Abstract
Many preclinical and clinical observations support that functional magnetic resonance imaging (MRI), such as diffusion weighted (DW) and dynamic contrast enhanced (DCE) MRI, might have a predictive value for radiotherapy. The aim of this review was to assess the current status of quantitative MRI on hybrid MR-Linacs. In a literature research, four publications were identified, investigating technical feasibility, accuracy, repeatability and reproducibility of DW and DCE-MRI in phantoms and first patients. Accuracy and short term repeatability was < 5% for DW-MRI in current MR-Linac systems. Consequently, quantitative imaging providing accurate and reproducible functional information seems possible in MR-Linacs.
Keywords: MR-Linac, MR-guided radiotherapy, Quantitative MRI, Diffusion-weighted MRI
1. Introduction
Functional magnetic resonance imaging (MRI) has been shown in a number of recent studies to provide not only beneficial information for target volume delineation [1], [2], but more importantly also prognostic information with respect to outcome after radiotherapy (RT) [3], [4]. In terms of quantitative MRI techniques in a clinical context, so far mainly diffusion weighted imaging (DWI) and dynamic contrast enhanced (DCE) MRI have been used to predict RT response [5], [6]. DWI is a functional MRI technique to visualize diffusion properties of water in tissues and thus cellular density [5], whereas DCE measures tissue perfusion and permeability [6].
There is growing evidence that diffusion weighted (DW) MRI may provide prognostic information to predict RT treatment outcome in head and neck cancer (HNC) [7], [8], [9], [10], [11]. Also for other tumor sites, such as brain tumors [12], [13], cervical cancer [14] or rectal cancer [15], the prognostic value of DW MRI for RT outcome prognosis has been shown. The majority of these studies correlated functional biomarkers derived from functional MRI before the start of RT with observed outcome following therapy. Some studies also investigated the optimal time point of imaging and showed that biomarkers acquired in the first weeks of therapy might be beneficial for prognosis prediction [16]. Vandecaveye et al. [16] reported that DW MRI during RT allowed for a more accurate response prediction compared to anatomical imaging and correlated significantly to two-year loco-regional control (LRC) in HNC.
Other studies showed that also DCE MRI may yield prognostic information with respect to RT outcome [6], [17]. For example, Halle et al. identified a prognostic parameter from DCE MRI data to predict RT response in cervical cancer [17]. Consequently, functional MRI yielded promising results in the light of developing prognostic models for RT outcome prediction. Such predictive models relating quantitative biomarkers derived from functional MRI to RT outcome might serve in the future as a basis for RT interventions aiming for personalized dose prescriptions. However, the direct use of functional imaging information for RT dose optimization requires quantitative imaging information [18], [19].
Studies on optimal imaging time points are ongoing. Those are challenging due to a limited availability of imaging appointments and high burden to the patient when using separate, stand-alone MRI scanners.
Recently, combined devices of MRI and linear accelerator (MR-Linac) have been introduced clinically [20], [21], [22], [23], [24], [25], which allow for daily online-adaptive MR-guided RT [26], [27], [28], [29], [30], [31]. Two different systems with magnetic field strengths of 0.35 T [32] and 1.5 T [33] are currently commercially available. In addition to daily anatomical MRI during RT, MR-Linac treatment machines provide an optimal basis for un-biased biomarker studies, as functional imaging can be done sequentially, at any time point during RT [34]. A recent review by Datta et al. [34] discussed challenges related to the technical implementation of functional MRI techniques on combined MR-Linac systems. However, the MR scanner design in combined MR-Linacs is different compared to diagnostic MR-scanners in terms of technical parameters such as gradient strength, slew rate and eventually also field strength as well as the use of radio-opaque radiofrequency coils adapted to the RT-specific patient positioning [32], [35]. Therefore, after implementation of functional MRI on MR-Linacs, their capacity to provide quantitative imaging and thus accurate, repeatable and reproducible quantitative imaging biomarkers (QIB) needs to be investigated before initiating clinical studies [36], [37]. Consequently, the aim of this work was to review the current status regarding technical and clinical validation of quantitative imaging using hybrid MR-Linac systems.
2. Methods
A literature research was performed in the database PubMed (www.pubmed.ncbi.nlm.nih.gov) using the search terms “MR-Linac / MRI-Linac” AND “functional / quantitative MRI” OR “diffusion-weighted imaging” to assess the current status of functional MRI using combined MR-Linacs. Search results were screened for original papers. Reviews and studies investigating issues related to stand-alone MRI scanners during RT were excluded from analysis.
3. Results
A total of eight articles were found during the literature research, out of which two were review articles focusing on the management of lung cancer using MR-Linacs [31] and on the technical challenges when implementing functional MRI [34]. Two articles were not related to combined MR-Linac systems. After curation of the search results, four articles were reviewed and will be discussed in the following.
3.1. 0.35 T MR-Linac / MR-guided tri-cobalt 60 system
Yang et al. [38] have demonstrated the general feasibility of acquiring longitudinal DWI on the combined 0.35 T MRI-guided tri-cobalt 60 radiotherapy system, the precursor of today’s 0.35 T MRI-Linac. In this study, a multi-slice spin echo single-shot echo planar imaging (EPI) pulse sequence to measure diffusion was implemented and tested in phantom studies. Tests were performed on the diffusion phantom recommended by the Quantitative Imaging Biomarker Alliance (QIBA) [37] which contains inserts with different known diffusion coefficients. Results of this phantom study demonstrated that apparent diffusion coefficients (ADC) measured on the 0.35 T MRI-guided RT system matched with the reference ADC values within < 5% error. Furthermore, this study provided proof-of-principle data in six patients with head-and-neck cancer (HNC) or sarcoma, where ADC value changes during fractionated RT were acquired and reported [38].
As DW MRI using single-shot EPI suffers not only from limited resolution but also from severe spatial distortion and potentially inaccurate ADC value determination at low field strengths, the same group developed a diffusion prepared turbo spin echo (TSE) readout sequence to measure diffusion in tumors [39]. Similarly, a QIBA diffusion phantom was used to benchmark the accuracy and repeatability of the TSE-DW MRI sequence in addition to assessing geometrical distortions using a spatial integrity phantom. The spatial integrity of the TSE sequence was shown to be < 1 mm within a radius of 100 mm which was much lower compared to the EPI sequence for DW imaging [39]. Experiments with the diffusion phantom demonstrated that both sequences provided accurate quantitation of ADC at a temperature of 0 °C, which was < 3% with respect to the reference values. At room temperature, EPI-based ADC values showed 8% of variation compared to reference, whereas TSE based ADC determination was accurate within 4%. TSE experiments showed reasonable repeatability with a coefficient of variation < 5% [39]. Also in this study, proof of concept was provided for clinical usability of DW MRI using TSE sequences, although using longer acquisition times compared to EPI-based DWI, by reporting preliminary clinical experience on six patients.
Wojcieszynski et al. reported on the semi-quantitative use of MRI contrast agents to improve tumor visibility for online contouring and tracking on a 0.35 T MRI-guided RT system in 5 patients [40]. This initial report on clinical feasibility demonstrated increased signal-to-noise ratios of contrast-enhanced MRI at hybrid MRI-guided RT machines, which may be a first step towards future usage of DCE-MRI at MRI-Linac systems.
3.2. 1.5 T MR-Linac
In a recent multi-center phantom study, the performance of the 1.5 T MR-Linac system regarding quantitative MRI was assessed [41]. In this study, four MR-Linac systems at four different institutes were used to investigate accuracy, repeatability and reproducibility of measuring T1- and T2-relaxation times, ADC values and DCE imaging using dedicated phantoms. Kooreman et al. showed bias and limits of agreement (LoA) of 11 ± 238 ms and −6 ± 63 ms for T1- and T2-relaxometry, respectively. For ADC determination, a bias of (0.007 ± 0.027)·10-3 mm2/s were found in addition to a short term repeatability of 0 ± 9%. A median relative coefficient of variation of 0.6 was reported to DCE MRI on the MR-Linac systems. Reproducibility was shown to be 11.2% for T1, 2.9% for T2, 2.2% for ADC, and 18.4% for DCE [41]. Consequently, this study demonstrated that quantitative MRI on the 1.5 T MR-Linac is feasible, repeatable, and accurate within the observed limits. These are important requirements for a future use of MR-Linac for response monitoring and RT interventions based on daily quantitative MRI.
4. Discussion
This literature review demonstrates that so far only a few studies were carried out to investigate the use of MR-Linac systems for the acquisition of functional and quantitative imaging [38], [39], [40], [41]. Those few studies report that on both currently commercially available MR-Linac systems, DWI and also DCE MRI seems to be technically feasible in dedicated phantoms and also in first proof-of-concept patient examinations. Accuracy and short term repeatability was shown to be below 5% for DW MRI in the 0.35 T and also in the 1.5 T MR-Linac. Fig. 1 presents an example, where accuracy and repeatability of DWI were assessed on a 1.5 T MR-Linac using a dedicated phantom. Clinical usage of combined MR-Linacs seems to be feasible even though the systems present with MR system specifications (e.g. gradient strength, slew rate, etc.) which are different to state-of-the-art diagnostic systems, as accuracy and reproducibility of quantitative imaging has been demonstrated in first experiments.
Fig. 1.
Assessment of accuracy and repeatability with respect to ADC determination based on DW MRI at the 1.5 T MR-Linac using a dedicated diffusion phantom. (A) Diffusion Phantom (Model 128, Qalibre MD, Boulder, USA) containing inserts prepared with different known diffusion coefficients. (B) Regions of interest used to analyze ADC values in the different probe areas, acquired with an EPI-based DW MRI sequence (b = 0, 200, 500 s/mm2; TE/TR = 117/6683 ms; voxel size: 1.53 × 1.53 × 4 mm3). (C,D) Bland-Altman plots showing accuracy and short term repeatability of ADC assessed in four measurements on two different days, including bias (full line) and LoA (limits of agreement, dashed line). (C) Accuracy of ADC assessment with a bias of −2.5·10-6 mm2/s and LoA ± 26.8·10-6 mm2/s. (D) Repeatability analysis presents a bias of 0.6%, LoA = ±7.6%. Median accuracy and reproducibility were −0.31% and 0.31%, respectively.
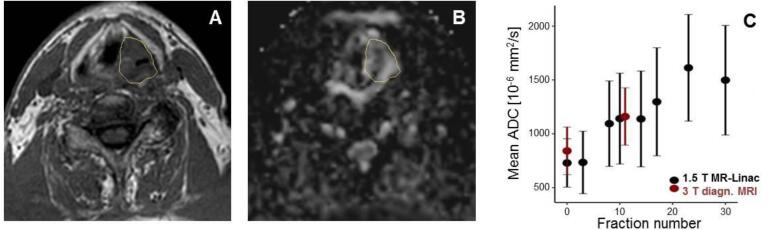
Nevertheless, most studies so far investigated quantitative imaging characteristics of MR-Linacs only in phantom experiments. In order to overcome the first translational gap according to [36], not only technical validation in phantom studies but also clinical validation studies of proposed quantitative imaging biomarkers are needed. Therefore, systematic assessment of sequential quantitative MRI in patients with different tumors needs to be carried out as a next research step towards quantitative MRI-based personalized RT interventions. To realize this, the MR-Linac is an optimal tool, as it inherently provides the opportunity for sequential imaging during fractionated RT. Test-retest studies in patient cohorts are required to investigate reproducibility of quantitative parameters measured on combined MR-Linacs. Such test–retest experiments in addition to studies assessing cross-correlations with quantitative MRI obtained from diagnostic MRI scanners are absolutely necessary before biomarker studies with outcome correlations can be done. In Fig. 2, a proof-of-concept data set is shown where DWI was performed in a HNC patient during RT on the MR-Linac and mean tumor ADC values were compared to those measured with a diagnostic MR system. In addition, also questions about optimal imaging time points for assessing quantitative information with prognostic information and optimal timing of quantitative MR-based interventions may be answered with patient studies at the MR-Linac in the near future.
Fig. 2.
Sequential DWI measurements acquired on the 1.5 T MR-Linac (Unity, Elekta AB, Stockholm, Sweden) in a HNC patient. This patient was treated in the context of a phase 2 feasibility trial (NCT04172753), which had been approved by the ethics committee of the University Hospital Tübingen, and gave written informed consent. (A) Anatomical T1-weighted MRI acquired on the 1.5 T MR-Linac after two weeks of RT (fraction 10) with gross tumor volume (GTV) delineated. (B) ADC map derived from EPI-based DW MRI (b = 200, 500, 800 s/mm2, TE/TR = 107/10392 ms, voxel size: 3 × 3 × 4.8 mm3). (C) Mean ADC values in the tumor region sequentially assessed during the course of fractionated RT, in comparison to two DW-MRI scans acquired at a diagnostic 3 T MR scanner (Vida, Siemens Healthineers, Erlangen, Germany). The error bars visualize the standard deviation of the ADC distribution inside the GTV. ADC values are continuously increasing during RT, which may be a sign of individual treatment response.
So far published data on accuracy and repeatability of DW MRI on hybrid MR-Linacs were concerning mean ADC values averaged over bulk tumor or phantom regions [38], [39], [41]. However, biological interventions such as e.g. dose painting based on functional MR information measured on combined MR-Linacs require spatially resolved information about diffusion properties of the tumor to identify resistant sub-regions. To quantify the level of accuracy and reproducibility of voxel-based DW MRI on MR-Linacs, further phantom and patient studies are needed. Furthermore, not only quantitative information acquired accurately, but also reproducibly and with a high level of repeatability is required. In addition, geometrically accurate images are needed for dose painting interventions. Consequently, DWI needs to be characterized in terms of geometrical distortions. Due to potentially significant geometric distortions of > 1 mm, Gao et al. [39] have developed and implemented a diffusion prepared TSE sequence on the 0.35 T MR-guided tri-cobalt 60 system. This sequence does not suffer from geometrical distortions but comes with much lower signal-to-noise ratios, which causes much longer acquisition times. Therefore, optimal quantitative imaging protocols suitable for longitudinal patient studies need to be carefully selected to provide optimal trade-offs for different aspects providing geometrically useful, accurate information measured in a realistic time frame. However, sequence parameters need to be chosen in a way to take into account the technical characteristics and related limitations of the respective MR systems and may thus differ from settings of comparable sequences on modern diagnostic MRI scanners.
In conclusion, first studies have shown the potential of combined MR-Linacs for quantitative imaging providing accurate and reproducible functional information in tumor tissues comparable to diagnostic MR scanners. With the ability of providing sequential functional information during the whole course of fractionated RT, geometrically aligned with the RT plan, future use for investigating prognostic quantitative imaging biomarkers appears feasible. However, as most studies so far presented only phantom experiments, test–retest studies in clinical cohorts are required as a next step towards quantitative MR-based RT interventions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The MR-Linac program at the University of Tübingen is funded by the German Research Council (DFG, grants no. ZI 736/2-1, TH 1528/6-1, ZI 736/4-1, GA 2996/1-1).
The authors report institutional collaborations with Elekta, Philips, Siemens and PTW Freiburg.
This paper is part of a special issue that contains contributions originally submitted to the scientific meeting MR in RT, which was planned to take place 05/2020, organized by the German Research Center (DKFZ) in Heidelberg.
References
- 1.Gurney-Champion O.J., Kieselmann J.P., Wong K.H., Ng-Cheng-Hin B., Harrington K., Oelfke U. A convolutional neural network for contouring metastatic lymph nodes on diffusion-weighted magnetic resonance images for assessment of radiotherapy response. Phys Imaging Radiation Oncol. 2020;15:1–7. doi: 10.1016/j.phro.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ligtenberg H, Schakel T, Dankbaar JW, Ruiter LN, Peltenburg B, Willems SM, et al. Target Volume Delineation Using Diffusion-weighted Imaging for MR-guided Radiotherapy: A Case Series of Laryngeal Cancer Validated by Pathology. Cureus. 2018;10:e2465. [DOI] [PMC free article] [PubMed]
- 3.Leibfarth S., Winter R.M., Lyng H., Zips D., Thorwarth D. Potentials and challenges of diffusion-weighted magnetic resonance imaging in radiotherapy. Clin Transl Radiation Oncol. 2018;13:29–37. doi: 10.1016/j.ctro.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martens R.M., Noij D.P., Ali M., Koopman T., Marcus J.T., Vergeer M.R., de Vet H., de Jong M.C., Leemans C.R., Hoekstra O.S., de Bree R., de Graaf P., Boellaard R., Castelijns J.A. Functional imaging early during (chemo)radiotherapy for response prediction in head and neck squamous cell carcinoma; a systematic review. Oral Oncol. 2019;88:75–83. doi: 10.1016/j.oraloncology.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Iima M., Le Bihan D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology. 2016;278(1):13–32. doi: 10.1148/radiol.2015150244. [DOI] [PubMed] [Google Scholar]
- 6.Zahra M.A., Hollingsworth K.G., Sala E., Lomas D.J., Tan L.T. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 2007;8(1):63–74. doi: 10.1016/S1470-2045(06)71012-9. [DOI] [PubMed] [Google Scholar]
- 7.Martens R.M., Noij D.P., Koopman T., Zwezerijnen B., Heymans M., de Jong M.C., Hoekstra O.S., Vergeer M.R., de Bree R., Leemans C.R., de Graaf P., Boellaard R., Castelijns J.A. Predictive value of quantitative diffusion-weighted imaging and 18-F-FDG-PET in head and neck squamous cell carcinoma treated by (chemo)radiotherapy. Eur J Radiol. 2019;113:39–50. doi: 10.1016/j.ejrad.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Kim S., Loevner L., Quon H., Sherman E., Weinstein G., Kilger A., Poptani H. Diffusion-Weighted Magnetic Resonance Imaging for Predicting and Detecting Early Response to Chemoradiation Therapy of Squamous Cell Carcinomas of the Head and Neck. Clin Cancer Res. 2009;15(3):986–994. doi: 10.1158/1078-0432.CCR-08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambrecht M., Van Calster B., Vandecaveye V., De Keyzer F., Roebben I., Hermans R., Nuyts S. Integrating pretreatment diffusion weighted MRI into a multivariable prognostic model for head and neck squamous cell carcinoma. Radiother Oncol. 2014;110(3):429–434. doi: 10.1016/j.radonc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 10.King A.D., Mo F.K.F., Yu K.-H., Yeung D.K.W., Zhou H., Bhatia K.S., Tse G.M.K., Vlantis A.C., Wong J.K.T., Ahuja A.T. Squamous cell carcinoma of the head and neck: diffusion-weighted MR imaging for prediction and monitoring of treatment response. Eur Radiol. 2010;20(9):2213–2220. doi: 10.1007/s00330-010-1769-8. [DOI] [PubMed] [Google Scholar]
- 11.King A.D., Chow K.-K., Yu K.-H., Mo F.K.F., Yeung D.K.W., Yuan J., Bhatia K.S., Vlantis A.C., Ahuja A.T. Head and Neck Squamous Cell Carcinoma: Diagnostic Performance of Diffusion-weighted MR Imaging for the Prediction of Treatment Response. Radiology. 2013;266(2):531–538. doi: 10.1148/radiol.12120167. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood F., Johannesen H.H., Geertsen P., Hansen R.H. Repeated diffusion MRI reveals earliest time point for stratification of radiotherapy response in brain metastases. Phys. Med. Biol. 2017;62(8):2990–3002. doi: 10.1088/1361-6560/aa5249. [DOI] [PubMed] [Google Scholar]
- 13.Karami E., Soliman H., Ruschin M., Sahgal A., Myrehaug S., Tseng C.-L., Czarnota G.J., Jabehdar-Maralani P., Chugh B., Lau A., Stanisz G.J., Sadeghi-Naini A. Quantitative MRI Biomarkers of Stereotactic Radiotherapy Outcome in Brain Metastasis. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-56185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scalco E., Marzi S., Sanguineti G., Vidiri A., Rizzo G. Characterization of cervical lymph-nodes using a multi-parametric and multi-modal approach for an early prediction of tumor response to chemo-radiotherapy. Physica Med. 2016;32(12):1672–1680. doi: 10.1016/j.ejmp.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Pham T.T., Liney G.P., Wong K., Barton M.B. Functional MRI for quantitative treatment response prediction in locally advanced rectal cancer. BJR. 2017;90(1072):20151078. doi: 10.1259/bjr.20151078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandecaveye V., Dirix P., De Keyzer F., Op de Beeck K., Vander Poorten V., Roebben I., Nuyts S., Hermans R. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. Eur Radiol. 2010;20(7):1703–1714. doi: 10.1007/s00330-010-1734-6. [DOI] [PubMed] [Google Scholar]
- 17.Halle C., Andersen E., Lando M., Aarnes E.-K., Hasvold G., Holden M., Syljuasen R.G., Sundfor K., Kristensen G.B., Holm R., Malinen E., Lyng H. Hypoxia-Induced Gene Expression in Chemoradioresistant Cervical Cancer Revealed by Dynamic Contrast-Enhanced MRI. Cancer Res. 2012;72(20):5285–5295. doi: 10.1158/0008-5472.CAN-12-1085. [DOI] [PubMed] [Google Scholar]
- 18.Gurney-Champion O.J., Mahmood F., van Schie M., Julian R., George B., Philippens M.E.P., van der Heide U.A., Thorwarth D., Redalen K.R. Quantitative imaging for radiotherapy purposes. Radiother Oncol. 2020;146:66–75. doi: 10.1016/j.radonc.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Heide U.A., Thorwarth D. Quantitative Imaging for Radiation Oncology. International Journal of Radiation Oncology*Biology*Physics. 2018;102(4):683–686. doi: 10.1016/j.ijrobp.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Nachbar M., Monnich D., Boeke S., Gani C., Weidner N., Heinrich V. Partial breast irradiation with the 1.5 T MR-Linac: First patient treatment and analysis of electron return and stream effects. Radiother Oncol. 2020;145:30–35. doi: 10.1016/j.radonc.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Werensteijn-Honingh A.M., Kroon P.S., Winkel D., Aalbers E.M., van Asselen B., Bol G.H. Feasibility of stereotactic radiotherapy using a 1.5T MR-linac: Multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol. 2019;134:50–54. doi: 10.1016/j.radonc.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Bertelsen A.S., Schytte T., Møller P.K., Mahmood F., Riis H.L., Gottlieb K.L., Agergaard S.N., Dysager L., Hansen O., Gornitzka J., Veldhuizen E., ODwyer D.B., Christiansen R.L., Nielsen M., Jensen H.R., Brink C., Bernchou U. First clinical experiences with a high field 1.5 T MR linac. Acta Oncol. 2019;58(10):1352–1357. doi: 10.1080/0284186X.2019.1627417. [DOI] [PubMed] [Google Scholar]
- 23.Eccles C.L., Adair Smith G., Bower L., Hafeez S., Herbert T., Hunt A., McNair H.A., Ofuya M., Oelfke U., Nill S., Huddart R.A. Magnetic resonance imaging sequence evaluation of an MR Linac system; early clinical experience. Technical Innovations & Patient Support in Radiation Oncology. 2019;12:56–63. doi: 10.1016/j.tipsro.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elter A, Dorsch S, Mann P, Runz A, Johnen W, Spindeldreier CK, et al. End-to-end test of an online adaptive treatment procedure in MR-guided radiotherapy using a phantom with anthropomorphic structures. Phys Med Biol. 2019;64:225003. [DOI] [PubMed]
- 25.Finazzi T., van Sörnsen de Koste J.R., Palacios M.A., Spoelstra F.O.B., Slotman B.J., Haasbeek C.J.A., Senan S. Delivery of magnetic resonance-guided single-fraction stereotactic lung radiotherapy. Physics and Imaging in Radiation Oncology. 2020;14:17–23. doi: 10.1016/j.phro.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall W.A., Paulson E.S., van der Heide U.A., Fuller C.D., Raaymakers B.W., Lagendijk J.J.W., Li X.A., Jaffray D.A., Dawson L.A., Erickson B., Verheij M., Harrington K.J., Sahgal A., Lee P., Parikh P.J., Bassetti M.F., Robinson C.G., Minsky B.D., Choudhury A., Tersteeg R.J.H.A., Schultz C.J. The transformation of radiation oncology using real-time magnetic resonance guidance: A review. Eur J Cancer. 2019;122:42–52. doi: 10.1016/j.ejca.2019.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurz C., Buizza G., Landry G., Kamp F., Rabe M., Paganelli C., Baroni G., Reiner M., Keall P.J., van den Berg C.A.T., Riboldi M. Medical physics challenges in clinical MR-guided radiotherapy. Radiat Oncol. 2020;15(1) doi: 10.1186/s13014-020-01524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kontaxis C., de Muinck Keizer D.M., Kerkmeijer L.G.W., Willigenburg T., den Hartogh M.D., van der Voort van Zyp J.R.N., de Groot-van Breugel E.N., Hes J., Raaymakers B.W., Lagendijk J.J.W., de Boer H.C.J. Delivered dose quantification in prostate radiotherapy using online 3D cine imaging and treatment log files on a combined 1.5T magnetic resonance imaging and linear accelerator system. Physics and Imaging in Radiation Oncology. 2020;15:23–29. doi: 10.1016/j.phro.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunlop A., Mitchell A., Tree A., Barnes H., Bower L., Chick J., Goodwin E., Herbert T., Lawes R., McNair H., McQuaid D., Mohajer J., Nilawar R., Pathmanathan A., Smith G., Hanson I., Nill S., Oelfke U. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: Initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clinical and Translational Radiation Oncology. 2020;23:35–42. doi: 10.1016/j.ctro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulson E.S., Ahunbay E., Chen X., Mickevicius N.J., Chen G.-P., Schultz C., Erickson B., Straza M., Hall W.A., Li X.A. 4D-MRI driven MR-guided online adaptive radiotherapy for abdominal stereotactic body radiation therapy on a high field MR-Linac: Implementation and initial clinical experience. Clinical and Translational Radiation Oncology. 2020;23:72–79. doi: 10.1016/j.ctro.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bainbridge H., Salem A., Tijssen R.H.N., Dubec M., Wetscherek A., Van Es C., Belderbos J., Faivre-Finn C., McDonald F. Magnetic resonance imaging in precision radiation therapy for lung cancer. Transl. Lung Cancer Res. 2017;6(6):689–707. doi: 10.21037/tlcr.2017.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clinical and Translational Radiation Oncology. 2019;18:98–101. doi: 10.1016/j.ctro.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winkel D., Bol G.H., Kroon P.S., van Asselen B., Hackett S.S., Werensteijn-Honingh A.M., Intven M.P.W., Eppinga W.S.C., Tijssen R.H.N., Kerkmeijer L.G.W., de Boer H.C.J., Mook S., Meijer G.J., Hes J., Willemsen-Bosman M., de Groot-van Breugel E.N., Jürgenliemk-Schulz I.M., Raaymakers B.W. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clinical and Translational Radiation Oncology. 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datta A., Aznar M.C., Dubec M., Parker G.J.M., O'Connor J.P.B. Delivering Functional Imaging on the MRI-Linac: Current Challenges and Potential Solutions. Clinical Oncology. 2018;30(11):702–710. doi: 10.1016/j.clon.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Tijssen R.H.N., Philippens M.E.P., Paulson E.S., Glitzner M., Chugh B., Wetscherek A., Dubec M., Wang J., van der Heide U.A. MRI commissioning of 1.5T MR-linac systems – a multi-institutional study. Radiother Oncol. 2019;132:114–120. doi: 10.1016/j.radonc.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor J.P.B., Aboagye E.O., Adams J.E., Aerts H.J.W.L., Barrington S.F., Beer A.J., Boellaard R., Bohndiek S.E., Brady M., Brown G., Buckley D.L., Chenevert T.L., Clarke L.P., Collette S., Cook G.J., deSouza N.M., Dickson J.C., Dive C., Evelhoch J.L., Faivre-Finn C., Gallagher F.A., Gilbert F.J., Gillies R.J., Goh V., Griffiths J.R., Groves A.M., Halligan S., Harris A.L., Hawkes D.J., Hoekstra O.S., Huang E.P., Hutton B.F., Jackson E.F., Jayson G.C., Jones A., Koh D.-M., Lacombe D., Lambin P., Lassau N., Leach M.O., Lee T.-Y., Leen E.L., Lewis J.S., Liu Y., Lythgoe M.F., Manoharan P., Maxwell R.J., Miles K.A., Morgan B., Morris S., Ng T., Padhani A.R., Parker G.J.M., Partridge M., Pathak A.P., Peet A.C., Punwani S., Reynolds A.R., Robinson S.P., Shankar L.K., Sharma R.A., Soloviev D., Stroobants S., Sullivan D.C., Taylor S.A., Tofts P.S., Tozer G.M., van Herk M., Walker-Samuel S., Wason J., Williams K.J., Workman P., Yankeelov T.E., Brindle K.M., McShane L.M., Jackson A., Waterton J.C. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14(3):169–186. doi: 10.1038/nrclinonc.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shukla-Dave A, Obuchowski NA, Chenevert TL, Jambawalikar S, Schwartz LH, Malyarenko D, et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging. 2019;49:e101-e2. [DOI] [PMC free article] [PubMed]
- 38.Yang Y., Cao M., Sheng K.e., Gao Y.u., Chen A., Kamrava M., Lee P., Agazaryan N., Lamb J., Thomas D., Low D., Hu P. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system: Longitudinal diffusion MRI using an MRI-guided radiotherapy system. Med. Phys. 2016;43(3):1369–1373. doi: 10.1118/1.4942381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Y.u., Han F., Zhou Z., Cao M., Kaprealian T., Kamrava M., Wang C., Neylon J., Low D.A., Yang Y., Hu P. Distortion-free diffusion MRI using an MRI-guided Tri-Cobalt 60 radiotherapy system: Sequence verification and preliminary clinical experience. Med. Phys. 2017;44(10):5357–5366. doi: 10.1002/mp.12465. [DOI] [PubMed] [Google Scholar]
- 40.Wojcieszynski A.P., Rosenberg S.A., Brower J.V., Hullett C.R., Geurts M.W., Labby Z.E., Hill P.M., Bayliss R.A., Paliwal B., Bayouth J.E., Harari P.M., Bassetti M.F. Gadoxetate for direct tumor therapy and tracking with real-time MRI-guided stereotactic body radiation therapy of the liver. Radiother Oncol. 2016;118(2):416–418. doi: 10.1016/j.radonc.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 41.Kooreman E.S., van Houdt P.J., Nowee M.E., van Pelt V.W.J., Tijssen R.H.N., Paulson E.S., Gurney-Champion O.J., Wang J., Koetsveld F., van Buuren L.D., ter Beek L.C., van der Heide U.A. Feasibility and accuracy of quantitative imaging on a 1.5 T MR-linear accelerator. Radiother Oncol. 2019;133:156–162. doi: 10.1016/j.radonc.2019.01.011. [DOI] [PubMed] [Google Scholar]