Abstract
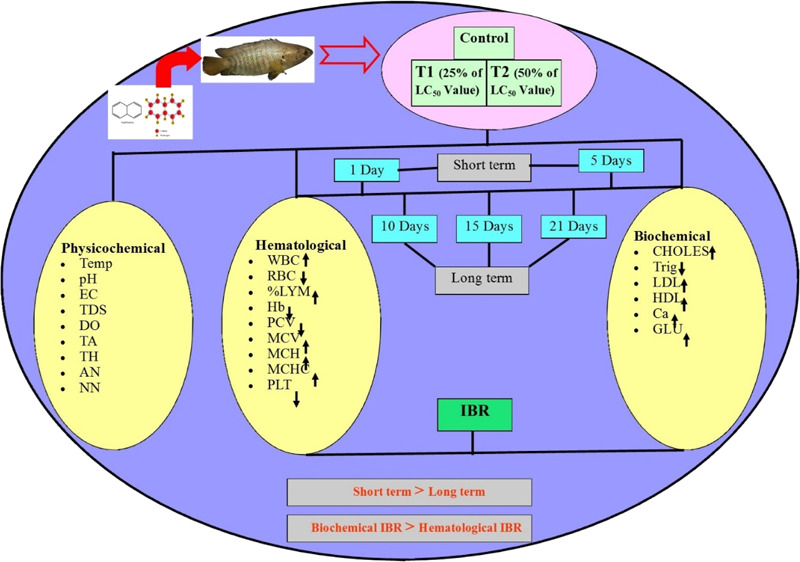
Polyaromatic compounds are the major, widespread contaminants in the aquatic environment. However, the adverse impacts of these compounds on blood pathophysiology (hematological profiling and serum biochemical responses) are poorly understood. As a consequence, this study was intended to evaluate the toxic effects of naphthalene, one of the polycyclic aromatic hydrocarbons, on the blood pathophysiology of Anabas testudineus using multiple end-point biomarker approach. A. testudineus was exposed to short-term (1 and 5 d) and long-term (10, 15, and 21 d) naphthalene concentrations, that is, T1 (0.71 mg/L indicates 25% of LC50) and T2 (1.42 mg/L indicates 50% of LC50 value). The results disclosed significant decrease in red blood cells, hemoglobin (Hb), packed cell volume, and platelet levels, while other blood parameters, namely, white blood cells, percent lymphocyte, mean cell volume, mean corpuscular Hb, and mean corpuscular Hb concentration showed enhanced levels under naphthalene intoxication. Results were more detrimental under T2 concentration. Cholesterol, glucose, calcium, high-density lipoprotein, and low-density lipoprotein levels gradually increased throughout the different exposure periods under T1 and T2 concentrations, while the triglyceride level gradually decreased during exposure periods. Finally, integrated biomarker responses (IBR) analysis indicated that serum biochemical parameters are more powerful than hematological parameters for determining the naphthalene-induced fish health status. Additionally, the IBR study clearly identified that long-term (>5 d) exposure was more harmful than short-term (<5 d) naphthalene exposure. So, these responses may be derived as biomarkers for monitoring naphthalene pollution in an aquatic ecosystem.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are generally organic pollutants containing two or more condensed aromatic rings.1 They are considered as the ninth most threatening compound to human health.2 Contamination of environment by PAHs is now becoming a serious problem worldwide because of massive and irregular extraction of natural resources from the earth. PAHs are widely distributed in aquatic environment including sediments, benthic invertebrates, fish, sea birds, and mammals.3,4 In particular, the naphthalene concentration in sediments ranges between 440 and 264,000 pg/g, in water, it ranges from 0.1 to 10 ng/L, and in biological samples, it ranges from 0.030 to 1.004 μg/g.5,6 Naphthalene addressed here is a PAH that is widely distributed in soil, water, air, and aquatic environments.7,8 Generally, in aquatic environment, PAHs originated from four different sources: petrogenic fuels, incomplete combustion (pyrogenic), organic metabolism (biogenic), and diagenetic transformation in sediments.9 Among these, petrogenic and pyrogenic sources are the major contributors of aquatic pollution by PAHs.10
However, the major cascading aspect of PAHs is their mutagenic and carcinogenic properties.5 First, the hydrophobic nature of PAHs induces gene expression of cytochrome P450 (CYP) family after its entry into cells.11,12 In the next step, the expressed CYP enzyme family metabolizes PAHs to produce either intermediate or final metabolites, which bind directly with DNA to become mutagenic/carcinogenic.10 The International Agency for Research on Cancer classified the PAHs under three major categories and grouped under carcinogenic chemicals (group 2A): benzo[a]pyrene, dibenz[a,h]anthracene, and benzo[a]anthracene. Additionally, the United States Environmental Protection Agency identified 16 major representatives of PAHs as priority one from different sources of emissions. Naphthalene, among them, is the very simplest one which has very low photo-oxidation capability and is highly persistent in aquatic environment.13
Different studies regarding developmental toxicity, oxidative stress, carcinogenicity, immunotoxicity, mutagenicity, and endocrine disruption caused by naphthalene have been documented on aquatic organisms.14−17 However, a dearth of information is still there, particularly on the adverse effects of naphthalene on hematological profiling and serum biochemical responses in fish species, particularly, on air-breathing Indian carnivorous teleosts. Blood parameters play a crucial role in monitoring fish health status under environmental contamination.18 Additionally, our previous study demonstrated the usefulness of blood biochemistry and erythrocyte pathology to assess the adverse effects of a large number of xenobiotics, including naphthalene, and considered them as a convenient and reliable diagnostic tool.19 Further, dose-and-time dependent studies of naphthalene toxicity on hematological and serum biochemical parameters are very scanty. The teleost, Anabas testudineus (Bloch), possesses both ephemeral and aestivating (burying under moist ground) behavior against competition or contamination defenses. This behavior specifically is considered as a key characteristic of A. testudineus for successful survival under contaminated environment. Generally, the impact of these xenobiotics is concerned not only with specific toxicity but also involvement of multiple processes.10 Therefore, linking information from multiple endpoint biomarker responses can provide valuable insight to evaluate the impact of PAHs in aquatic environment.20 In this regard, integrated biomarker responses (IBRs) serve as a prominent tool to evaluate adverse impacts of xenobiotic compounds. Apart from this, there is no available information on mechanistic linkage among biomarkers based on hematological profiling and serum biochemical parameters under naphthalene intoxication in A. testudineus. Accordingly, hematological profiling and serum biochemical parameters could be considered as early warning indicators of PAH pollution to understand the adverse outcomes as they are detected at very low exposures.21,22
This study, for the first time, reports naphthalene-induced hematological profiling and serum biochemical responses in A. testudineus under short-term (1 and 5 d) and long-term (10, 15, and 21 d) exposures. Second, this study will explore the relationship among multiple endpoint biomarkers through IBR analysis and finally establish A. testudineus as the bioindicator species of naphthalene intoxication. A. testudineus was considered as a model organism because of its capability to provide early warning signal under both laboratory and field exposures of xenobiotics.18,23 Additionally, it is widely distributed in natural environment, easily acclimated under laboratory environment, and has high commercial value as a protein source.18
2. Results and Discussion
2.1. Limnological Parameters
During experimentation, there was no mortality of fish under naphthalene exposure and control condition. Results of limnological parameters during the entire exposure period are presented in Table 1. The pH, electrical conductivity (EC), and total hardness (TH) are considered as important factors regulating the bioavailability and associated toxicity of xenobiotic compounds.24,25 In our study, these parameters (except hardness) showed significant changes; the dissolved oxygen (DO) concentration was significantly reduced under naphthalene exposure compared with the control condition, but it was well above the standard value of 4 mg/L which is considered as critical for fish health.26 This indicated that fish experienced higher metabolic activity as adaptive responses to compensate the stress since it is directly linked with oxygen consumption.27,28 Accordingly, the fecal matter production was increased, which was evidenced as higher ammonia-nitrogen (AN) level.24 Therefore, these inclinations in water quality parameters are linked to the toxic responses on blood because of naphthalene exposure on A. testudineus.
Table 1. Analysis of Physicochemical Parameters of Water under Control and Naphthalene Exposuresa.
| parameters | control | T1 | T2 |
|---|---|---|---|
| temperature (°C) | 22.0 ± 0.94 | 22.1 ± 1.35 | 21.8 ± 0.33 |
| pH | 7.1 ± 0.05a | 7.1 ± 0.01b | 7.2 ± 0.03c |
| electrical conductivity (EC), μS/cm | 448.9 ± 3.17a | 460.3 ± 3.74b | 451.5 ± 3.14a |
| total dissolved solids (TDS), mg/L | 320.8 ± 3.14 | 321.4 ± 2.08 | 319.2 ± 1.17 |
| dissolved oxygen (DO), mg/L | 6.9 ± 0.09a | 6.6 ± 0.19ab | 6.4 ± 0.31b |
| total hardness (TH, CaCO3), mg/L | 134.1 ± 2.94 | 144.3 ± 13.18 | 143.5 ± 14.18 |
| ammonia nitrogen (AN), mg/L | 1.7 ± 0.12 | 1.7 ± 0.21 | 1.8 ± 0.09 |
| nitrate nitrogen (NN), mg/L | 0.4 ± 0.03a | 0.4 ± 0.03b | 0.5 ± 0.03c |
Different letters represent significant differences among different exposure conditions for each parameter at p < 0.05 (one-way ANOVA).
2.2. Hematological Responses
During the naphthalene exposure, the white blood cell (WBC) count in A. testudineus increased compared with the control, and this increment was comparatively higher under T2 dose (Table 2 and S1). This increment indicated higher production of lymphocytes (LYMs) from lymphomyeloid tissues through the process of stimulated lymphopoiesis.29 However, the increment was gradually decreased in a time-dependent way under both T1 and T2 conditions (Table 2 and S1). Decreased WBC trend, also called lymphopenia, was also defined in Lepomis sp. by Lohner et al.(30) and in Prochilodus linestus by Bacchetta et al.(31) during over exposure. These alterations in WBC count may lead to development of immunotoxic effects in A. testudineus by naphthalene exposure.
Table 2. Hematological Parameters in A. testudineus under Naphthalene Exposuresa.
| WBC |
RBC |
%LYM |
Hb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| condition | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 |
| 1 day | 268.23 ± 1.72a | 279.35 ± 1.31b | 294.63 ± 1.43c | 3.92 ± 0.08a | 3.76 ± 0.05b | 3.68 ± 0.03b | 92.52 ± 0.54a | 93.84 ± 0.13b | 94.27 ± 0.06c | 10.11 ± 0.18a | 8.84 ± 0.18b | 7.96 ± 0.11c |
| 5 days | 268.23 ± 1.73a | 277.77 ± 1.19b | 291.12 ± 1.03c | 3.92 ± 0.08a | 3.71 ± 0.04b | 3.62 ± 0.05b | 92.52 ± 0.54a | 93.69 ± 0.07b | 93.92 ± 0.11b | 10.11 ± 0.18a | 8.69 ± 0.13b | 7.85 ± 0.09c |
| 10 days | 268.23 ± 1.74a | 276.29 ± 1.11b | 289.58 ± 1.29c | 3.92 ± 0.08a | 3.66 ± 0.05b | 3.54 ± 0.03c | 92.52 ± 0.54a | 93.57 ± 0.06b | 93.68 ± 0.08b | 10.11 ± 0.18a | 8.61 ± 0.09b | 7.73 ± 0.16c |
| 15 days | 268.23 ± 1.75a | 275.92 ± 1.21b | 289.25 ± 1.15c | 3.92 ± 0.08a | 3.58 ± 0.08b | 3.48 ± 0.06b | 92.52 ± 0.54a | 93.39 ± 0.11b | 93.47 ± 0.06b | 10.11 ± 0.18a | 8.53 ± 0.11b | 7.64 ± 0.07c |
| 21 days | 268.23 ± 1.76a | 274.98 ± 0.97b | 289.06 ± 0.92c | 3.92 ± 0.08a | 3.49 ± 0.05b | 3.41 ± 0.04b | 92.52 ± 0.54a | 93.04 ± 0.08b | 93.36 ± 0.07b | 10.11 ± 0.18a | 8.47 ± 0.12b | 7.52 ± 0.09c |
| PCV |
MCV |
MCH |
MCHC |
PLT |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| condition | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 |
| 1 day | 41.17 ± 0.79a | 37.04 ± 0.29b | 35.12 ± 0.18c | 104.97 ± 1.21a | 119.72 ± 0.97b | 128.07 ± 1.17c | 30.72 ± 0.42a | 35.07 ± 0.13b | 40.92 ± 0.36c | 28.87 ± 0.49a | 30.54 ± 0.21b | 31.96 ± 0.33c | 49.12 ± 0.69a | 45.21 ± 0.21b | 42.87 ± 0.14c |
| 5 days | 41.17 ± 0.79a | 36.89 ± 0.13b | 34.93 ± 0.13c | 104.97 ± 1.21a | 120.31 ± 0.31b | 128.98 ± 1.03c | 30.72 ± 0.42a | 35.81 ± 0.17b | 41.21 ± 0.21c | 28.87 ± 0.49a | 31.11 ± 0.29b | 32.82 ± 0.45c | 49.12 ± 0.69a | 44.78 ± 0.17b | 42.02 ± 0.17c |
| 10 days | 41.17 ± 0.79a | 36.51 ± 0.19b | 34.69 ± 0.17c | 104.97 ± 1.21a | 120.97 ± 1.07b | 129.76 ± 0.92c | 30.72 ± 0.42a | 36.24 ± 0.11b | 41.75 ± 0.27c | 28.87 ± 0.49a | 31.69 ± 0.33b | 33.97 ± 0.27c | 49.12 ± 0.69a | 44.19 ± 0.23b | 41.54 ± 0.09c |
| 15 days | 41.17 ± 0.79a | 36.07 ± 0.15b | 34.42 ± 0.21c | 104.97 ± 1.21a | 121.83 ± 0.83b | 130.42 ± 1.07c | 30.72 ± 0.42a | 36.78 ± 0.22b | 42.64 ± 0.29c | 28.87 ± 0.49a | 32.04 ± 0.41b | 34.64 ± 0.22c | 49.12 ± 0.69a | 43.64 ± 0.19b | 39.96 ± 0.11c |
| 21 days | 41.17 ± 0.79a | 35.94 ± 0.17b | 34.04 ± 0.15c | 104.97 ± 1.21a | 122.54 ± 1.17b | 131.05 ± 1.11c | 30.72 ± 0.42a | 37.17 ± 0.19b | 43.02 ± 0.16c | 28.87 ± 0.49a | 32.79 ± 0.24b | 35.01 ± 0.36c | 49.12 ± 0.69a | 42.98 ± 0.17b | 39.42 ± 0.23c |
Different letters represent significant differences among different naphthalene exposure conditions within each exposure day for a particular parameter at p < 0.05 (one-way ANOVA).
Contrarily, the red blood cell (RBC) count in A. testudineus under naphthalene exposure (both T1 and T2) was significantly reduced in comparison to the control condition (Table 2 and S1). Reduced RBC level indicated that impaired erythropoies is due to lower oxygen carrying capacity, which helps to develop both anemia and hypoxic condition.23 Comparatively, higher reduction was observed in T2 dose than T1 (Table 2 and S1), which indicated that higher dose caused more adverse impacts and reduced compensatory mechanism. Additionally, reduced number of RBC indicated the poor metabolism of iron.32 Furthermore, there was a time-dependent monotonically decreased trend of RBC count under both T1 and T2 doses, which indicated the serious impairment of fish health under long-term naphthalene exposure. Similar findings have also been reported in different fish species under xenobiotic exposures by several authors.33−35
During the naphthalene exposure in A. testudineus, the LYM count gradually decreased compared with the control, and this reduction was comparatively higher in T2 (Table 2 and S1). During short-term exposure, higher LYM count indicated higher adaptive capability against xenobiotic stress by A. testudineus through immune stimulation and lymphocytosis.36 However, the time-dependent gradual reduction under long-term T1 and T2 exposures indicated that long-term naphthalene exposure adversely affects the fish health. Time-dependent reduced LYM counts recorded under this study was also defined in Oreochromis niloticus by Samanta et al.(23)
Hemoglobin (Hb) concentration in A. testudineus under T1 and T2 conditions gradually decreased significantly in comparison to the control condition (Table 2 and S1). Reduction in Hb concentration indicated impaired supply of oxygen to different tissues, resulting in decreased metabolic function and reduced energy generation, which ultimately resulted in reduced adaptive responses to naphthalene intoxication.23 Additionally, lower Hb level promotes the development of anemia and hypoxic condition within fish.23 Accordingly, reduced Hb level could be the possible reason for lower RBC count in this study. Likely, Suvetha et al.(37) in their study correlated reduced Hb concentration with lower RBC level because of impaired formation of RBC. Furthermore, time-dependent gradual decrease indicated impaired fish health under long-term naphthalene exposure. Comparatively higher reduction under T2 dose than T1 indicated more adverse impacts of naphthalene intoxication and reduced compensatory mechanism.
Likely, the packed cell volume (PCV) level in A. testudineus under naphthalene exposure gradually decreased significantly in a time-dependent manner in comparison to the control condition (Table 2 and S1). Reduced PCV was caused due to deformation of RBC and/or due to reduced RBC synthesis.23 In addition, this is because it is influenced both by the number of blood cells and their size.35 This reduced PCV level indicated impaired metabolic activities and lower adaptive capability. Therefore, higher the reduction, higher is the metabolic disruption and deterioration of fish health. Accordingly, T2 dose could be more detrimental to A. testudineus than T1. Additionally, lower PCV level in combination with reduced RBC and Hb levels indicated development of anemia within erythropoietic tissues.23,38, Hussein et al.(39) also demonstrated the higher reduction in PCV value in Carassius auratus and O. nitoticus at 6 mg/L atrazine than 3 mg/L atrazine exposure because of changes in the RBC turnover rate. The mean cell volume (MCV) showed significant gradually increased time-dependent symptoms after naphthalene intoxication as compared to the control value, with higher increment in T2 than in T1 dose (Table 2 and S1), which indicated the stress condition of fish. Therefore, higher the MCV value, higher is the stress that is reflected under long-term exposure duration and T2 dose. Generally, increased MCV value under naphthalene exposure is an indicator of either hypoxic or anoxic condition for fish.23 Harding and Hoglund40 explained this enhanced response of MCV with swelled RBC and development of anemia (macrocytic nesmochromic type). In another study, Nikinmaa and Jensen41 corroborated swelled erythrocytes with physiological adjustment mainly due to changes in blood-oxygen tension pressure.
The mean corpuscular Hb concentration (MCHC) and the mean corpuscular hemoglobin (MCH) level in A. testudineus under naphthalene exposure (both T1 and T2) gradually increased significantly under time-dependent exposure in comparison to the control condition (Tables 2 and S1), which indicated that A. testudineus is under stress condition. This increment in MCH and MCHC levels was comparatively higher under T2 than under T1 dose. Generally, increased MCH and MCHC is linked with RBCs’ proliferation and anemic situation (macrocytic nesmochromic type), which might have influenced the Hb synthesis.23,37 Ada et al.(35) demonstrated that enhanced MCH level prevents blood cell multiplication to maintain constant Hb level within each cell, including the blood cell. In particular, enhanced MCH count indicated that stress situation resulted from pathological lesions of the concerned tissues,42 while MCHC is considered as a significant indicator of RBC proliferation.23 Collectively, these increasing trends of MCHC and MCH might have collapsed the Hb level (too low) because of changes in the RBC turnover rate in A. testudineus under naphthalene intoxication.
Platelet (PLT) count in A. testudineus under naphthalene exposure gradually decreased significantly in comparison to the control condition (Table 2 and S1). This reduction in PLT level was comparatively higher in T2 condition than in T1 condition. This reduced platelet content may be because of thrombostain content.23 In general, under stress conditions, the thrombocyte count increased significantly as the blood clotting process becomes more effective.23
2.3. Serum Biochemical Responses
Resistance to xenobiotic exposure generally depends on immediate response carried out by serum soluble molecules in fish.43 Data on serum biochemical responses of A. testudineus under naphthalene exposure is presented in Table 3. A time-dependent significant gradual increase in cholesterol (CHOLES) level, a major component of total lipids, in A. testudineus under naphthalene exposure was recorded in comparison to the control condition (Table 3 and S2). Additionally, comparatively higher CHOLES level was recorded under T2 than under T1 dose. A similar assessment was also done by Gaber et al.(44) in Clarias gariepinus collected from El-Rahawy drainage canal. This elevated CHOLES level could probably be due to higher metabolic cost either through higher oxidation or gradual instauration of lipid content.45 Membrane degeneration could probably be another reason for its elevation because they are an integral component of the cell membrane.46 Apart from this protein catabolism, glycogenolysis process, disturbance of lipid metabolism, and liver dysfunction might influence the higher production of CHOLES level.46,47 In addition, this higher CHOLES level might influence the impairment of signal transduction processes through alteration of different molecular recognition pathways.46,48 On the contrary, triglyceride (Trig), the other major component of total lipids, showed a time-dependent significant gradual reduction under naphthalene exposure, showing higher reduction in T2 dose (Table 3 and S2). During short-term exposure (1 and 5 d), the Trig level showed positive elevation, but after that, the Trig level was minimally reduced compared with the control value. The reduced value, that is, higher mobilization of Trigs during long-term (>5 d) naphthalene intoxication, could be explained by the constant energy demand to compensate the stress as they acted as lipid depots.46 This could also be corroborated with their role in membrane biogenesis to recover from membrane degeneration caused by the elevated level of CHOLES.48,49 Additionally, lowered Trig level might also be because of lower intake of feed and their subsequent lower absorption into gut lumen because of damage in the digestive tract and improper liver synthesis.46 Therefore, these changes in CHOLES and Trig levels could be considered as important diagnostic characters of naphthalene toxicity.
Table 3. Serum Biochemical Parameters in A. testudineus under Naphthalene Exposuresa.
| CHOLES (mg/dl) |
Trig (mg/dl) |
LDL (mg/dl) |
HDL (mg/dl) |
Ca (mg/dl) |
GLU (mg/dl) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| condition | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 | C | T1 | T2 |
| 1 day | 581 ± 5.28a | 783 ± 7.69b | 744 ± 6.21c | 470.6 ± 11.68a | 517.5 ± 12.61b | 545 ± 12.69c | 138.4 ± 1.52a | 114.6 ± 1.31b | 107.6 ± 1.28c | 72.4 ± 1.42a | 62.43 ± 1.48b | 58.7 ± 1.39c | 5.8 ± 0.24a | 4.8 ± 0.52b | 4.6 ± 0.47b | 172.2 ± 3.14a | 204 ± 4.22b | 209 ± 4.39b |
| 5 days | 581 ± 5.28a | 847 ± 8.36b | 880 ± 8.67c | 470.6 ± 11.68a | 495 ± 11.79b | 502 ± 11.97b | 138.4 ± 1.52a | 139.7 ± 1.47a | 141.2 ± 1.53a | 72.4 ± 1.42a | 66.5 ± 1.67b | 71.9 ± 1.73a | 5.8 ± 0.24a | 5.1 ± 0.38b | 5.4 ± 0.31b | 172.2 ± 3.14a | 218.5 ± 5.06b | 229.6 ± 4.97c |
| 10 days | 581 ± 5.28a | 896 ± 8.91b | 949 ± 7.48c | 470.6 ± 11.68a | 446 ± 12.07b | 468 ± 12.31ab | 138.4 ± 1.52a | 148.3 ± 1.46b | 152.3 ± 1.31c | 72.4 ± 1.42a | 81.2 ± 1.8b | 86.5 ± 1.71c | 5.8 ± 0.24a | 5.9 ± 0.27ab | 6.3 ± 0.29b | 172.2 ± 3.14a | 239.4 ± 3.58b | 258.2 ± 3.16c |
| 15 days | 581 ± 5.28a | 926 ± 9.07b | 983 ± 8.94c | 470.6 ± 11.68a | 407 ± 12.21b | 427 ± 11.92c | 138.4 ± 1.52a | 156.4 ± 1.21b | 161.9 ± 1.29c | 72.4 ± 1.42a | 99.8 ± 1.32b | 97.6 ± 1.48c | 5.8 ± 0.24a | 6.5 ± 0.34b | 6.8 ± 0.31c | 172.2 ± 3.14a | 247 ± 4.02b | 265.5 ± 4.31c |
| 21 days | 581 ± 5.28a | 956 ± 7.53b | 1003 ± 7.02c | 470.6 ± 11.68a | 396 ± 10.29b | 412 ± 10.41b | 138.4 ± 1.52a | 167.6 ± 1.61b | 173.8 ± 1.68c | 72.4 ± 1.42a | 106.4 ± 1.5b | 114.8 ± 1.56c | 5.8 ± 0.24a | 7.2 ± 0.41b | 7.5 ± 0.44c | 172.2 ± 3.14a | 263 ± 4.19b | 278.4 ± 4.46c |
Different letters represent significant differences among different naphthalene exposure conditions within each exposure day for a particular parameter at p < 0.05 (one-way ANOVA)
Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) are Trig/CHOLES-rich lipoproteins; therefore, their concentrations are dependent on Trig/CHOLES fraction.48 In this study, LDL and HDL levels showed significant gradual increase in a time-dependent manner under naphthalene exposure compared with the control condition (Tables 3 and S2). Higher naphthalene dose, that is, T2, displayed higher LDL and HDL levels. Increasing patterns of LDL and HDL levels were also observed in a time-dependent manner in A. testudineus under anthracene exposure.50 Additionally, Kojima et al.(51) corroborated higher LDL with changes in hepatic enzyme gene expression such as hydroxyl-methylglutaryl-CoA reductase, which suppresses the expression of LDL-receptor gene. However, during short-term exposure, the LDL (1 d) and HDL (1 and 5 d) levels were decreased significantly compared with the control value. Javed et al.(46) explained this reduced HDL during short-term exposure as due to abrupt development of lipid peroxidation since HDL scavenges CHOLES from the liver tissue. Therefore, decrease in the HDL level in the present study could be corroborated with increased CHOLES contents.52 Contrarily, higher HDL level in this study during long-term exposure could be explained by higher CHOLES level as HDL played a vital role in transporting either reserve CHOLES or excess CHOLES from nonhepatic cells to the liver through the blood.53 On the other hand, decrease in the LDL level during short-term naphthalene exposure in the present study could be corroborated with increased Trig contents. As time proceeds, that is, during long-term naphthalene exposure, the LDL level increased gradually, which could be correlated with a decrease in the Trig level. Therefore, it can be concluded that the LDL level is indirectly proportional to the Trig fraction, while the HDL level is directly proportional to the CHOLES fraction.
Calcium content in A. testudineus during short-term naphthalene exposure (1 and 5 d) reduced significantly, but after that it gradually increased in a time-dependent manner (Tables 3 and S2). The maximum increment and reduction were found after 21 d followed by 1 d exposure under T2 dose (Table 3 and S2). Naphthalene exposure in this study showed a diverse transient effect on calcium metabolism and homeostasis.54,55 During short-term exposure, lower calcium content may be possibly due to enhanced calcium excretion.41 Similar findings have also been reported by Ko et al.(56) in Platichthys stellatus under chromium exposure. As time proceeds (after 5 d), the calcium level gradually increased, which indicated that naphthalene might have influenced the hypercalcemic hormone from the pituitary gland.57,58 Generally, prolactin serves as a precursor of hypercalcemic hormone in teleosts58,59 to improve the calcium reserve. Therefore, it can be concluded that naphthalene regulated calcium metabolism differently to maintain the homeostasis as a compensatory response. In the present study, glucose level significantly increased in a time-dependent manner under naphthalene exposure. Higher the naphthalene dose, higher was the response, that is, under T2 dose. Serum glucose is a reliable biomarker of xenobiotic exposure including PAHs and is generally increased by carbohydrate metabolism.56 A gradual increase in the glucose level was indicative of intensive glycogenolysis (a process of releasing glucose into blood by breakdown of energy reserves, namely, proteins and HDL stored as glycogen in the liver and muscle), initiated generally by adrenocorticotrophic and glucagon hormones such as cortisol, glucose 6-phosphatase, catecholamines, and so forth.60−62 Additionally, higher glucose level indicated higher stress condition as adaptive responses. These findings indicated disrupted carbohydrate metabolism under naphthalene intoxication.
2.4. IBR
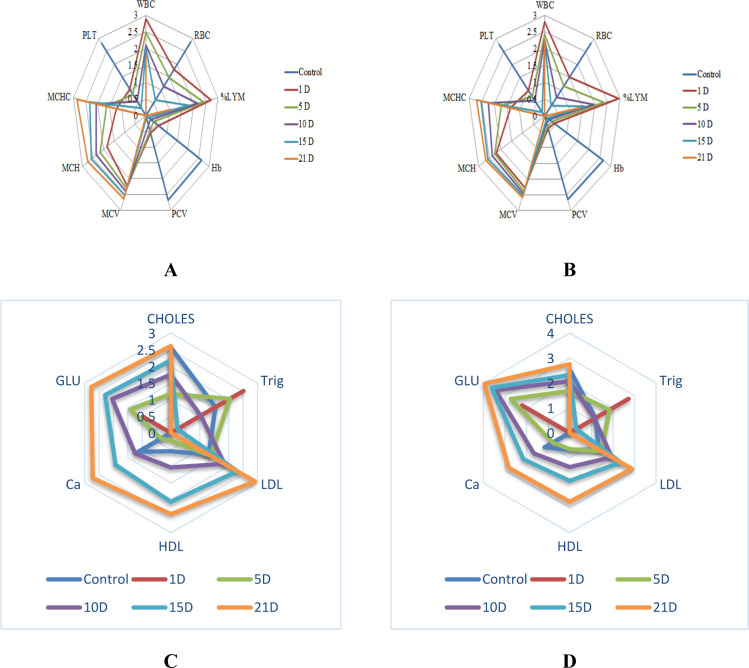
The IBR index determined by integrating individual biomarker responses is tabulated in (Tables 4 and 5). The values widely varied for different conditions. At the serum biochemical level, comparatively higher IBR value was observed at higher concentration under different treatment conditions, indicating that long-term (>5 d) naphthalene exposure was more detrimental to A. testudineus. During short-term exposure (<5 d), the IBR value was less compared with that of the control, indicating that short-term naphthalene exposure was less detrimental. Contrarily, higher IBR value was observed during short-term (<5 d) naphthalene exposure, and after that, it was gradually decreasing in a time-dependent manner. Recorded IBRs in this study were also in consonance with23 the IBR values calculated by using hematological measurements, viz., RBC, WBC, PCV, MCV, %LYM, MCH, MCHC, Hb, and PLT. Moreover, higher IBR values were recorded at T2 dose compared to T1 for both serum biochemical and hematological markers, indicating that higher naphthalene concentration (T2) was more detrimental to A. testudineus than lower concentrations (T1). Furthermore, higher IBR values for serum biochemical parameters suggested that serum biochemical parameters were more determinant (as reflected in the correlation study) for evaluating the naphthalene impacts on fish health compared to hematological parameters, as higher IBR value indicates higher stress. Likely, star plot representation revealed a totally different pattern (Figure 1A–D) for both serum biochemical and hematological parameters, which was generally used to identify predominant markers of xenobiotic stress.63 Star plot representation depicted that glucose and LDL were highly responsive among the serum biochemical markers followed by CHOLES, HDL, and calcium under T1 dose. At T2 dose, glucose and CHOLES were more responsive followed by LDL, calcium, and HDL. On the other hand, among the hematological markers, RBC and LYM were highly responsive followed by WBC and PLT under both T1 and T2 conditions in the descending order, while MCV and MCH were prominent in the ascending order.
Table 4. Standardized Biomarker Response Values of Hematological Parameters in A. testudineus During Naphthalene Exposures and Corresponding IBR Values.
| condition | duration | RBC | WBC | %LYM | Hb | PCV | MCV | MCH | MCHC | PLT | IBR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 2.88 | 0 | 2.65 | 2.67 | 0 | 0 | 0 | 2.82 | 4.25 | |
| 1D | 2.8975 | 1.81 | 2.71 | 0.59 | 0.56 | 2.22 | 1.84 | 1.22 | 1.03 | 11.75 | |
| T1 | 5D | 2.48 | 1.48 | 2.39 | 0.36 | 0.48 | 2.31 | 2.16 | 1.64 | 0.83 | 10.62 |
| 10D | 2.09 | 1.14 | 2.15 | 0.23 | 0.29 | 2.41 | 2.34 | 2.06 | 0.56 | 9.69 | |
| 15D | 2.00 | 0.60 | 1.78 | 0.09 | 0.07 | 2.53 | 2.57 | 2.32 | 0.30 | 8.89 | |
| 21D | 1.76 | 0 | 1.07 | 0 | 0 | 2.64 | 2.73 | 2.87 | 0 | 8.89 | |
| 0 | 0 | 2.83 | 0 | 2.64 | 2.65 | 0 | 0 | 0 | 2.786 | 4.21 | |
| 1D | 2.80 | 1.49 | 2.94 | 0.45 | 0.40 | 2.28 | 2.19 | 1.36 | 0.99 | 10.88 | |
| T2 | 5D | 2.43 | 1.16 | 2.35 | 0.34 | 0.33 | 2.37 | 2.26 | 1.74 | 0.74 | 9.67 |
| 10D | 2.27 | 0.72 | 1.95 | 0.21 | 0.24 | 2.45 | 2.38 | 2.25 | 0.61 | 9.12 | |
| 15D | 2.23 | 0.39 | 1.59 | 0.12 | 0.14 | 2.51 | 2.57 | 2.54 | 0.15 | 8.63 | |
| 21D | 2.21 | 0 | 1.41 | 0 | 0 | 2.57 | 2.65 | 2.71 | 0 | 8.34 |
Table 5. Standardized Biomarker Response Values of Serum Biochemical Parameters in A. testudineusDuring Naphthalene Exposures and Corresponding IBR Values.
| condition | duration | CHOLES | Trig | LDL | HDL | Ca | GLU | IBR |
|---|---|---|---|---|---|---|---|---|
| 0 | 2.57 | 1.55 | 1.31 | 0.55 | 1.13 | 0 | 3.46 | |
| 1D | 0 | 2.52 | 0 | 0 | 0 | 0.97 | 2.41 | |
| T1 | 5D | 1.17 | 2.05 | 1.38 | 0.23 | 0.34 | 1.41 | 2.77 |
| 10D | 1.75 | 1.04 | 1.86 | 1.04 | 1.24 | 2.04 | 7.89 | |
| 15D | 2.18 | 0.23 | 2.31 | 2.07 | 1.92 | 2.28 | 11.71 | |
| 21D | 2.61 | 0 | 2.92 | 2.44 | 2.71 | 2.76 | 17.35 | |
| 0 | 2.55 | 1.2 | 1.35 | 0.67 | 1.16 | 0 | 3.48 | |
| 1D | 0 | 2.73 | 0 | 0 | 0 | 2.2 | 2.41 | |
| T2 | 5D | 1.68 | 1.85 | 1.47 | 0.65 | 0.78 | 2.72 | 6.70 |
| 10D | 2.07 | 1.15 | 1.95 | 1.37 | 1.65 | 3.43 | 12.76 | |
| 15D | 2.32 | 0.31 | 2.37 | 1.92 | 2.13 | 3.61 | 15.34 | |
| 21D | 2.75 | 0 | 2.89 | 2.76 | 2.81 | 3.94 | 21.91 |
Figure 1.
(A–D): Star plots for hematological parameters (A,B) and serum biochemical parameters (C,D) of A. testudineus under T1 and T2 naphthalene exposures, respectively.
Correlation study between limnological parameters and biochemical and hematological parameters was varied in A. testudineus under different naphthalene exposures (Table 6). In particular, the hematological IBR value in A. testudineus was significantly correlated with total dissolved solids (TDS, −0.967). The negative correlation indicated the negligible effect of TDS on the hematological alterations in fish. In addition, the positive correlation was recorded between biochemical IBR and water parameters, namely, TH, AN, and nitrate nitrogen (NN) under both naphthalene conditions. The positive correlation indicates that these variables caused energetic stress in A. testudineus, and the observed biochemical and hematological inductions, at least in part, were attributable to these parameters. Samanta et al.(63) reported that similar results have been reported in Z. koreanus collected from Bucheon stream. Moreover, the comparatively higher positive correlation between water parameters and biochemical IBR values than hematological IBR values indicated that biochemical IBR values are more determinant and independent than hematological parameters. These findings indicated that integration of different individual biomarker responses was better to explain the adverse effects than individual responses. Additionally, it can be stated that the IBR index could also be used as a powerful technique to distinguish the short-term and long-term toxicological effects, specifically to a particular type of xenobiotic, naphthalene, in aquatic environment.
Table 6. Correlation Coefficients (n = 6) Between Limnological Parameters and IBR Index of Hematological and Biochemical Alterations in A . testudineus under Naphthalene Exposures.
| hematological
IBR |
biochemical
IBR |
|||
|---|---|---|---|---|
| parameters | T1 | T2 | T1 | T2 |
| temperature | –0.797 | 0.207 | –0.150 | –0.052 |
| pH | –0.145 | –0.307 | –0.325 | 0.084 |
| EC | –0.192 | 0.006 | –0.184 | 0.365 |
| TDS | –0.192 | –0.967a | –0.184 | 0.174 |
| DO | 0.091 | –0.035 | 0.356 | –0.249 |
| TH | –0.246 | –0.725 | 0.420 | 0.259 |
| AN | 0.536 | –0.161 | 0.553 | 0.206 |
| NN | 0.064 | 0.027 | 0.346 | 0.259 |
p < 0.05, p < 0.01.
3. Conclusions
The present findings established the time-dependent chronic effects of naphthalene (PAH) on hematological and serum biochemical levels of A. testudineus (Bloch). Results inferred that responses were exposure-specific as well as dose-specific, and fish species were adversely affected by naphthalene toxicosis. IBR analysis depicted that serum biochemical parameters were more determinant for evaluating the naphthalene impacts than hematological parameters. Gradual detrimental symptoms were more adverse under long-term (>5 d) exposure than under short-term (<5 d) naphthalene exposure. So, these responses may be deliberated as a biomarker for monitoring PAH pollution, especially, naphthalene, in aquatic ecosystem.
4. Experimental Section
4.1. Fish Collection and Nourishment
Air-breathing, carnivorous, adult freshwater Indian teleost A. testudineus(Bloch) of both sexes having average weight and length of 34.24 ± 1.32 g and 10.68 ± 0.82 cm, respectively, were collected from a local authorized fish farm (n = 150) and acclimatized in laboratory conditions for 21 days in a 350 L capacity aquarium. Fish collection and nourishment were performed following the guidelines described by Dey et al.(50) Water was replaced every alternate day during the experimentation period.
4.2. Laboratory Setup for the Experiment
Fresh healthy acclimatized fish were grouped under three categories: one group representing control and other two groups for naphthalene exposure, that is, T1 (0.71 mg/L) and T2 (1.42 mg/L), respectively. T1 and T2 concentrations represent 25 and 50% of the LC50 value, that is, 2.83 mg/L, respectively. The LC50 value used in this study was determined and recommended by dos Santos et al.64 Each condition was run as triplicate for 1, 5, 10, 15, and 21 day(s), and each aquarium (60 L capacity) contained 10 fish. One (1) and 5 days were considered as short-term exposure condition, while 10, 15, and 21 d were considered as long-term exposure condition. Primarily, naphthalene as per required dose was dissolved in 2 mL of alcohol (95%). Later, stock solutions were prepared in sufficient quantity by dissolving the required quantities of solution in water. We considered 10 fish per aquarium to determine the statistical differences more accurately. The experiment was conducted at the Department of Environmental Science, The University of Burdwan following the toxicological protocol laid down by the university. Water was replaced every alternate day.
4.3. Sampling for Hematological Study
During the entire experiment, water quality was maintained and monitored on regular basis every alternate day.65 Blood samples seven in number in replica were collected from the same tagged fish during the total experimental schedule periods, viz., 1 and 5 d (short-term) and 10, 15, and 21 d (long-term) from all sets. The sampling of the blood sample for the hematological study was done following the methodology established by Dey et al.,50 and blood parameters, namely, WBC, RBC, Hb, percent lymphocyte (%LYM), PCV, MCV, MCH, MCHC, and (PLT), were analyzed by a SYSMEX KX-21 N hematology analyzer.
For serum biochemical analysis, blood samples were collected and prepared based on our previous published paper.66 The different bioenzymological parameters were measured as follows: CHOLES by ERBA kit (BLT00035); Trig by ERBA kit (BLT00058); HDL by ERBA kit (BLT00028), LDL by ERBA kit (BLT00041); calcium (Ca) by ERBA kit (BLT00016); and glucose by ERBA kit (BLT00027).
4.4. IBR
IBR analysis was performed according to ref (67) with some modifications as per ref (50). At first, the mean (x) and standard deviation (SD) for each hematological and serum biochemical parameter under each condition were calculated. In the next step, the standardization value (Z) was calculated using the equation Z = (x – m)/SD, where, “m” is the mean of each biomarker value. The standardization value (Z) will be positive for activation or enhancement, while Z will be negative in case of inhibition or reduction. Using standardized data, the final score (S) value was analyzed by the following equation to calculate the area value (star plot)
where S ≥ 0 and |Min| were minimum absolute values. This area value was used to calculate the IBR value by the equation
where Si and Si+1 are star plot radius coordinates between two clockwise consecutive values and n is the number of corresponding biomarker radii. Higher IBR value indicates higher toxic stress and more determinant to toxicant exposure.63
4.5. Statistical Analysis of the Study
One-way analysis of variance (ANOVA) followed by Tukey test was used to check the differences among naphthalene exposure conditions within each exposure day and among exposure durations within each naphthalene exposure (mean ± SD) using SPSS (v25) at p < 0.05. “Two-sampled paired t-test” was used to check the differences between control and treatment conditions within each exposure at p < 0.05. Pearson’s correlation study was also performed by SPSS (v25) at p < 0.05.).
Acknowledgments
The authors are indebted to the Department of Environmental Science, The University of Burdwan for providing us the infrastructure support through the laboratory facility furnished under the sponsorship of DST-FIST program.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04603.
Percentage of increase (+) or decrease (−) of different hematological parameters in A. testudineus under naphthalene exposures and percentage of increase (+) or decrease (−) of different serum biochemical parameters in A. testudineus under naphthalene exposures (PDF)
Author Present Address
§ Ecotoxicology Lab, Department of Environmental Science, The University of Burdwan, Golapbag, Burdwan 713104, West Bengal, India.
The authors declare no competing financial interest.
Supplementary Material
References
- Collier T. K.; Anulacion B. F.; Arkoosh M.; Dietrich J.; Incardona J.; Johnson L.; Ylitalo G.; Myers M. Effects on Fish of Polycyclic Aromatic Hydrocarbons (PAHS) and Naphthenic Acid Exposures. Fish Physiol. Organic Chem.Toxicol. Fish. 2013, 33, 195–255. 10.1016/b978-0-12-398254-4.00004-2. [DOI] [Google Scholar]
- King S.; Meyer J. S.; Andrews A. R. J. Screening method forpolycyclic aromatic hydrocarbons in soil using hollow fibremembrane solvent microextraction. J. Chromatogr. A 2002, 982, 201–208. 10.1016/s0021-9673(02)01594-7. [DOI] [PubMed] [Google Scholar]
- Hu G.; Sun C.; Li J.; Zhao Y.; Wang H.; Li Y. POPs accumulated in fish and benthos bodies taken from Yangtze River in Jiangsu area. Ecotoxicol 2009, 18, 647–651. 10.1007/s10646-009-0341-2. [DOI] [PubMed] [Google Scholar]
- Miki S.; Uno S.; Ito K.; Koyama J.; Tanaka H. Distributions of polycyclic aromatic hydrocarbons and alkylated polycyclic aromatic hydrocarbons in Osaka Bay, Japan. Mar. Pollut. Bull. 2014, 85, 558–565. 10.1016/j.marpolbul.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Hossain M. A.; Yeasmin F.; Rahman S. M. M.; Rana S. Naphthalene, a polycyclic aromatic hydrocarbon, in the fish samples from the Bangsai river of Bangladesh by gas chromatograph–mass spectrometry. Arabian J. Chem. 2014, 7, 976–980. 10.1016/j.arabjc.2010.12.014. [DOI] [Google Scholar]
- McGoldrick D. J.; Pelletier M.; de Solla S. R.; Marvin C. H.; Martin P. A. Legacy of legacies: Chlorinated naphthalenes in Lake Trout, Walleye, Herring Gull eggs and sediments from the Laurentian Great Lakes indicate possible resuspension during contaminated sediment remediation. Sci. Total Environ. 2018, 634, 1424–1434. 10.1016/j.scitotenv.2018.04.077. [DOI] [PubMed] [Google Scholar]
- Slezakova K.; Pires J. C. M.; Castro D.; Alvim-Ferraz M. C. M.; Delerue-Matos C.; Morais S.; Pereira M. C. PAH air pollution at a Portuguese urban area: Carcinogenic risks and sources identification. Environ. Sci. Pollut. Res. 2013, 20, 3932–3945. 10.1007/s11356-012-1300-7. [DOI] [PubMed] [Google Scholar]
- Nakata H.; Uehara K.; Goto Y.; Fukumura M.; Shimasaki H.; Takikawa K.; Miyawaki T. Polycyclicaromatic hydrocarbons in oysters and sediments from the Yatsushiro Sea, Japan: Comparison of potentialrisks among PAHs, dioxins and dioxin-like compounds in benthic organisms. Ecotoxicol. Environ. Saf. 2014, 99, 61–68. 10.1016/j.ecoenv.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Hylland K. Polycyclic Aromatic Hydrocarbon (PAH) Ecotoxicology in Marine Ecosystems. J. Toxicol. Environ. Health, Part A 2006, 69, 109–123. 10.1080/15287390500259327. [DOI] [PubMed] [Google Scholar]
- Honda M.; Suzuki N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. 10.3390/ijerph17041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekki K.; Toriba A.; Tang N.; Kameda T.; Hayakawa K. Biological effects of polycyclic aromatic hydrocarbon derivatives. J. UOEH 2013, 35, 17–24. 10.7888/juoeh.35.17. [DOI] [PubMed] [Google Scholar]
- Ikenaka Y.; Oguri M.; Saengtienchai A.; Nakayama S. M. M.; Ijiri S.; Ishizuka M. Characterization of phase-II conjugation reaction of polycyclic aromatic hydrocarbons in fish species: Unique pyrene metabolism and species specificity observed in fish species. Environ. Toxicol. Pharmacol. 2013, 36, 567–578. 10.1016/j.etap.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Vijayavel K.; Gomathi R. D.; Durgabhavani K.; Balasubramanian M. P. Sublethal effect of naphthalene on lipid peroxidation and antioxidant status in the edible marine crab Scylla serrata. Mar. Pollut. Bull. 2004, 48, 429–433. 10.1016/j.marpolbul.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Bekki K.; Takigami H.; Suzuki G.; Tang N.; Hayakawa K. Evaluation of toxic activities of polycyclic aromatic hydrocarbon derivatives using in vitro bioassays. J. Health Sci. 2009, 55, 601–610. 10.1248/jhs.55.601. [DOI] [Google Scholar]
- Hannam M. L.; Bamber S. D.; John Moody A.; Galloway T. S.; Jones M. B. Immunotoxicity and oxidative stress in the Arctic scallop Chlamys islandica: effects of acute oil exposure. Ecotoxicol. Environ. Saf. 2010, 73, 1440–1448. 10.1016/j.ecoenv.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Macdonald G. Z.; Hogan N. S.; Köllner B.; Thorpe K. L.; Phalen L. J.; Wagner B. D.; van den Heuvel M. R. Immunotoxic effects of oil sands-derived naphthenic acids to rainbow trout. Aquat. Toxicol. 2013, 126, 95–103. 10.1016/j.aquatox.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Cherr G. N.; Fairbairn E.; Whitehead A. Impacts of petroleum-derived pollutants on fish development. Annu. Rev. Anim. Biosci. 2017, 5, 185–203. 10.1146/annurev-animal-022516-022928. [DOI] [PubMed] [Google Scholar]
- Samanta P.; Pal S.; Mukherjee A. K.; Senapati T.; Jung J.; Ghosh A. R. Assessment of adverse impacts of glyphosate-based herbicide, Excel Mera 71 by integrating multi-level biomarker responses in fishes. Int. J. Environ. Sci. Technol. 2018, 16, 6291–6300. 10.1007/s13762-018-2013-3. [DOI] [Google Scholar]
- Dey S.; Ballav P.; Mandal A.; Samanta P.; Patra A.; Das S.; Mondal A. K.; Ghosh A. R. Blood Biochemical and Erythrocytic Morpho-pathological Consequences of Naphthalene Intoxication in Indian Teleost, Anabas testudineus (Bloch). Environ. Toxicol. Pharmacol. 2020, 80, 103490. 10.1016/j.etap.2020.103490. [DOI] [PubMed] [Google Scholar]
- Samanta P.; Im H.; Shim T.; Na J.; Jung J. Linking multiple biomarker responses in Daphnia magna under thermal stress. Environ. Pollut. 2020, 263, 114432. 10.1016/j.envpol.2020.114432. [DOI] [PubMed] [Google Scholar]
- Coelho S.; Oliveira R.; Pereira S.; Musso C.; Domingues I.; Bhujel R. C.; Soares A. M. V. M.; Nogueira A. J. A. Assessing lethal and sub-lethal effects of trichlorfon on different trophic levels. Aquat. Toxicol. 2011, 103, 191–198. 10.1016/j.aquatox.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Moreira R. A.; de Araujo G. S.; Silva A. R. R. G.; Daam M. A.; Rocha O.; Soares A. M. V. M.; Loureiro S. Effects of abamectin-based and difenoconazole-based formulations and their mixtures in Daphnia magna: a multiple endpoint approach. Ecotoxicol 2020, 29, 1486–1499. 10.1007/s10646-020-02218-z. [DOI] [PubMed] [Google Scholar]
- Samanta P.; Pal S.; Mukherjee A. K.; Senapati T.; Jung J.; Ghosh A. R. Multi-level integrative biomarker responses in freshwater teleostean fishes exposed to almix herbicide. Int. J. Environ. Res. 2017, 11, 475–487. 10.1007/s41742-017-0042-2. [DOI] [Google Scholar]
- Rand G. M.; Wells P. G.; McCarty L. S.. Introduction to Aquatic Toxicology. In Fundamentals of Aquatic Toxicology. Effects, Environmental Fate, and Risk Assessment, Rand G. M., Ed.; Taylor and Francis: Washington, DC, 1995; pp 3–67. [Google Scholar]
- de la Torre F. R.; Salibián A.; Ferrari L. Assessment of the pollution impact on biomarkers of effect of a freshwater fish. Chemosphere 2007, 68, 1582–1590. 10.1016/j.chemosphere.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Samanta P.; Im H.; Lee H.; Hwang S.-J.; Kim W.; Ghosh A. R.; Jung J. Impact assessment of sewage effluent on freshwater crucian carp Carassius auratus using biochemical and histopathological biomarkers. J. Korean Soc. Water Environ. 2016, 32, 419–432. 10.15681/kswe.2016.32.5.419. [DOI] [Google Scholar]
- Pörtner H. O. Climate variations and the physiological basis of temperature dependent biogeography: Systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 2002, 132, 739–761. 10.1016/s1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Klumpen E.; Hoffschröer N.; Zeis B.; Gigengack U.; Dohmen E.; Paul R. J. Reactive oxygen species (ROS) and the heat stress response of Daphniapulex: ROS-mediated activation of hypoxia-inducible factor 1 (HIF-1) and heat shock factor 1 (HSF-1) and the clustered expression of stress genes. Biol. Cell. 2016, 109, 39–64. 10.1111/boc.201600017. [DOI] [PubMed] [Google Scholar]
- El-Sayed Y. S.; Saad T. T.; El-Bahr S. M. Acute intoxication of deltamethrin in monosex Nile tilapia, Oreochromis niloticus with special reference to the clinical, biochemical and haematological effects. Environ. Toxicol. Pharmacol. 2007, 24, 212–217. 10.1016/j.etap.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Lohner T. W.; Reash R. J.; Williams M. Assessment of tolerant sunfish populations (Lepomis sp.) inhabiting selenium-laden coal ash effluents. Ecotoxicol. Environ. Saf. 2001, 50, 217–224. 10.1006/eesa.2001.2098. [DOI] [PubMed] [Google Scholar]
- Bacchetta C.; Cazenave J.; Parma M. J. Responses of biochemical markers in the fish Prochiloduslineatus exposed to a commercial formulation of endosulfan. Water, Air, Soil Pollut. 2010, 216, 39–49. 10.1007/s11270-010-0512-z. [DOI] [Google Scholar]
- Chowdhury M. J.; Mcdonald D. G.; Wood C. M. Gastrointestinal uptake and fate of cadmium in rainbow trout acclimated to sublethal dietary cadmium. Aquat. Toxicol. 2004, 69, 149–163. 10.1016/j.aquatox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Joshi P. K.; Bose M.; Harish D. Haematological changes in the blood of Clariasbatrachus exposed to mercuric chloride. J. Ecotoxicol. Environ. Monit. 2002, 12, 119–122. [Google Scholar]
- Lee H. J.; Shim W. J.; Lee J.; Kim G. B. Temporal and geographical trends in the genotoxic effects of marine sediments after accidental oil spill on the blood cells of striped beakperch (Oplegnathusfasciatus). Mar. Pollut. Bull. 2011, 62, 2264–2268. 10.1016/j.marpolbul.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Ada F. B.; Ekpenyong E.; Ayotunde E. O.. Haematological, biological and behavioural changes in Oreochromis niloticus(Linne 1757) juveniles exposed to Paraquat herbicide. J. Environ. Chem. Ecotoxicol. 2012, 4(). 10.5897/jece11.067 [DOI] [Google Scholar]
- Barreto-Medeiros J. M.; Feitoza E. G.; de Magalhães Lima K.; da Silva R. R.; Manhães-de-Castro F. M.; Manhães-de-Castro R.; De-Castro C. The expression of an intraspecific aggressive reaction in the face of a stressor agent alters the immune response in rats. Braz. J. Biol. 2005, 65, 203–209. 10.1590/s1519-69842005000200003. [DOI] [PubMed] [Google Scholar]
- Suvetha L.; Saravanan M.; Hur J.-H.; Ramesh M.; Bindu F. C. Responses of the Indian major carpLabeorohitato deltamethrin at acute and sublethal concentrations. Toxicol. Environ. Chem. 2015, 97, 186–199. 10.1080/02772248.2015.1031666. [DOI] [Google Scholar]
- Barcellos L. J. G.; Kreutz L. C.; Rodrigues L. B.; Fioreze I.; Quevedo R. M.; Cericato L.; Terra S. Haematological and biochemical characteristics of male jundia (Rhamdiaquelen Quoy&Gaimardpimelodidae): changes after acute stress. Aquacult. Res. 2003, 34, 1465–1469. 10.1111/j.1365-2109.2003.00972.x. [DOI] [Google Scholar]
- Hussein S. Y.; El-Nasser M. A.; Ahmed S. M. Comparative studies on the effects of herbicide atrazine on freshwater fish Oreochromis niloticus and Chrysichthyes auratus at Assiut, Egypt. Bull. Environ. Contam. Toxicol. 1996, 57, 503–510. 10.1007/s001289900218. [DOI] [PubMed] [Google Scholar]
- Härdig J.; Höglund L. B. Seasonal and ontogenetic effects on methaemoglobin and reduced glutathione contents in the blood of reared Baltic salmon. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 1983, 75, 27–34. 10.1016/0300-9629(83)90039-7. [DOI] [Google Scholar]
- Nikinmaa M.; Jensen F. B. Blood oxygen transport and acid-base status of stressed trout (Salmo gairdnerii): pre- and post-branchial values in winter fish. Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol. 1986, 84, 391–396. 10.1016/0300-9629(86)90634-1. [DOI] [Google Scholar]
- John P. J. Alteration of certain blood parameters of freshwater teleost Mystusvittatus after chronic exposure to Metasystox and Sevin. Fish Physiol. Biochem. 2006, 33, 15–20. 10.1007/s10695-006-9112-7. [DOI] [Google Scholar]
- Kreutz L. C.; Gil Barcellos L. J.; de Faria Valle S.; de Oliveira Silva T.; Anziliero D.; Davi dos Santos E.; Pivato M.; Zanatta R. Altered hematological and immunological parameters in silver catfish (Rhamdiaquelen) following short term exposure to sublethal concentration of glyphosate. Fish Shellfish Immunol. 2011, 30, 51–57. 10.1016/j.fsi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Gaber SH. S.; El-Fatah El-Kasheif M. A.; Ibrahim S. A.; Authman M. M. N. Effect of water pollution in El-Rahawy drainage canal on hematology and organs of freshwater fish Clariasgariepinus. World Appl. Sci. J. 2013, 21, 329–341. 10.5829/idosi.wasj.2013.21.3.71192. [DOI] [Google Scholar]
- Vaseem H.; Banerjee T. K. Contamination of the River Ganga and its toxic implication in the blood parameters of the major carp Labeorohita (Ham). Environ. Sci. Pollut. Res. 2013, 20, 5673–5681. 10.1007/s11356-013-1570-8. [DOI] [PubMed] [Google Scholar]
- Javed M.; Ahmad M. I.; Usmani N.; Ahmad M. Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci. Rep. 2017, 7, 1675. 10.1038/s41598-017-01749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes B. S.; Clasen B.; Loro V. L.; Pretto A.; Toni C.; de Avila L. A.; Marchesan E.; de Oliveira Machado S. L.; Zanella R.; Reimche G. B. Toxicological responses of Cyprinus carpio after exposure to a commercial herbicide containing imazethapyr and imazapic. Ecotoxicol. Environ. Saf. 2011, 74, 328–335. 10.1016/j.ecoenv.2009.05.013. [DOI] [PubMed] [Google Scholar]
- van Meer G.; Voelker D. R.; Feigenson G. W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C. R.; Schulze A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- Dey S.; Samanta P.; Mondal N. S.; Kole D.; Mandal A.; Patra A.; Ghosh A. R. Dose specific responses of Anabas testudineus (Bloch) to anthracene (PAH): haematological and biochemical manifestation. Emerging Contam. Handb. 2019, 5, 232–239. 10.1016/j.emcon.2019.07.001. [DOI] [Google Scholar]
- Kojima M.; Masui T.; Nemoto K.; Degawa M. Lead nitrate-induced development of hypercholesterolemia in rats: sterol-independent gene regulation of hepatic enzymes responsible for cholesterol homeostasis. Toxicol. Lett. 2004, 154, 35–44. 10.1016/j.toxlet.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Abdou H. M.; Hassan M. A. Protective role of omega-3 polyunsaturated fatty acid against lead acetate-induced toxicity in liver and kidney of female rats. BioMed Res. Int. 2014, 2014, 1–11. 10.1155/2014/435857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall A. R. Plasma high density lipoproteins. metabolism and relationship to atherogenesis. J. Clin. Invest. 1990, 86, 379–384. 10.1172/jci114722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N.; Yamamoto M.; Watanabe K.; Kambegawa A.; Hattori A. Both mercury and cadmium directly influence calcium homeostasis resulting from the suppression of scale bone cells: the scale is a good model for the evaluation of heavy metals in bone metabolism. J. Bone Miner. Metabol. 2004, 22, 439–446. 10.1007/s00774-004-0505-3. [DOI] [PubMed] [Google Scholar]
- Van Winkle L. S.; Johnson Z. A.; Nishio S. J.; Brown C. D.; Plopper C. G. Early events in naphthalene-induced acute Clara cell toxicity: comparison of membrane permeability and ultrastructure. Am. J. Respir. Cell Mol. Biol. 1999, 21, 44–53. 10.1165/ajrcmb.21.1.3630. [DOI] [PubMed] [Google Scholar]
- Ko H.-D.; Park H.-J.; Kang J.-C.. Change of growth performance, hematological parameters, and plasma component by hexavalent chromium exposure in starry flounder, Platichthysstellatus. Fisher. Aquat. Sci., 2019, 22(). 10.1186/s41240-019-0124-5 [DOI] [Google Scholar]
- Dacke C. G.Calcium regulation in the osteichthyes. In Calcium Regulation in Sub-Mammalian Vertebrates; Dacke C. G., Ed.; Academic Press: London, 1979; pp 99–122. [Google Scholar]
- Carta P.; Flore C.; Alinovi R.; Ibba A.; Tocco M.; Aru G.; Carta R.; Girei M.; Mutti A.; Randaccio F. R. Neuroendocrine and neurobehavioral effects associated with exposure to low doses of mercury from habitual consumption of marine fish. Med. Lav. 2002, 93, 215–224. [PubMed] [Google Scholar]
- Flik G.; Rentier-Delrue F.; Wendelaar Bonga S. E.. Calciotropic effects of recombinant prolactins in Oreochromis mossambicus. Am. J. Physiol.: Regul., Integr. Comp. Physiol. 1994, 266(). 10.1152/ajpregu.1994.266.4.r1302 [DOI] [PubMed] [Google Scholar]
- Fırat Ö.; Cogun H. Y.; Yüzereroğlu T. A.; Gök G.; Fırat Ö.; Kargin F.; Kötemen Y. A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2011, 37, 657–666. 10.1007/s10695-011-9466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydarnejad M. S.; Khosravian-Hemamai M.; Nematollahi A.. Effects of cadmium at sub-lethal concentration on growth and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Ir. Vet. J. 2013, 66(). 10.1186/2046-0481-66-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Banerjee T. K. Arsenic induced hematological and biochemical responses in nutritionally important catfish Clariasbatrachus (L.). Toxicol. Rep. 2016, 3, 148–152. 10.1016/j.toxrep.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta P.; Im H.; Yoo J.; Lee H.; Kim N.-Y.; Kim W.; Hwang S.-J.; Kim W.-K.; Jung J. Comparative assessment of the adverse outcome of wastewater effluents by integrating oxidative stress and histopathological alterations in endemic fish. J. Hazard. Mater. 2018, 344, 81–89. 10.1016/j.jhazmat.2017.10.016. [DOI] [PubMed] [Google Scholar]
- dos Santos T. d. C. A.; Ngan P. V.; Passos M. J. A. C. R.; Gomes V. Effects of naphthalene on metabolic rate and ammonia excretion of juvenile Florida pompano, Trachinotuscarolinus. J. Exp. Mar. Biol. Ecol. 2006, 335, 82–90. 10.1016/j.jembe.2006.02.019. [DOI] [Google Scholar]
- APHA, AWWA, WPCF , Standard Methods for the Examination of Water and Wastewater. 21st ed.; APHA-AWWA-WPCF: Washington, DC, 2005. [Google Scholar]
- Dey S.; Ghosh A. R. Dose-specific biochemical and erythrocytic alterations of anthracene exposure on blood of Anabas testudineus (Bloch). Environ. Toxicol. Pharmacol. 2019, 72, 103247. 10.1016/j.etap.2019.103247. [DOI] [PubMed] [Google Scholar]
- Beliaeff B.; Burgeot T. Integrated biomarker response: a useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. 10.1002/etc.5620210629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.