpMRI) is defined as the combination of an anatomical T1w and T2w imaging and functional imaging techniques. The functional imaging is usually DWI and DCE-MRI, but MRS has also been included in the concept mpMRI. However, in presence of DWI and DCE-MRI, MRS is considered complex and of less added value and therefore often ignored [61]. In addition, DWI is often preferred to DCE-MRI for characterization of lesions, since enhancement in malign lesions can be hard to distinguish from benign conditions, such as prostatitis and prostatic hyperplasia [62].
A lesion is typically depicted as hypointense region on a T2w image, high intensity on DWI (or low ADC), and enhancement on DCE-MRI. Detailed instructions to interpret the images are available in the PI-RADS v.2 document [1], which unfortunately does not provide any recommendations for definition of radiotherapy targets. The mpMRI can be used for tumor detection, staging, assessment of aggressiveness and treatment monitoring [61], [63], [64]. For radiotherapy, mpMRI can have a significant impact on the choice of the treatment. In two studies based on mpMRI findings, 30% of the patients experienced a change in their initial risk group compared to the clinical tumor categorization [65], [66].
After radical prostatectomy or after radiotherapy mpMRI shows a potential to visualize post treatment changes and to detect localized recurrence [47]. For this case, DCE-MRI is of great value and sensitive for detection of small lesions.
3.6. Technical aspects
There are some technical aspects on functional MRI imaging of the prostate when the examination is performed in a radiotherapy setting, especially in treatment position. For technical aspects in a standard diagnostic radiology setting, see recent review by Purysko et al. [62]. A flat tabletop in the MR-scanner may create an extra distance between the receiver coil and the body. If the body is kept in a dedicated mould, i.e. treatment position, it can be difficult to use the standard and optimized receiver coils. Both these adjustments may reduce the signal to noise ratio (SNR). At present, there is an urge to create radiotherapy planning workflow based entirely on MRI and exclude the CT. With no density maps from CT, the corresponding information needs to be provided from MR images. There are several methods presented to generate density maps from MR-images [67], and a few are also available commercially [68], [69], and have recently been validated for prostate cancer radiotherapy planning [4], [70]. If the intended use of the examination is to replace the CT and generate density maps from MR-images, it is very important to keep the outer surface of the body intact. One way to accomplish this is to place a dedicated stiff bridge holder between the body and the coil [4], which again may reduce SNR. Altogether, the adjustments to a radiotherapy setting can result in a lower signal to noise ratio (SNR) compared to a standard setting in a radiology. This will affect the quality of functional measurement negatively. For example, the outcome of an ADC measurement is critically dependent on the SNR of the acquired images. In addition, the geometric distortions needs to be monitored and may be of special interest if the functional images are used in for target volume decisions. Typically, this can be an issue for DWI, a method in which MRI sequences sensitive to distortions are often used [71].
For prostate radiotherapy, fiducial markers are often used for positioning the anatomy of the patient accurately for every fraction during the treatment time course [72]. The markers are made of metal in order to create high contrast on X-ray images. Thereby, the markers also create susceptibility artefacts in the MR-images. For normal T1w or T2w imaging this is a minor issue. Certain MRI sequences are very sensitive to susceptibility effects and metallic fiducial markers may prevent reliable measurements of for example diffusion [73]. Other materials such as radiopaque hydrogel are under development to address this issue [74].
In order to obtain quantitative functional data used for example repeated follow-up or adaptive treatment, it can be of extra value to perform the bowel preparation carefully. DWI is very sensitive to gas and to changes of gas filling during the examination. Similarly, even a small improvement on the image quality from using antiperistaltic agents may be considered, when the data should be used for treatment decisions rather than diagnostic procedures [75].
4. Discussion
Magnetic resonance imaging has played an important role in the diagnosis and management of prostate cancer since it was clinically introduced. Recently, the interest to apply MRI at all stages in the radiotherapy process, i.e. after the diagnostic procedure has been completed, has increased. This was emphasized in an editorial, in which the undeserved needs also were reviewed [76]. One of these needs, i.e. education of the radiation oncology community on MRI and in this case more specifically FMRI, has been addressed in the present review. The recently introduced MR-linac will even further emphasize the need for efficient MR methods in all stages of the radiotherapy workflow [77].
In order to use FMRI methods appropriately and efficiently, it is important to understand both the underlying biological process in the tumor and risk organs during therapy, and how these processes relate to FMRI. In addition, understanding the limitations of what the FMRI techniques are actually measuring, are important for the interpretation of the results. For example, ADCs, initially increase during radiotherapy, as a sign of response, rise to a level above normal tissue due to necrosis, and thereafter return to an intermediate value. Obviously, this indicates that the time point for the measurement, as well as the region of interest for the analysis, is crucial to the interpretation of the results. Additionally, it is reasonable to assume that even in a single tumor there will be an inhomogeneous structure, with a multitude of physiological changes. This increases the complexity and may require more than one functional MRI readout to characterize the tumor. Therefore, multiparametric imaging with combined FMRI techniques will be of especially high value as well as complementary measurements from other imaging modalities. Although, not yet applied to radiotherapy explicitly, the inhomogeneous structure of the tumors has also been successfully addressed with texture analysis of the image data. Several texture features in ADC and mpMRI were able to differentiate between non-cancerous and cancerous tissue including the transition zone and correlate with Gleason score [78], [79].
A well-recognized problem in manual delineation of target structures is the interobserver variability [80], [81]. New methods are being developed for increasing the constancy of tumor delineation [82]. Of special interest is the latest development on artificial intelligence (AI) or machine learning, which may supersede texture analysis in finding other and valuable features for tumor characterization [83]. This could be of particular interest for applications to the MR-linac, when the information, including the functional measures need to be rapidly extracted from the images, while the patient is waiting for the treatment in the machine.
As indicated in this overview several functional MR-methods are available. Although many of the methods have been available in diagnostic radiology for decades, not many methods have been established as a standard tool in clinical routine or even in clinical research trials. There are several explanations. When the functional methods need to be quantitative, they often become complicated and technically challenging both for manufacturers of equipment and for the operators. The methods may require dedicated analyzing software not supplied or available from the vendors. There are limited established standards for data acquisition and data analyzing. However, it is worth to underline that one of the strengths of the functional methods is that they do result in quantitative data. If these functional measures can be brought into robust and validated biomarkers for the imaging community, their working potential is hard to overestimate. More data under different conditions and during different kinds of treatments are needed to reach this level of confidence.
There are several on-going initiatives to standardize FMRI. In Europe there is the European Imaging Biomarkers Alliance [84] within the European Society of Radiology and in the US Radiological Society of North America started the Quantitative Imaging Biomarkers Alliance, QIBA [85]. Within the latter framework, task groups are working on standardization of for example MR-perfusion, diffusion and flow. There are other initiatives on this subject from the American Association of Physicists in Medicine and the National Cancer Institute, although reports are still pending [86].
The different FMRI techniques are of different maturity. The literature on DWI, DCE-MRI and MRS is vast within oncology with applications in most relevant organs. The applications to radiotherapy are less frequent, but steadily increasing. For these FMRI techniques, most vendors provide dedicated sequences as well as tools for analysis. However, there are few standards and the techniques may not be validated.
The remaining techniques for measurement of hypoxia, MTR, hyperpolarization and elastography are more at an experimental level. The measurement techniques varies and the links between read-outs and the biology are less established. An exception may be the method for hypoxia based on DWI [27]. DWI is a method already implemented in most MRI protocols for prostate cancer and therefore hypoxia measurement with DWI may be easier to provide clinically. In addition, new FMRI methods keep emerging, such as the luminal water imaging. A method with a potential to both detect and grade prostate cancer [87].
In parallel to the rapid development of FMRI for radiotherapy, other imaging modalities are gaining interest. Prostate specific PET methods using tracers such 68Ga-PSMA are promising [88]. Future hybrid imaging protocols combining FMRI and prostate specific PET tracers may further increase the possibility to delineate intraprostatic lesions as well as malignant lymph nodes and local relapses. However, the discussion on modalities besides MRI is out of the scope of the present paper.
In conclusion, the role of MR-methods for functional imaging of the prostate as a tool for characterization of tissue and monitoring of response is promising but not established for radiotherapy applications. In this rapidly expanding area of research, as has been outlined in this review, there are many indications that FMRI could play an important role in many of the steps in the radiotherapy process.
Conflict of interest
None of the authors has any conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2019.02.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Weinreb J.C., Barentsz J.O., Choyke P.L., Cornud F., Haider M.A., Macura K.J. PI-RADS prostate imaging – Reporting and data system: 2015, Version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dirix P., Haustermans K., Vandecaveye V. The value of magnetic resonance imaging for radiotherapy planning. Semin Radiat Oncol. 2014;24:151–159. doi: 10.1016/j.semradonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Salembier C., Villeirs G., De Bari B., Hoskin P., Pieters B.R., Van Vulpen M. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol. 2018;127:49–61. doi: 10.1016/j.radonc.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Persson E., Gustafsson C., Nordstrom F., Sohlin M., Gunnlaugsson A., Petruson K. MR-OPERA: a multicenter/multivendor validation of magnetic resonance imaging-only prostate treatment planning using synthetic computed tomography images. Int J Radiat Oncol Biol Phys. 2017;99:692–700. doi: 10.1016/j.ijrobp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Tyagi N., Fontenla S., Zelefsky M., Chong-Ton M., Ostergren K., Shah N. Clinical workflow for MR-only simulation and planning in prostate. Radiat Oncol. 2017;12:1–12. doi: 10.1186/s13014-017-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlsson M., Karlsson M.G., Nyholm T., Amies C., Zackrisson B. Dedicated magnetic resonance imaging in the radiotherapy clinic. Int J Radiat Oncol Biol Phys. 2009;74:644–651. doi: 10.1016/j.ijrobp.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 7.McPartlin A.J., Li X.A., Kershaw L.E., Heide U., Kerkmeijer L., Lawton C. MRI-guided prostate adaptive radiotherapy – A systematic review. Radiother Oncol. 2016;119:371–380. doi: 10.1016/j.radonc.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Lips I., van der Heide U., Haustermans K., van Lin E., Pos F., Franken S. Single blind randomized phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): study protocol for a randomized controlled trial. Trials. 2011:255–264. doi: 10.1186/1745-6215-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Z., Wang C. Treatment assessment of radiotherapy using MR functional quantitative imaging. World J Radiol. 2015;7:1–6. doi: 10.4329/wjr.v7.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima M., Le Bihan D. Clinical intravoxel incoherent motion and diffusion mr imaging: past, present, and future. Radiology. 2016;278:13–32. doi: 10.1148/radiol.2015150244. [DOI] [PubMed] [Google Scholar]
- 11.Molinelli V., Angeretti M.G., Duka E., Tarallo N., Bracchi E., Novario R. Role of MRI and added value of diffusion-weighted and gadolinium-enhanced MRI for the diagnosis of local recurrence from rectal cancer. Abdom Radiol. 2018;43:2903–2911. doi: 10.1007/s00261-018-1518-z. [DOI] [PubMed] [Google Scholar]
- 12.Maurer M.H., Heverhagen J.T. Diffusion weighted imaging of the prostate-principles, application, and advances. Transl Androl Urol. 2017;6:490–498. doi: 10.21037/tau.2017.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamada T., Sone T., Jo Y., Yamamoto A., Ito K. Diffusion-weighted MRI and its role in prostate cancer. NMR Biomed. 2014;27:25–38. doi: 10.1002/nbm.2956. [DOI] [PubMed] [Google Scholar]
- 14.Tsien C., Cao Y., Chenevert T. Clinical applications for diffusion magnetic resonance imaging in radiotherapy. Semin Radiat Oncol. 2014;24:218–226. doi: 10.1016/j.semradonc.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato S., Kamijima S., Inaoka T., Kamiya N., Sasai D., Terada H. Quantitative evaluation of the relative apparent diffusion coefficient values on multiparametric magnetic resonance imaging to predict higher Gleason score prostate cancer. Scand J Urol. 2018;52:180–185. doi: 10.1080/21681805.2018.1481143. [DOI] [PubMed] [Google Scholar]
- 16.Groenendaal G., van den Berg C.A., Korporaal J.G., Philippens M.E., Luijten P.R., van Vulpen M. Simultaneous MRI diffusion and perfusion imaging for tumor delineation in prostate cancer patients. Radiother Oncol. 2010;95:185–190. doi: 10.1016/j.radonc.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Park S.Y., Kim C.K., Park B.K., Park W., Park H.C., Han D.H. Early changes in apparent diffusion coefficient from diffusion-weighted MR imaging during radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83:749–755. doi: 10.1016/j.ijrobp.2011.06.2009. [DOI] [PubMed] [Google Scholar]
- 18.Decker G., Murtz P., Gieseke J., Traber F., Block W., Sprinkart A.M. Intensity-modulated radiotherapy of the prostate: dynamic ADC monitoring by DWI. Radiother Oncol. 2014;113:115–120. doi: 10.1016/j.radonc.2014.07.016. at 3.0 T. [DOI] [PubMed] [Google Scholar]
- 19.Pasquier D., Hadj Henni A., Escande A., Tresch E., Reynaert N., Colot O. Diffusion weighted MRI as an early predictor of tumor response to hypofractionated stereotactic boost for prostate cancer. Sci Rep. 2018;8:10407. doi: 10.1038/s41598-018-28817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenkrantz A.B., Padhani A.R., Chenevert T.L., Koh D.M., De Keyzer F., Taouli B. Body diffusion kurtosis imaging: basic principles, applications, and considerations for clinical practice. J Magn Reson Imaging. 2015;42:1190–1202. doi: 10.1002/jmri.24985. [DOI] [PubMed] [Google Scholar]
- 21.Mazzoni L.N., Lucarini S., Chiti S., Busoni S., Gori C., Menchi I. Diffusion-weighted signal models in healthy and cancerous peripheral prostate tissues: comparison of outcomes obtained at different b-values. J Magn Reson Imaging. 2014;39:512–518. doi: 10.1002/jmri.24184. [DOI] [PubMed] [Google Scholar]
- 22.Rosenkrantz A.B.S.E., Johnson G., Babb J.S., Mussi T.C., Melamed J., Taneja S.S. Prostate cancer: feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology. 2012;264:126–135. doi: 10.1148/radiol.12112290. [DOI] [PubMed] [Google Scholar]
- 23.Si Y., Liu R.B. Diagnostic performance of monoexponential DWI versus diffusion kurtosis imaging in prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2018;211:358–368. doi: 10.2214/AJR.17.18934. [DOI] [PubMed] [Google Scholar]
- 24.Hu F., Tang W., Sun Y., Wan D., Cai S., Zhang Z. The value of diffusion kurtosis imaging in assessing pathological complete response to neoadjuvant chemoradiation therapy in rectal cancer: a comparison with conventional diffusion-weighted imaging. Oncotarget. 2017;8:10. doi: 10.18632/oncotarget.17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bihan D., Breton E., Lallemand D., Grenier P., Cabanis E., Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 26.Pang Y., Turkbey B., Bernardo M., Kruecker J., Kadoury S., Merino M.J. Intravoxel incoherent motion MR imaging for prostate cancer: an evaluation of perfusion fraction and diffusion coefficient derived from different b-value combinations. Magn Reson Med. 2013;69:553–562. doi: 10.1002/mrm.24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hompland T., Hole K.H., Ragnum H.B., Aarnes E.K., Vlatkovic L., Lie A.K. Combined MR imaging of oxygen consumption and supply reveals tumor hypoxia and aggressiveness in prostate cancer patients. Cancer Res. 2018;78:4774–4785. doi: 10.1158/0008-5472.CAN-17-3806. [DOI] [PubMed] [Google Scholar]
- 28.Di Paola V., Cybulski A., Belluardo S., Cavicchioli F., Manfredi R. Pozzi Mucelli R. Evaluation of periprostatic neurovascular fibers before and after radical prostatectomy by means of 1.5 T MRI diffusion tensor imaging. Br J Radiol. 2018;91:10. doi: 10.1259/bjr.20170318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazaheri Y., Akin O., Hricak H. Dynamic contrast-enhanced magnetic resonance imaging of prostate cancer: a review of current methods and applications. World J Radiol. 2017;9:416–425. doi: 10.4329/wjr.v9.i12.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tofts P.S.B.G., Buckley D.L., Evelhoch J.L., Henderson E., Knopp M.V., Larsson H.B.W. estimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 31.Lovegrove C.E., Matanhelia M., Randeva J., Eldred-Evans D., Tam H., Miah S. Prostate imaging features that indicate benign or malignant pathology on biopsy. Transl Androl Urol. 2018;7:S420–S435. doi: 10.21037/tau.2018.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haider M.A., Chung P., Sweet J., Toi A., Jhaveri K., Menard C. Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:425–430. doi: 10.1016/j.ijrobp.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Sung Y.S., Kwon H.J., Park B.W., Cho G., Lee C.K., Cho K.S. Prostate cancer detection on dynamic contrast-enhanced MRI: computer-aided diagnosis versus single perfusion parameter maps. AJR Am J Roentgenol. 2011;197:1122–1129. doi: 10.2214/AJR.10.6062. [DOI] [PubMed] [Google Scholar]
- 34.Fütterer J.J.H.S., Scheenen T.W., Veltman J., Huisman H.J., Vos P., Hulsbergen-Van de Kaa C.A., Witjes J.A., Krabbe P.F., Heerschap A., Barentsz J.O. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology. 2006;241:449–458. doi: 10.1148/radiol.2412051866. [DOI] [PubMed] [Google Scholar]
- 35.Ling C., Humm J., Larson S., Amols H., Fuks Z., Leibel S. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47:551–559. doi: 10.1016/s0360-3016(00)00467-3. [DOI] [PubMed] [Google Scholar]
- 36.Bentzen S. Dose painting and theragnostic imaging: towards the prescription, planning and delivery of biologically targeted dose distributions in external beam radiation oncology. Cancer Treat Res. 2008;130:41–61. [PubMed] [Google Scholar]
- 37.Monninkhof E.M., van Loon J.W.L., van Vulpen M., Kerkmeijer L.G.W., Pos F.J., Haustermans K. Standard whole prostate gland radiotherapy with and without lesion boost in prostate cancer: toxicity in the FLAME randomized controlled trial. Radiother Oncol. 2018;127:74–80. doi: 10.1016/j.radonc.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Cao Y. The promise of dynamic contrast-enhanced imaging in radiation therapy. Semin Radiat Oncol. 2011;21:147–156. doi: 10.1016/j.semradonc.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinseifer I.K., Philips B.W., Gagoski B., Weiland E., Scheenen T.W., Heerschap A. Flexible proton 3D MR spectroscopic imaging of the prostate with low-power adiabatic pulses for volume selection and spiral readout. Magn Reson Med. 2017;77:928–935. doi: 10.1002/mrm.26181. [DOI] [PubMed] [Google Scholar]
- 40.Tayari N., Heerschap A., Scheenen T.W.J., Kobus T. In vivo MR spectroscopic imaging of the prostate, from application to interpretation. Anal Biochem. 2017;529:158–170. doi: 10.1016/j.ab.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Caivano R.C.P., Balestra A., Lotumolo A., Fortunato G., Macarini L., Zandolino A. Prostate cancer in magnetic resonance imaging: diagnostic utilities of spectroscopic sequences. J Med Imaging Radiat Oncol. 2012;56:606–616. doi: 10.1111/j.1754-9485.2012.02449.x. [DOI] [PubMed] [Google Scholar]
- 42.Fusco R., Sansone M., Granata V., Setola S.V., Petrillo A. A systematic review on multiparametric MR imaging in prostate cancer detection. Infect Agent Cancer. 2017;12:14. doi: 10.1186/s13027-017-0168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payne G.S., Leach M.O. Applications of magnetic resonance spectroscopy in radiotherapy treatment planning. Br J Radiol. 2006;79:S16–S26. doi: 10.1259/bjr/84072695. [DOI] [PubMed] [Google Scholar]
- 44.Riches S.F., Payne G.S., Desouza N.M., Dearnaley D., Morgan V.A., Morgan S.C. Effect on therapeutic ratio of planning a boosted radiotherapy dose to the dominant intraprostatic tumour lesion within the prostate based on multifunctional MR parameters. Br J Radiol. 2014;87:20130813. doi: 10.1259/bjr.20130813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen M.L., Willows B., Khan R., Chi A., Kim L., Nour S.G. The potential role of magnetic resonance spectroscopy in image-guided radiotherapy. Front Oncol. 2014;4:1–6. doi: 10.3389/fonc.2014.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurhanewicz J., Vigneron D.B. Advances in MR spectroscopy of the prostate. Magn Reson Imaging Clin N Am. 2008;16:697–710. doi: 10.1016/j.mric.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaur S., Turkbey B. Prostate MR imaging for posttreatment evaluation and recurrence. Urol Clin North Am. 2018;45:467–479. doi: 10.1016/j.ucl.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Mistry N.Q. CRC Press, Taylor&Francis Group; Boca Raton, FL, USA: 2012. CC. Imaging tissue oxygenation status with MRI; pp. 193–202. [Google Scholar]
- 49.Burrell J.S., Walker-Samuel S., Baker L.C., Boult J.K., Jamin Y., Halliday J. Exploring DeltaR(2)* and DeltaR(1) as imaging biomarkers of tumor oxygenation. J Magn Reson Imaging. 2013;38:429–434. doi: 10.1002/jmri.23987. [DOI] [PubMed] [Google Scholar]
- 50.Chopra S., Foltz W.D., Milosevic M.F., Toi A., Bristow R.G., Menard C. Comparing oxygen-sensitive MRI (BOLD R2*) with oxygen electrode measurements: a pilot study in men with prostate cancer. Int J Radiat Biol. 2009;85:805–813. doi: 10.1080/09553000903043059. [DOI] [PubMed] [Google Scholar]
- 51.O'Connor J.P., Naish J.H., Jackson A., Waterton J.C., Watson Y., Cheung S. Comparison of normal tissue R1 and R*2 modulation by oxygen and carbogen. Magn Reson Med. 2009;61:75–83. doi: 10.1002/mrm.21815. [DOI] [PubMed] [Google Scholar]
- 52.O'Connor J.P., Naish J.H., Parker G.J., Waterton J.C., Watson Y., Jayson G.C. Preliminary study of oxygen-enhanced longitudinal relaxation in MRI: a potential novel biomarker of oxygenation changes in solid tumors. Int J Radiat Oncol Biol Phys. 2009;75:1209–1215. doi: 10.1016/j.ijrobp.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 53.Kumar V., Jagannathan N.R., Kumar R., Thulkar S., Gupta S.D., Hemal A.K. Evaluation of the role of magnetization transfer imaging in prostate: a preliminary study. Magn Reson Imaging. 2008;26:644–649. doi: 10.1016/j.mri.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 54.Barrett T., McLean M., Priest A.N., Lawrence E.M., Patterson A.J., Koo B.C. Diagnostic evaluation of magnetization transfer and diffusion kurtosis imaging for prostate cancer detection in a re-biopsy population. Eur Radiol. 2018;28:3141–3150. doi: 10.1007/s00330-017-5169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golman K., Zandt R.I., Lerche M., Pehrson R., Ardenkjaer-Larsen J.H. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 2006;66:10855–10860. doi: 10.1158/0008-5472.CAN-06-2564. [DOI] [PubMed] [Google Scholar]
- 56.Nelson S.J., Kurhanewicz J., Vigneron D.B., Larson P.E., Harzstark A.L., Ferrone M. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci. Transl Med. 2013;5:198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arani A., Da Rosa M., Ramsay E., Plewes D.B., Haider M.A., Chopra R. Incorporating endorectal MR elastography into multi-parametric MRI for prostate cancer imaging: initial feasibility in volunteers. J Magn Reson Imaging. 2013;38:1251–1260. doi: 10.1002/jmri.24028. [DOI] [PubMed] [Google Scholar]
- 58.Sahebjavaher R.S., Nir G., Honarvar M., Gagnon L.O., Ischia J., Jones E.C. MR elastography of prostate cancer: quantitative comparison with histopathology and repeatability of methods. NMR Biomed. 2015;28:124–139. doi: 10.1002/nbm.3218. [DOI] [PubMed] [Google Scholar]
- 59.Dittmann F., Reiter R., Guo J., Haas M., Asbach P., Fischer T. Tomoelastography of the prostate using multifrequency MR elastography and externally placed pressurized-air drivers. Magn Reson Med. 2018;79:1325–1333. doi: 10.1002/mrm.26769. [DOI] [PubMed] [Google Scholar]
- 60.McGrath D.M., Lee J., Foltz W.D., Samavati N., van der Kwast T., Jewett M.A. MR elastography to measure the effects of cancer and pathology fixation on prostate biomechanics, and comparison with T 1, T 2 and ADC. Phys Med Biol. 2017;62:1126–1148. doi: 10.1088/1361-6560/aa52f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bittencourt L., Sabbagh de Hollanda E., de Oliveira R., Multiparametric M.R. Multiparametric MR imaging for detection and locoregional staging of prostate cancer. Top Magn Reson Imaging. 2016;25:109–117. doi: 10.1097/RMR.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 62.Purysko A.S., Rosenkrantz A.B. Technique of Multiparametric MR Imaging of the Prostate. Urol Clin North Am. 2018;45:427–438. doi: 10.1016/j.ucl.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Duvnjak P., Schulman A.A., Holtz J.N., Huang J., Polascik T.J., Gupta R.T. Multiparametric Prostate MR Imaging: impact on Clinical Staging and Decision Making. Urol Clin North Am. 2018;45:455–466. doi: 10.1016/j.ucl.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Winfield J.M., Payne G.S., Weller A., deSouza N.M. DCE-MRI, DW-MRI, and MRS in cancer: challenges and advantages of implementing qualitative and quantitative multi-parametric imaging in the clinic. Top Magn Reson Imaging. 2016;25:245–254. doi: 10.1097/RMR.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Counago F., Del Cerro E., Diaz-Gavela A.A., Marcos F.J., Recio M., Sanz-Rosa D. Tumor staging using 3.0 T multiparametric MRI in prostate cancer: impact on treatment decisions for radical radiotherapy. Springerplus. 2015;4:789. doi: 10.1186/s40064-015-1596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panje C., Panje T., Putora P.M., Kim S.K., Haile S., Aebersold D.M. Guidance of treatment decisions in risk-adapted primary radiotherapy for prostate cancer using multiparametric magnetic resonance imaging: a single center experience. Radiat Oncol. 2015;10:1–9. doi: 10.1186/s13014-015-0338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edmund J.M., Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat Oncol. 2017;12:28–43. doi: 10.1186/s13014-016-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siversson C., Nordstrom F., Nilsson T., Nyholm T., Jonsson J., Gunnlaugsson A. Technical note: MRI only prostate radiotherapy planning using the statistical decomposition algorithm. Med Phys. 2015;42:6090–6097. doi: 10.1118/1.4931417. [DOI] [PubMed] [Google Scholar]
- 69.Köhler M.V., Van Grootel M., Hoogeveen R., Kemppainen R., Kemppainen R., Renisch S. MR-only simulation for radiotherapy planning. Philips White paper. 2015;16 [Google Scholar]
- 70.Tyagi N., Fontenla S., Zhang J., Cloutier M., Kadbi M., Mechalakos J. Dosimetric and workflow evaluation of first commercial synthetic CT software for clinical use in pelvis. Phys Med Biol. 2017;62:2961–2975. doi: 10.1088/1361-6560/aa5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nketiah G., Selnaes K.M., Sandsmark E., Teruel J.R., Kruger-Stokke B., Bertilsson H. Geometric distortion correction in prostate diffusion-weighted MRI and its effect on quantitative apparent diffusion coefficient analysis. Magn Reson Med. 2018;79:2524–2532. doi: 10.1002/mrm.26899. [DOI] [PubMed] [Google Scholar]
- 72.O'Neill A.G., Jain S., Hounsell A.R., O'Sullivan J.M. Fiducial marker guided prostate radiotherapy: a review. Br J Radiol. 2016;89:20160296. doi: 10.1259/bjr.20160296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rylander S., Thornqvist S., Haack S., Pedersen E.M. Muren LP. Intensity profile based measurement of prostate gold markers influence on 1.5 and 3T diffusion-weighted MR images. Acta Oncol. 2011;50:866–872. doi: 10.3109/0284186X.2011.590523. [DOI] [PubMed] [Google Scholar]
- 74.Damato A.L., Kassick M., Viswanathan A.N. Rectum and bladder spacing in cervical cancer brachytherapy using a novel injectable hydrogel compound. Brachytherapy. 2017;16:949–955. doi: 10.1016/j.brachy.2017.04.236. [DOI] [PubMed] [Google Scholar]
- 75.Thoeny H.C., Barbieri S., Froehlich J.M., Turkbey B., Choyke P.L. Functional and targeted lymph node imaging in prostate cancer: current status and future challenges. Radiology. 2017;285:728–743. doi: 10.1148/radiol.2017161517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGee K.P., Hu Y., Tryggestad E., Brinkmann D., Witte B., Welker K. MRI in radiation oncology: underserved needs. Magn Reson Med. 2016;75:11–14. doi: 10.1002/mrm.25826. [DOI] [PubMed] [Google Scholar]
- 77.Winkel D., Bol G.H., Kiekebosch I.H., Van Asselen B., Kroon P.S., Jurgenliemk-Schulz I.M. Evaluation of online plan adaptation strategies for the 1.5T MR-linac based on “First-In-Man” treatments. Cureus. 2018;10 doi: 10.7759/cureus.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wibmer A., Hricak H., Gondo T., Matsumoto K., Veeraraghavan H., Fehr D. Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol. 2015;25:2840–2850. doi: 10.1007/s00330-015-3701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sidhu H.S., Benigno S., Ganeshan B., Dikaios N., Johnston E.W., Allen C. Textural analysis of multiparametric MRI detects transition zone prostate cancer. Eur Radiol. 2017;27:2348–2358. doi: 10.1007/s00330-016-4579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nyholm T., Jonsson J., Söderström K., Bergström P., Carlberg A., Frykholm G. Variability in prostate and seminal vesicle delineations defined on magnetic resonance images, a multi-observer, -center and -sequence study. Radiat Oncol. 2013;126:126–137. doi: 10.1186/1748-717X-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Schie M.A., Dinh C.V., Houdt P.J.V., Pos F.J., Heijmink S., Kerkmeijer L.G.W. Contouring of prostate tumors on multiparametric MRI: evaluation of clinical delineations in a multicenter radiotherapy trial. Radiother Oncol. 2018;128:321–326. doi: 10.1016/j.radonc.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 82.Dinh C.V., Steenbergen P., Ghobadi G., van der Poel H., Heijmink S.W., de Jong J. Multicenter validation of prostate tumor localization using multiparametric MRI and prior knowledge. Med Phys. 2017;44:949–961. doi: 10.1002/mp.12086. [DOI] [PubMed] [Google Scholar]
- 83.Yang X., Liu C., Wang Z., Yang J., Min H.L., Wang L. Co-trained convolutional neural networks for automated detection of prostate cancer in multi-parametric MRI. Med Image Anal. 2017;42:212–227. doi: 10.1016/j.media.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 84.European Imaging Biomarker Alliance (EIBALL). European Society of Radiology 2015; Available from: https://www.myesr.org.
- 85.Quantitative Imaging Biomarker Alliance of RSNA (QIBA). 2014; Available from: http://qibawiki.rsna.org.
- 86.National Cancer Institute (NCI). Quantitative Imaging Network. 2014; Available from: htttp://imaging.cancer.gov.
- 87.Sabouri S., Chang S.D., Savdie R., Zhang J., Jones E.C., Goldenberg S.L. Luminal water imaging: a new MR imaging T2 mapping technique for prostate cancer diagnosis. Radiology. 2017;284:451–459. doi: 10.1148/radiol.2017161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emmett L., Metser U., Bauman G., Hicks R.J., Weickhardt A., Davis I.D. A prospective, multi-site, international comparison of F-18 fluoro-methyl-choline, multi-parametric magnetic resonance and Ga-68 HBED-CC (PSMA-11) in men with high-risk features and biochemical failure after radical prostatectomy: clinical performance and patient outcomes. J Nucl Med. 2018;15 doi: 10.2967/jnumed.118.220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.