Abstract
The von Hippel–Lindau (VHL) tumor suppressor associates with transcription factors elongin-C and elongin-B to form the VHL–elongin-C–elongin-B protein complex and carry out its functions, such as degradation of hypoxia-inducible factors. VHL ligands are used not only to modulate hypoxia-signaling pathways and potentially treat chronic anemia or ischemia but also to form bivalent ligands as proteolysis-targeting chimeras to degrade proteins for potential therapeutic applications. Sensitive and selective VHL-based binding assays are critical for identifying and characterizing VHL ligands with high-throughput screening approaches. VHL ligand-binding assays, such as isothermal titration calorimetry, surface plasmon resonance, and fluorescence polarization assays, are reported but with limitations. Isothermal titration calorimetry requires higher protein concentrations with a lower throughput than fluorescence-based assays do. Surface plasmon resonance requires protein immobilization, which introduces variation and is not suitable for testing a large number of ligands. Fluorescence polarization can be sensitive with high-throughput capability but is susceptible to assay interference, and small-molecule-based fluorescent probes are not available. We developed the first small-molecule-based high-affinity VHL fluorescent probe BODIPY FL VH032 (5), with a Kd of 3.01 nM, for a time-resolved fluorescence resonance energy-transfer assay. This new assay is sensitive, selective, resistant to assay interference, and capable of characterizing VHL ligands with a wide range of affinities. It is also suitable for VHL ligand identification and characterization with high-throughput screening.
Introduction
The von Hippel–Lindau (VHL) tumor suppressor associates with transcription factors elongin-C and elongin-B to form the VHL–elongin-C–elongin-B (VCB) protein complex,1,2 which is critical for VHL functions, including degradation of its hypoxia-inducible factor (HIF) substrates.3 VHL ligands can be used to modulate VHL–HIF1α interactions4−8 and regulate hypoxia-signaling pathways, with potential applications for chronic anemia or ischemia treatment. The VHL ligands VH032 (1) and VH298 (2) are hydroxyproline (Hyp) derivatives, and the Hyp564 of HIF-1α is critical for its interaction with VHL.9,10 VHL ligands are also widely used to generate bivalent molecules such as proteolysis-targeting chimeras (PROTACs) to degrade proteins for potential therapeutic applications.11 MZ1 (3) is one such PROTAC,12 which joins the bromodomain and extraterminal domain (BET) inhibitor (+)-JQ1 (4)13 with VH032 (1) by a polyethylene glycol (PEG) linker.
To develop ligands for VHL, sensitive and selective assays that measure the binding affinities of VHL ligands are critical. Several assays have been reported, including direct binding assays of isothermal titration calorimetry (ITC),4,6−8,12,14,15 surface plasmon resonance (SPR),8,14 and competitive fluorescence polarization (FP), which use fluorescently labeled HIF-1α peptides (i.e., FAM-DEALAHyp-YIPD, 10 mer, MW: 1477.48 Da and FAM-DEALAHyp-YIPMDDDFQLRSF, 19 mer, M + H: 2617.167 Da) as fluorescent binding partners.4−6,8,14,16,17 In all reported ITC, SPR, and FP assays, the VCB complex (in which elongin-C and elongin-B associate with VHL and the association is critical for maintaining the function of VHL2) was used to determine optimal interactions. ITC assays typically require high VCB protein concentrations (e.g., 100 μM),4 whereas FP assays can use VBC protein concentrations as high as 1 μM16 or as low as 15 nM.8 In contrast, SPR assays require protein immobilization, which introduces variations and is not suitable for high-throughput ligand testing.8,14
FP assays can be of high throughput but with drawbacks. FP typically requires higher concentrations of target protein than that of the labeled probe,4−6,8,14,16,17 which may increase the protein demand when large numbers of ligands are tested.18,19 Furthermore, FP assays are typically more susceptible to assay interference than time-resolved fluorescence energy-transfer (TR-FRET) assays,20 causing higher rates of false positives and false negatives.21 In contrast, TR-FRET assays usually require much less protein (typically of low nM concentrations) than that of the labeled probe.22−25 Moreover, TR-FRET assays have the additional advantages of low-assay interference and less well-to-well variation because of their time-delayed measurements26 and ratiometric nature of detection,27 respectively.
We develop the first small-molecule-based VHL fluorescent probe, BODIPY FL VH032 (5), which has a high binding affinity (Kd = 3.01 nM) to the VCB protein complex in a TR-FRET binding assay. In the BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay, the terbium-labeled anti-GST antibody (Tb-anti-GST) binds with GST of the GST–VCB protein complex, and terbium is the donor fluorophore. BODIPY FL is the acceptor fluorophore and VH032 (1) is a high-affinity VHL ligand. Binding of BODIPY FL VH032 (5) to VHL of the GST–VCB protein complex places terbium and BODIPY FL in close proximity. When excited at 340 nm, terbium emits light centered at 490 nm28 with a bandwidth of ca. 10 nm which overlaps well with the excitation spectrum of BODIPY FL (with a peak at 504 nm and a relatively broad bandwidth).29 The acceptor fluorophore BODIPY FL is then excited and emits light at 520 nm. The 520 nm emission signal is due to the TR-FRET. In the presence of another VHL ligand, BODIPY FL VH032 (5) is displaced from the VHL binding site, resulting in a diminished TR-FRET signal because the donor fluorophore and the acceptor fluorophore no longer maintain close proximity. In the VHL FP assay, the BODIPY FL VH032 (5) probe yields a Kd value of 100.8 nM to the VCB protein complex. We then use BODIPY FL VH032 (5)-based TR-FRET and FP assays to test a panel of reported VHL ligands (Figure 1), including VH032 (1), VH298 (2), MZ1 (3), VH032 amine (6), Me-VH032 amine (7), BOC-VH032 (8), VH032 phenol (9), and VH032-PEG4-amine (10).12,14,30−32 We also test non-VHL ligands (Figure 2), including (+)-JQ1 (4), thalidomide-4′-oxyacetamido-alkylC4-amine (11, cereblon E3 ligase ligand), and dBET1 (12, bivalent BRD-CRBN PROTAC).33 Only the VHL ligands exhibit binding, demonstrating the specificity of the BODIPY FL VH032 (5)-mediated TR-FRET and FP assays. The BODIPY FL VH032 (5)-mediated FP assay demonstrates similar sensitivity to that of a previously reported FP assay with a FAM-labeled HIF-1α peptide (FAM-DEALAHyp-YIPMDDDFQLRSF, 19 mer). However, the BODIPY FL VH032 (5)-mediated TR-FRET assay is more sensitive and consumes less VCB protein than does the FP assays. In addition, the BODIPY FL VH032 (5)-mediated TR-FRET assay is resistant to assay interference, capable of detecting VHL ligands with a wide range of binding affinities and displays acceptable statistics with an average Z′ value of 0.91 in a pilot screen. Therefore, the new high-affinity VHL fluorescent probe BODIPY FL VH032 (5) can be used in a TR-FRET assay that is sensitive, selective, resistant to assay interference, and suitable for VHL ligand identification and characterization in large-scale screening.
Figure 1.

Structures of a panel of VHL ligands.
Figure 2.

Structures of a panel of non-VHL ligands.
Results and Discussion
Synthesis of VH032 (1)
VH032 (1) is a potent VHL inhibitor8,14 and is the ligand moiety in our designed fluorescent probe BODIPY FL VH032 (5) (Figure 3). We prepared VH032 (1) by acetylating VH032 amine (6) with acetic anhydride (Ac2O) in the presence of N,N-diisopropylethylamine (DIPEA), with dichloromethane (DCM) as the solvent. The yield was 60.6% after purification with a Prep-HPLC system (Scheme 1).
Figure 3.
Design of BODIPY FL VH032 (5).
Scheme 1. Synthesis of VH032 (1).
Design of BODIPY FL VH032 (5)
The BODIPY FL VH032 (5) probe was designed on the basis of MZ1 (3). MZ1 (3) is a bivalent BRD–VHL PROTAC molecule, with the (+)-JQ1 (4) moiety joined to the VH032 (1) moiety with a PEG linker.12 MZ1 maintains binding affinities to both BRD and VHL proteins via its corresponding (+)-JQ1 (4) and VH032 (1) moieties. We rationalized that a high-affinity VHL fluorescent probe will be obtained if the (+)-JQ1 (4) moiety in MZ1 (3) is replaced with a fluorescent moiety, such as a BODIPY fluorophore, and the PEG linker and VH032 moiety portions remain intact (Figure 1). We thus designed the BODIPY FL VH032 (5) probe accordingly (Figure 3).
Synthesis of BODIPY FL VH032 (5)
The BODIPY FL VH032 (5) probe was prepared by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide- and hydroxybenzotriazole-mediated coupling34 between VH032-PEG4-amine (10) and BODIPY FL propionic acid (13) in the presence of DIPEA at room temperature, with a yield of 64.3% after flash column chromatography purification (Scheme 2).
Scheme 2. Synthesis of BODIPY FL VH032 (5).

BODIPY FL VH032 (5) Displays High Binding Affinity to VHL in a TR-FRET Assay
To measure the binding affinity of BODIPY FL VH032 (5) to VHL, we incubated dilutions of BODIPY FL VH032 (5, 1 to 2 dilutions, with an optimized concentration range of 0.06–500 nM) with 2 nM terbium (Tb)-labeled anti-GST antibody in the presence of 2 nM GST–VCB. We also included groups of samples without GST–VCB or with additional VH298 (2, 30 μM) in the presence of 2 nM GST–VCB to determine the background interactions between BODIPY FL VH032 (5) and the Tb-anti-GST antibody or between the BODIPY FL VH032 (5) and the complex of Tb-anti-GST antibody and GST–VCB protein in the presence of VH298 (2). We then measured the TR-FRET signals every 30 min, from 30 to 300 min.
We first analyzed the TR-FRET signals of the groups with 2 nM GST–VCB and without GST–VCB by fitting them with the equation for a one-site total binding with nonspecific interactions in GraphPad PRISM software (Figure 4A). In the presence of Tb-anti-GST, the interaction between BODIPY FL VH032 (5) and GST–VCB increased exponentially in the BODIPY FL VH032 (5) concentration range of 0.06–15.6 nM. The interaction then increased in a linear manner in the BODIPY FL VH032 (5) concentration range of 15.6–500 nM (Figure 4A, top panel curves). The Kd values were derived from the 2 nM GST–VCB group and were 3.61, 3.22, 3.01, 3.04, 3.01, 2.98, 2.96, 2.98, 2.99, and 3.04 nM for the incubation times of 30, 60, 90, 120, 150, 180, 210, 240, 270, and 300 min, respectively. The Kd values were generally very stable at approximately 3.0 nM from 90 to 300 min, indicative of a high-affinity interaction between BODIPY FL VH032 (5) and GST–VCB. To our knowledge, BODIPY FL VH032 (5) is the first small-molecule-based VHL fluorescent probe. In the absence of GST–VCB, we observed a linear and very low TR-FRET interaction between BODIPY FL VH032 (5) and Tb-anti-GST in the entire BODIPY FL VH032 (5) concentration range of 0.06 to 500 nM, indicative of low nonspecific background interactions (Figure 4A, bottom curves).22,23
Figure 4.
TR-FRET binding affinity of BODIPY FL VH032 (5, 1 to 2 dilutions, at an optimized concentration range of 0.06–500 nM) to GST–VCB. (A) Binding interaction of BODIPY FL VH032 (5) to 2 nM Tb-anti-GST in the presence of 2 nM GST–VCB or in the absence of GST–VCB at the designated incubation times. “+” and “–” (after each time point) represent “with GST–VCB” and “without GST–VCB”, respectively. The TR-FRET signals were expressed as relative TR-FRET units (RTU), which were calculated using 10,000 × 520 nm/490 nm. (B) Binding interaction of BODIPY FL VH032 (5) and 2 nM Tb-anti-GST, with 2 nM GST–VCB, 2 nM GST–VCB + VH298 (2, 30 μM), or without GST–VCB + DMSO at the 90-min incubation time. (C) Fold changes in the TR-FRET signals of BODIPY FL VH032 (5) with (2 nM GST–VCB + DMSO) to (2 nM GST–VCB + VH298) (2, 30 μM) (blue curve) or to (without GST–VCB + DMSO) (red curve).
Because 90 min of incubation time was the earliest stable time point, we used the 90 min time point for further examination with 2 nM GST–VCB + VH298 (2, 30 μM). The three groups of data—2 nM GST–VCB + dimethyl sulfoxide (DMSO), 2 nM GST–VCB + VH298 (2, 30 μM), and without GST–VCB + DMSO—at 90 min were graphed by fitting with the equation for a one-site total binding with nonspecific interactions in GraphPad PRISM (Figure 4B). The curve derived from the group of data with 2 nM GST–VCB + DMSO represented the total interaction between BODIPY FL VH032 (5) and GST–VCB in the presence of Tb-anti-GST, with a Kd value of 3.01 nM (Figure 4B, top blue curve). In the presence of VH298 (2, 30 μM), a potent VHL inhibitor,8,14 the TR-FRET signal between BODIPY FL VH032 (5) and GST–VCB in the presence of Tb-anti-GST (Figure 4B, red curve) was very similar to that of the group without GST–VCB (Figure 4B, green curve). The overlap of the VH298 (2, 30 μM)-inhibited curve with the background curve demonstrated minimal nonspecific interactions between BODIPY FL VH032 (5) and GST–VCB. For comparison, we also calculated the Kd value by subtracting the background interaction (green curve in Figure 4B from the “without GST–VCB + DMSO” group) from the total interaction (blue curve in Figure 4B from the “2 nM GST–VCB + DMSO” group) to obtain the specific interaction between BODIPY FL VH032 (5) and GST–VCB. This analysis yielded a Kd value of 3.39 nM, which is very similar to the Kd of 3.01 nM derived from the total interaction curve (blue curve in Figure 4B).
To characterize VHL ligands for their inhibitory binding activities in the TR-FRET assay, the concentration of the BODIPY FL VH032 (5) fluorescent probe is very important. To elucidate the most sensitive BODIPY FL VH032 (5) concentration for the TR-FRET assay, we plotted the fold changes in the TR-FRET signals at various BODIPY FL VH032 (5) concentrations between two different groups: (1) [2 nM GST–VCB + DMSO] and [2 nM GST–VCB + VH298 (2, 30 μM)] (Figure 4C, blue curve) and (2) [2 nM GST–VCB + DMSO] and [without GST–VCB + DMSO] (Figure 4C, red curve). The curves of the signal fold change overlapped well, with only very small differences. The highest signal fold changes were observed at 3.9 or 7.8 nM BODIPY FL VH032 (5), with the [2 nM GST–VCB + DMSO] and [2 nM GST–VCB + VH-298 (2, 30 μM)] curves (blue) at 7.8 nM (15.1-fold) and the [2 nM GST–VCB + DMSO] and [without GST–VCB + DMSO] curve (red) at 3.9 nM (16.2-fold). Because using probe concentrations closer to the Kd concentration leads to less deviation in Ki calculation of the tested ligands,35 we used 4 nM BODIPY FL VH032 (5) (Kd = 3.01 nM) for further TR-FRET assay development.
BODIPY FL VH032 (5) VHL TR-FRET Signal Is Stable
Signal stability is very important for obtaining consistent activities of tested compounds. We established the BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay with the following assay conditions: 4 nM BODIPY FL VH032 (5), 2 nM GST–VCB, 2 nM Tb-anti-GST, and a tentative 90 min of incubation time based on the signal stability observed without competing compounds.
To further evaluate signal stability in the presence of competing compounds, we used the potent VHL inhibitor VH298 (2, 30 μM) as positive control and DMSO as negative control because the compound stock solutions were prepared in DMSO. In addition, the group of samples with 4 nM BODIPY FL VH032 (5), 2 nM Tb-anti-GST, and DMSO, but without GST–VCB, was included as the background control. The performance of controls at different incubation time points was summarized in the form of relative TR-FRET units (RTUs) (Figure 5A) and the signal fold change from 2 nM GST–VCB + VH298 (2, 30 μM) (Figure 5B). The positive control group had TR-FRET signals (278.7 ± 4.5) and a signal fold change to 2 nM GST–VCB + VH298 (2, 30 μM) (1.00 ± 0.03) that were very similar to those (276.6 ± 5.4 for TR-FRET signals and 0.99 ± 0.03 for the signal fold change) of the background control group at all incubation times (Figure 5B). The overall TR-FRET signals for the negative control group were slightly lower at 30-min and 60-min incubation time points, with respective RTUs of 3451 and 3679 counts, but very stable RTUs of 3858 ± 46 from 90 to 300 min (Figure 5A). In terms of signal-fold change to 2 nM GST–VCB + VH298 (2, 30 μM) (Figure 5B), the negative control group exhibited 12.05- and 12.94-fold changes at 30- and 60-min incubation times, respectively. The signal was then stable at 13.93 ± 0.09, with a coefficient of variance of 0.67%, from 90 to 300 min. Thus, the BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay maintains stable signals from incubation times of 90–300 min.
Figure 5.
Signal stability of the BODIPY FL VH032 (5)-based VHL TR-FRET assay. (A) TR-FRET interaction of 4 nM BODIPY FL VH032 (5) and 2 nM Tb-anti-GST with 2 nM GST–VCB + DMSO (negative control), 2 nM GST–VCB + VH298 (2, 30 μM) (positive control), or without GST–VCB + DMSO (background control) at specified incubation time points. (B) TR-FRET signal-fold change to 2 nM GST–VCB + VH298 (2, 30 μM) of 2 nM GST–VCB + DMSO (negative control), 2 nM GST–VCB + VH298 (2, 30 μM) (positive control), or without GST–VCB + DMSO (background control) in the presence of 4 nM BODIPY FL VH032 (5) and 2 nM Tb-anti-GST at specified incubation time points. (C) Dose–response inhibition curves of VH298 (2, 1–3 dilutions, in the concentration range of 2.1 pM to 30 μM) at specified incubation time points in the presence of 4 nM BODIPY FL VH032 (5), 2 nM GST–VCB, and 2 nM Tb-anti-GST.
We further evaluated the signal stability of the assay with the positive control VH298 (2) in a dose-dependent manner (1–3 dilutions, with an optimized concentration range of 2.09 pM to 30 μM) (Figure 5C). We observed half-maximal inhibitory concentration (IC50) values of 42.17, 43.27, 44.46, 42.93, 43.20, 44.04, 43.86, 45.74, 45.13, and 48.27 nM at the respective time points from 30 to 300 min, with an average IC50 value of 44.31 nM, standard deviation of 1.75 nM, and 3.95% coefficient of variation. Our results further demonstrated that the BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay yields stable signals over a wide range of incubation times.
BODIPY FL VH032 (5)-Mediated VHL TR-FRET Binding Assay Is Sensitive and Selective
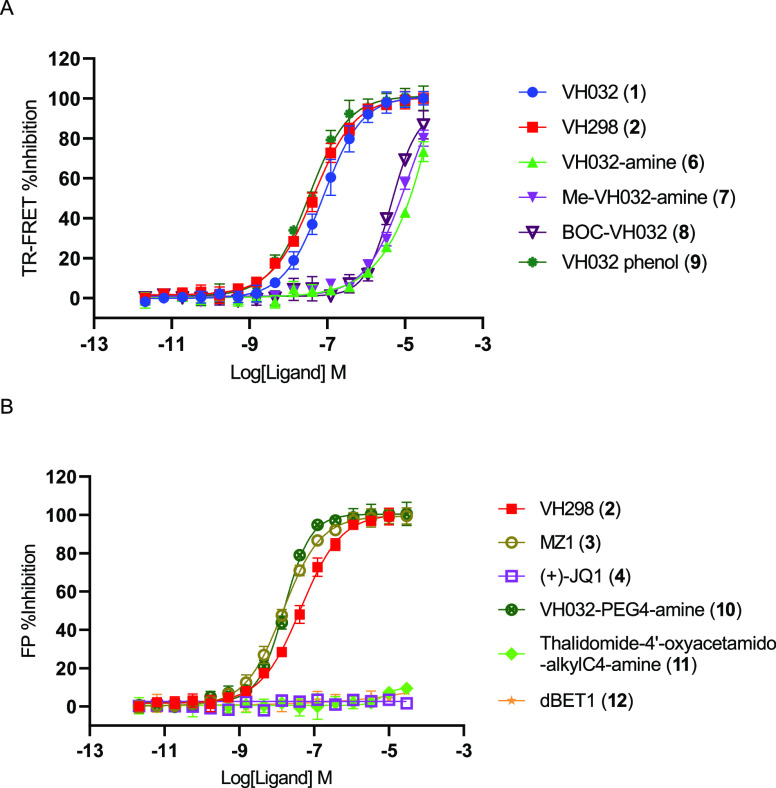
We next tested a panel of reported VHL ligands and non-VHL ligands for their ability to inhibit VHL. The VHL ligands included VH032 (1), VH298 (2), VH032 amine (6), Me-VH032 amine (7), BOC-VH032 (8), VH032 phenol (9), VH032-PEG4-amine (10), and the dual VHL and BRD PROTAC ligand MZ1 (3). The non-VHL ligands included (+)-JQ1 (4), thalidomide-4′-oxyacetamido-alkylC4-amine (11), and dBET1 (12). We used the optimized assay conditions of 4 nM BODIPY FL VH032 (5), 2 nM GST–VCB, 2 nM Tb-anti-GST, and a 90-min incubation time, along with a DMSO negative control and a VH298 (2, 30 μM) positive control. The dose–response curves of VH032 (1), VH298 (2), VH032 amine (6), Me-VH032 amine (7), BOC-VH032 (8), and VH032 phenol (9) are summarized in Figure 6A. The dose–response curves of MZ1 (3), dBET1 (12), (+)-JQ1 (4), VH032-PEG4-amine (10), thalidomide-4′-oxyacetamido-alkylC4-amine (11), and VH298 (2) positive control are depicted in Figure 6B.
Figure 6.
Dose–response curves of a panel of VHL ligands and non-VHL ligands in the presence of BODIPY FL VH032 (5, 4 nM), 2 nM GST–VCB, and 2 nM Tb-anti-GST at a 90-min incubation time. Ligand-relative TR-FRET units (RTUs) at their individual concentrations were normalized to that of VH298 (2, 30 μM, positive control, 100% inhibition) and DMSO (negative control, 0% inhibition) and fitted to a sigmoidal equation with GraphPad PRISM to derive the IC50 values, if applicable. The Ki values were calculated with the Cheng–Prusoff equation.35 (A) Dose–response curves of VHL ligands VH032 (1), VH298 (2), VH032 amine (6), Me-VH032 amine (7), BOC-VH032 (8), and VH032 phenol (9). (B) Dose–response curves of VHL ligands VH298 (2), MZ1 (3), and VH032-PEG4-amine (10) and of non-VHL ligands (+)-JQ1 (4), thalidomide-4′-oxyacetamido-alkylC4-amine (11), and dBET1 (12).
VH032 (1), VH298 (2), VH032 amine (6), Me-VH032 amine (7), BOC-VH032 (8), VH032 phenol (9), VH032-PEG4-amine (10), and MZ1 (3) exhibited IC50 values of 77.8 nM, 44.0 nM, 13.3 μM, 7.9 μM, 4.9 μM, 34.0 nM, 5.9 nM, and 14.7 nM, respectively, and Ki values of 33.4 nM, 18.9 nM, 5.7 μM, 3.4 μM, 2.1 μM, 14.6 nM, 6.8 nM, and 6.3 nM, respectively. Among the VHL ligands tested, MZ1 (3) was the most potent, with a Ki value of 6.3 nM. The least potent ligand was VH032 amine (6), with a Ki value of 5.7 μM. An over 904-fold activity difference occurred between the most and least potent ligands, indicating that the BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay is sensitive enough to characterize VHL ligands with high-, medium-, or low-inhibitory activities. As expected, the non-VHL ligands (+)-JQ1 (4), thalidomide-4′-oxyacetamido-alkylC4-amine (11), and dBET1 (12) were inactive (Figure 6B), demonstrating that the assay selectively detects only VHL ligands.
Optimizing BODIPY FL VH032 (5) Concentration for a VHL FP Assay
To compare the TR-FRET and FP assay formats, we also developed a VHL FP assay with BODIPY FL VH032 (5) as the fluorescent probe. To establish an FP assay that is both sensitive and robust, an optimal probe concentration is critical because too much probe will decrease the sensitivity, and insufficient probe levels will reduce the robustness. We optimized the probe concentration by incubating dilutions of GST–VCB (1 to 2 dilutions; optimal concentration range of 0.03–1000 nM) with BODIPY FL VH032 (5) at 70, 60, 50, 40, 30, 20, 10, 5, 2, and 1 nM (Figure 7). BODIPY FL VH032 (5) probe concentrations ranging from 70 nM to 10 nM (Figure 7A) did not affect the GST–VCB concentration curves, except that slight FP signal increases occurred at certain GST–VCB concentrations (125, 250, and 500 nM) with 10 nM BODIPY FL VH032 (5). However, lower concentrations of BODIPY FL VH032 (5) (i.e., 5, 2, or 1 nM) (Figure 7B) caused substantially increased background at lower GST–VCB concentrations (Figure 7B), with background interactions increasing from 21 to 33, 52, and 71 mP for BODIPY FL VH032 (5) concentrations of 10, 5, 2, and 1 nM, respectively. In addition, we observed marked FP signal variations at lower BODIPY FL VH032 (5) concentrations, especially at 2 and 1 nM (Figure 7B). Therefore, we used 10 nM BODIPY FL VH032 (5) for the FP assay.
Figure 7.
BODIPY FL VH032 (5) concentration optimization in a VHL FP assay with GST–VCB (1 to 2 dilutions; in an optimized concentration range of 0.03–1000 nM) and a 90-min incubation time. (A) VHL FP assay performance with BODIPY FL VH032 (5) concentrations of 70, 60, 50, 40, 30, 20, and 10 nM. (B) VHL FP assay performance with BODIPY FL VH032 (5) concentrations of 10, 5, 2, and 1 nM.
BODIPY FL VH032 (5) Exhibits High VHL Affinity in an FP Assay
To determine the optimal GST–VCB concentration for the BODIPY FL VH032 (5)-mediated VHL FP assay, we incubated 10 nM BODIPY FL VH032 (5) with dilutions of GST–VCB (1 to 2 dilutions; in an optimized concentration range of 0.03–1000 nM) plus DMSO (total interactions) or VH298 (2, 30 μM) (GST–VCB-mediated nonspecific interactions). In addition, we used 10 nM BODIPY FL VH032 (5) with DMSO only and without GST–VCB to determine the background interaction signal. We fit the FP signals from the three groups to the equation for one-site total binding with nonspecific interactions in GraphPad PRISM (Figure 8). The curve derived from the total interaction group (i.e., DMSO group) represented the total FP interaction between BODIPY FL VH032 (5) and GST–VCB, with a Kd value of 100.8 nM (Figure 8, blue curve). The FP signals from the GST–VCB-mediated nonspecific interaction with VH298 (2, 30 μM) (Figure 8, red curve) were very similar to those of the background interaction without GST–VCB (Figure 8, green curve), except that the FP signal (43.0 mP) at the highest GST–VCB concentration (1000 nM) in the presence of VH298 (2, 30 μM) was slightly higher than the background FP signal (24.8 mP). Therefore, we used 100 nM GST–VCB to minimize GST–VCB-mediated nonspecific interactions. At 100 nM, the GST–VCB concentration approached the BODIPY FL VH032 (5) Kd value of 100.8 nM, balancing the sensitivity and signal window for the FP assay.36
Figure 8.
Determination of the binding affinity of BODIPY FL VH032 (5, 10 nM) to GST–VCB in an FP assay with GST–VCB (1 to 2 dilutions; in the optimal concentration range of 0.03–1000 nM) + DMSO (total interactions), GST–VCB (1 to 2 dilutions; in the optimal concentration range of 0.03–1000 nM) + VH298 (2, 30 μM) (i.e., GST–VCB-mediated nonspecific interactions), or DMSO without GST–VCB (i.e., background interactions) at a 90-min incubation time.
BODIPY FL VH032 (5)-Mediated VHL FP Assay Is Sensitive and Selective for Detecting VHL Ligands
We applied the FP assay with the established conditions of 10 nM BODIPY FL VH032 (5), 100 nM GST–VCB, and a 90-min incubation time to characterize the VHL ligands for their binding affinities. We subjected the DMSO negative control, VH298 (2, 30 μM) positive control, and dilutions (1–3 dilutions; in the concentration range of 2.1 pM to 30 μM) of the same panel of VHL ligands and non-VHL ligands used in the TR-FRET assay to the FP assay. The DMSO and VH298 (2, 30 μM) control samples exhibited FP signals of 143.75 and 14.5 mP, respectively (Figure 9A), and fold-change FP signals from VH298 (2, 30 μM) of 9.91 and 1.00, respectively (Figure 9B). The fold-change FP signal of 9.91 between the DMSO and VH298 (2, 30 μM) controls was slightly less than that of the TR-FRET assay (i.e., 13.88).
Figure 9.
Activities of controls and selected VHL or non-VHL ligands in the BODIPY FL VH032 (5)-mediated VHL FP assay with 10 nM BODIPY FL VH032 (5) and 100 nM GST–VCB at a 90-min incubation time. (A) FP assay signals of DMSO and VH298 (2, 30 μM). (B) FP signal fold change from VH298 (2, 30 μM) of DMSO and VH298 (2, 30 μM). (C) FP dose–response curves of the VHL ligands VH032 (1), VH298 (2), VH032 amine (6), Me-VH032 amine (7), BOC-VH032 (8), and VH032 phenol (9). (D) FP dose–response curves of VH298 (2), MZ1 (3), and VH032-PEG4-amine (10) and of the non-VHL ligands (+)-JQ1 (4), thalidomide-4′-oxyacetamido-alkylC4-amine (11), and dBET1 (12).
The dose–response curves of the VHL ligands VH032 (1), VH298 (2), VH032 amine (6), Me-VH032 amine (7), BOC-VH032 (8), and VH032 phenol (9) in the FP assay are depicted in Figure 9C. The dose–response curves for MZ1 (3), dBET1 (12), (+)-JQ1 (4), VH032-PEG4-amine (10), thalidomide-4′-oxyacetamido-alkylC4-amine (11), and VH298 (2) positive control are depicted in Figure 9D. VH032 (1), VH298 (2), MZ1 (3), BOC-VH032 (8), VH032 phenol (9), and VH032-PEG4-amine (10) exhibited IC50 values of 352.2 nM, 288.2 nM, 226.2 nM, 16.3 μM, 212.5 nM, and 430.8 nM, respectively, and Ki values of 142.1 nM, 110.4 nM, 79.7 nM, 8.0 μM, 77.9 nM, and 181.0 nM, respectively.
The maximal inhibition of VH032 amine (6) was only 36.6% at the maximum tested concentration of 30 μM; therefore, its IC50 and Ki values could not be determined. However, its corresponding TR-FRET IC50 and Ki values were 13.3 μM and 5.7 μM, respectively (Figure 6A), indicating that the TR-FRET assay is more sensitive than the FP assay for detecting VHL ligands with lower binding affinities. Furthermore, the TR-FRET assay was more robust than the FP assay for testing ligands that introduce assay interference. For example, Me-VH032 amine (7) disturbed FP assay detection at a concentration equal to or higher than 370 nM (Figure 9C, purple curve with solid inverted triangles). However, this assay interference did not occur in the TR-FRET assay (Figure 6A). We found the respective IC50 and Ki values of 7.9 μM and 3.4 μM for Me-VH032 amine (7) in the TR-FRET assay without any interference. Nevertheless, the FP assay was as selective as the TR-FRET assay because the non-VHL ligands (+)-JQ1 (4), thalidomide-4′-oxyacetamido-alkylC4-amine (11), and dBET1 (12) did not inhibit VHL in the FP assay.
Comparing the Newly Developed VHL TR-FRET and FP Assays with a Previously Reported VHL FP Assay
Fluorescently labeled peptides derived from HIF-1α protein were previously used to characterize VHL ligands in FP assays.4,8 Two versions of FAM- HIF-1α peptides—FAM-DEALAHyp-YIPD (10 mer, MW: 1477.48) and FAM-DEALAHyp-YIPMDDDFQLRSF (19 mer, M + H: 2617.167)—are reported. FAM-DEALAHyp-YIPD (10 mer) and FAM-DEALAHyp-YIPMDDDFQLRSF (19 mer) exhibited respective Kd values of 560 and 36 nM in one previously reported FP assay.4 Our small-molecule BODIPY FL VH032 (5, MW: 937.91 Da) probe exhibited a Kd value of 3.01 nM in the TR-FRET assay, which is similar to that of 3 nM for FAM-DEALAHyp-YIPMDDDFQLRSF (19 mer, M + H: 2617.167) in a previously reported FP assay,8,14 even though BODIPY FL VH032 (5) is smaller than FAM-DEALAHyp-YIPMDDDFQLRSF. Moreover, BODIPY FL VH032 (5) had a FP Kd value of 100.8 nM, which is better than the reported FP Kd value of 560 nM for the 10 mer FAM-DEALAHyp-YIPD HIF-1α peptide (10 mer, MW: 1477.48),4 although the FAM-DEALAHyp-YIPD HIF-1α peptide was derived from a protein of large molecular size.
We next tested the three VHL ligands VH032 (1), VH298 (2), and BOC-VH032 (8) using our new BODIPY FL VH032 (5)-based TR-FRET and FP assays and compared the activities with those from the reported FAM–HIF–1α peptide (19 mer)-based FP assay14 (Table 1). Although the affinities of VH298 (2), VH032 (1), and BOC-VH032 (8) exhibited the same rank order (high to low) among the three assays, the BODIPY FL VH032 (5)-based TR-FRET assay was the most sensitive. The most potent inhibitor VH298 (2) exhibited VHL inhibitory activity values of 80 nM (Kd), 18.9 nM (Ki), and 110.4 nM (Ki) in the FAM–HIF–1α peptide (19 mer)-mediated FP assay, BODIPY FL VH032 (5)-mediated TR-FRET assay, and FP assay, respectively. For VH298 (2) activity, our BODIPY FL VH032 (5)-based TR-FRET assay was more sensitive (4.23-fold) than the FAM–HIF–1α peptide (19 mer)-based FP assay. Similarly improved sensitivity of the TR-FRET assay also occurred for VH032 (1) and BOC-VH032 (8) (4.49-fold and 3.09-fold, respectively). In addition, less VCB protein (2 nM) was consumed in the TR-FRET assay than in the previously reported FP assay, which consumed 15 nM of VCB protein. However, our BODIPY FL VH032 (5)-based FP assay exhibited comparable sensitivity with the previously reported FP assay with the FAM–HIF–1α peptide (19 mer).
Table 1. Comparison of a Previously Reported VHL FP Assay and Our Newly Developed VHL TR-FRET and FP Assays.
| assay components and compounds tested | FP assay14 | TR-FRET assaya | FP assaya |
|---|---|---|---|
| probe used (Kd) | FAM–HIF–1α peptide (3 nM) | BODIPY FL VH032 (5) (3.01 nM) | BODIPY FL VH032 (5) (100.8 nM) |
| reagent concentration | 10 nM FAM–HIF–1α, 15 nM VCB | 4 nM BODIPY FL VH032 (5), 2 nM GST–VCB, 2 nM Tb-anti-GST | 10 nM BODIPY FL VH032 (5), 100 nM GST–VCB |
| VH032 (1) potency | Kd = 150 nM | Ki = 33.4 nM | Ki = 142.1 nM |
| VH298 (2) potency | Kd = 80 nM | Ki = 18.9 nM | Ki = 110.4 nM |
| MZ1 (3) potency | NAb | 6.3 nM | 79.7 nM |
| BOC-VH032 (8) potency | Kd = 6.5 μM | Ki = 2.1 μM | Ki = 8.0 μM |
| VH032 phenol (9) potency | NA | Ki = 14.6 nM | Ki = 77.9 nM |
| VH032-PEG4-amine (10) potency | NA | Ki = 6.8 nM | Ki = 181.0 nM |
Assay developed in this report.
NA, not available.
We also compared VHL ligand activity in both the BODIPY FL VH032 (5)-mediated TR-FRET and FP assays (Table 1). The TR-FRET assay was more sensitive (with lower Ki values) than the FP assay for all the active ligands, including VH032 (1), VH298 (2), MZ1 (3), BOC-VH032 (8), VH032 phenol (9), and VH032-PEG4-amine (10). The most potent ligand tested with the TR-FRET assay was MZ1 (3), with a Ki value of 6.3 nM. The weakest VHL ligand tested with the TR-FRET assay was VH032 amine (6), with a Ki value of 5.7 μM (Figure 6A). Thus, the TR-FRET assay detected compounds with Ki values ranging from 6.3 nM to 5.7 μM. The rank order of activity was generally similar for the tested VHL ligands in the two assays, although we did observe slight differences. The TR-FRET and FP assays may yield slightly different rank orders of activity when a common set of ligands are tested,37−39 but the TR-FRET assay appeared superior to the FP assay because of its lower assay variability, lower nonspecific interference, and better correlation with cell-based assays.40 A TR-FRET assay is more sensitive than the corresponding FP assay using the same fluorescent probe and generates lower IC50 or Ki values of ligands. This is mostly due to the combination of a lower probe Kd value and smaller amount of proteins and probes used in the TR-FRET assay than those used in a corresponding FP assay. A similar observation has been reported in another study.28
DMSO Tolerance Test for the BODIPY FL VH032 (5)-Mediated VHL Binding Assay
DMSO is a common solvent for chemicals used in biological assays. All tested chemicals in this report have been prepared as DMSO stock solutions before further diluted to the desired final concentrations with aqueous assay buffers. However, it has been reported that DMSO could negatively affect assays, especially at higher concentrations.22−25
To investigate the impact of DMSO on the BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay, we tested its performance at various DMSO concentrations using the established assay conditions of 4 nM BODIPY FL VH032 (5), 2 nM GST–VCB, 2 nM Tb-anti-GST, and a 90-min incubation time. The TR-FRET signals of the negative control DMSO and the positive control VH298 (2, 30 μM) were first measured in the presence of 0.2, 0.5, 1, 2, 5, or 10% DMSO (Figure 10A). The respective RTUs of the negative control DMSO group were 3729, 3685, 3491, 3108, 2258, and 1141 with the corresponding DMSO concentrations of 0.2, 0.5, 1, 2, 5, and 10%. Only a slight decrease in the TR-FRET signal was observed when the DMSO concentration increased from 0.2% (TR-FRET signal % RTU was set as 100%) to 0.5% (98.8%) or 1% (93.6%) (Figure 10B). The reduction in the TR-FRET signal % RTU accelerated when the DMSO concentration was increased further (83.3, 60.6, and 37.8% TR-FRET signal %RTU at 2%, 5%, and 10% DMSO, respectively) (Figure 10B). For the positive control VH298 (2, 30 μM) group in which the TR-FRET signal is always very low, the RTUs were 306, 282, 289, 287, 241, and 238 (Figure 10A) and % RTUs were 8.2, 7.6, 7.7, 7.7, 6.5, and 6.4% (Figure 10B) with the corresponding DMSO concentrations of 0.2, 0.5, 1, 2, 5, and 10%, respectively.
Figure 10.

DMSO tolerance of the BODIPY FL VH032 (5)-mediated VHL TR-FRET assay in the presence of 4 nM BODIPY FL VH032 (5), 2 nM GST–VCB, and 2 nM Tb-anti-GST at the 90-min incubation time point. (A) RTU of the negative control DMSO or the positive control VH298 (2, 30 μM) in the presence of 0.2, 0.5, 1, 2, 5, or 10% DMSO. (B) % RTU (% RTU of 0.2% DMSO was set as 100%) of the negative control DMSO or the positive control VH298 (2, 30 μM) in the presence of 0.2, 0.5, 1, 2, 5, or 10% DMSO. (C) Dose–response inhibition curves of VH298 (2, 1–3 dilutions; in the concentration range of 2.1 pM to 30 μM) in the presence of indicated DMSO concentrations.
We further evaluated the effect of DMSO on the BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay using the positive control VH298 (2) in a dose-dependent manner (1–3 dilutions; in the optimized concentration range of 2.09 pM to 30 μM) (Figure 10C). The observed IC50 value was 45.2, 45.5, 44.4, 57.9, 81.1, or 110.6 nM with the corresponding DMSO concentrations of 0.2, 0.5, 1, 2, 5, or 10%, respectively. The observed IC50 values of 45.2, 45.5, and 44.4 nM for the corresponding DMSO concentrations of 0.2, 0.5, and 1%, respectively, along with the minimal change in the TR-FRET signal at low VH298 (2) concentrations, were consistent with the minimal effect of DMSO concentration at or below 1%. A more dramatic IC50 value change was observed for DMSO concentrations of 2, 5, and 10% with respective observed IC50 values of 57.9, 81.1, and 110.6 nM, along with a more dramatic overall TR-FRET signal change at low VH298 (2) concentrations.
The DMSO tolerance test demonstrated that the effect of DMSO on the BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay is minimal when the DMSO concentration is 1% or less, but is more apparent with the DMSO concentration higher than 1%, such as 2, 5, or 10%. Similar DMSO effects on TR-FRET and FP assays have been reported,22−25,41,42 so it is reasonable to anticipate that DMSO affects the BODIPY FL VH032 (5)-mediated VHL FP and TR-FRET binding assays similarly.
Based on our DMSO tolerance test, we suggest that the BODIPY FL VH032 (5)-mediated VHL TR-FRET assay be performed at a DMSO concentration at or below 1%. In this report, we have used 0.2% DMSO concentration in all assays.
Pilot Screening Using the BODIPY FL VH032 (5)-Mediated VHL TR-FRET Binding Assay
To further evaluate the performance of the BODIPY FL VH032 (5)-based TR-FRET assay, we performed a pilot screening. We used a custom-assembled alpha-helix library from ChemDiv, Inc. (San Diego, CA) with 2,011 alpha-helix-mimetic small molecules, based on the knowledge that the currently reported potent VHL ligands were derived from proteins or peptides.4−8,14
For each 384-well assay plate, the tested chemicals (10 μM) were placed in columns 3–12 and columns 15–24; the positive control VH298 (2, 30 μM) group and the negative control DMSO group were, respectively, plated in columns 1 and 13 with 16 wells for each control group. For the seven screening plates (Figure 11A), the negative control had respective RTU [data were expressed as mean ± SD (CV)] of 4017 ± 77 (1.9%), 4066 ± 93 (2.3%), 4116 ± 82 (2.0%), 4114 ± 71 (1.7%), 4082 ± 130 (3.2%), 4116 ± 93 (2.3%), and 4112 ± 88 (2.1%); the positive control had the corresponding RTUs of 287 ± 25 (8.7%), 290 ± 18 (6.3%), 285 ± 26 (9.0%), 282 ± 12 (4.2%), 288 ± 16 (5.5%), 283 ± 19 (6.8%), and 276 ± 21 (7.8%), respectively. The average interplate negative control RTU was 4089 ± 91 (2.2%) and the average interplate positive control RTU was 284 ± 20 (6.9%). For both intraplate and interplate controls, the TR-FRET signals of negative or positive control were very much consistent with CV less than 5% for the negative control group and less than 10% for the positive control group, demonstrating the robustness of the BODIPY FL VH032 (5)-based TR-FRET assay. The signal fold change (RTUnegative/RTUpositive) for all seven assay plates was greater than 13-fold: 14.0 ± 0.3, 14.0 ± 0.3, 14.5 ± 0.3, 14.6 ± 0.3, 14.2 ± 0.5, 14.6 ± 0.3, and 14.9 ± 0.3 for the assay plates 1–7, respectively (Figure 11A). The Z-prime value for assay plates 1–7 was 0.92, 0.91, 0.92, 0.94, 0.88, 0.91, and 0.91 (Figure 11B), demonstrating acceptable statistics,43 and suggesting that the BODIPY FL VH032 (5)-based TR-FRET assay is suitable for high throughput screening.
Figure 11.
Pilot screening using the BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay in the presence of 4 nM BODIPY FL VH032 (5), 2 nM GST–VCB, and 2 nM Tb-anti-GST at the 90-min incubation time point. (A) Plate-by-plate negative (DMSO) and positive (VH298, 2, 30 μM) control performance. (B) Screening scatterplot of plate Z-prime. (C) Screening scatterplot of activity values, where the positive control is VH298 (2, 30 μM, green dots, 100% inhibition), the negative control is DMSO (red dots, 0% inhibition), active compounds (blue dots) are chemicals with % inhibition ≥30% with the 30% activity cutoff defined by the black dotted line, and inactive compounds (black dots) are chemicals with % inhibition <30%. (D) Chemical structures and VHL binding activities of compounds SJ000994241-1 (14), SJ000994129-1 (15), SJ000994509-1 (16), and SJ000994244-1 (17).
The normalized activities (% inhibition) of positive control, negative control, and all tested chemicals are summarized in Figure 11C. Four compounds showed greater than 30% inhibition at 10 μM screening concentration: SJ000994241-1 (14, 45.7%), SJ000994129-1 (15, 36.4%), SJ000994509-1 (16, 33.1%), and SJ000994244-1 (17, 31.1%) (Figure 11D). It is not unexpected that few compounds were identified as potent hits because the alpha-helix mimetics do not have the hydroxyproline moiety which might be required for a potent VHL ligand.4−8,14
In conclusion, we developed BODIPY FL VH032 (5) as the first small-molecule fluorescent probe with high affinity for VHL binding. Our BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay is more sensitive than previously reported FP assays. Both BODIPY FL VH032 (5)-mediated TR-FRET and FP assays selectively detected only VHL ligands. Assays based on high-affinity probes are reportedly more sensitive for detecting ligand binding with a wide range of inhibitory potencies.44 Our high-affinity VHL fluorescent probe BODIPY FL VH032 (5)-based TR-FRET assay is sensitive, selective, resistant to assay interference, capable of detecting ligands with a wide range of activities, and suitable for the identification of VHL ligands using an HTS approach. Therefore, it is suitable for identifying and characterizing VHL ligands in large-scale screenings. BODIPY FL VH032 (5, a high-affinity VHL fluorescent probe), together with BODIPY FL thalidomide (a high-affinity cereblon fluorescent probe reported by us recently),25 enable the development of highly sensitive TR-FRET binding assays for VHL and cereblon to facilitate PROTAC-based research and development.
Experimental Procedures
Chemistry
VH032 amine and VH032-PEG4-amine hydrochloride salt were purchased from MedChemExpress, LLC (Monmouth Junction, NJ). BODIPY FL propionic acid was purchased from BroadPharm (San Diego, CA). Acetic anhydride, DIPEA, DCM, DMSO, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, hydroxybenzotriazole, and all other basic chemical reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO). DMSO-d6 and chloroform-d were purchased from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA). Reported protocols23 were adopted to assess or verify the reaction progress and product purity and identity; to determine high-resolution mass spectra; and to record 1H and 13C NMR spectra (Figures S1–S6).
(2S,4R)-1-((S)-2-Acetamido-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (1, VH032)
We solubilized VH032 amine (6, 215 mg, 0.5 mmol) in a stirred solution of DCM (5 mL) and DIPEA (263 μL, 1.5 mmol) in an ice water bath. Ac2O (60 μL, 0.635 mmol) was then added. The ice water bath was removed after 5 min, and the reaction was continued for another 25 min at room temperature. The reaction mixture was diluted with DCM (20 mL) and quenched with brine (30 mL). After separation from the brine, the DCM solution was washed with brine (20 mL × 2) and dried with anhydrous Na2SO4. A white raw powder product was obtained after the solvent was removed from the DCM solution with an IKA RV 10 digital rotavapor (IKA Works, Inc., Wilmington, NC) and further purified with an Acquity prep-UPLC system (Waters Corporation, Milford, MA) equipped with an Acquity UPLC BEH C18 1.7 μm, 2.1 × 50 mm column to yield the product VH032 (1, 143 mg, 60.6% yield, and 98.0% purity) as a white solid. 1H NMR (500 MHz, DMSO-d6): δ 8.98 (s, 1H), 8.57 (t, J = 6.1 Hz, 1H), 7.95 (d, J = 9.3 Hz, 1H), 7.43–7.36 (m, 4H), 5.12 (d, J = 3.5 Hz, 1H), 4.54 (d, J = 9.4 Hz, 1H), 4.48–4.39 (m, 2H), 4.36–4.32 (m, 1H), 4.21 (dd, J = 15.9, 5.4 Hz, 1H), 3.72–3.60 (m, 2H), 2.44 (s, 3H), 2.08–2.00 (m, 1H), 1.93–1.89 (m, 1H), 1.88 (s, 3H), 0.93 (s, 9H). 13C NMR (126 MHz, DMSO-d6): δ 172.42, 170.14, 169.55, 151.94, 148.19, 139.99, 131.64, 130.10, 129.10, 127.88, 69.34, 60.23, 59.14, 56.86, 42.10, 38.43, 35.67, 26.83, 22.80, 16.42. ESI-TOF HRMS m/z: [M + H]+ calcd for C24H33N4O4S+, 473.2217; found, 473.2225.
(2S,4R)-1-((S)-2-(tert-Butyl)-21-(5,5-difluoro-7,9-dimethyl-5H-5λ4,6λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-3-yl)-4,19-dioxo-6,9,12,15-tetraoxa-3,18-diazahenicosanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (5, BODIPY FL VH032)
At room temperature, VH032-PEG4-amine hydrochloride (10, 50 mg, 0.075 mmol) was added to a solution of BODIPY FL propionic acid (13, 24.20 mg, 0.083 mmol) and DIPEA (29.2 mg, 0.226 mmol) in DMSO (1 mL). 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (21.66 mg, 0.113 mmol) and hydroxybenzotriazole (12.21 mg, 0.090 mmol) were then added. The reaction was stirred for 3 h, and the reaction mixture was then directly purified with a Biotage Isolera four-flash chromatography system (Biotage, LLC, Charlotte, NC) with an Sfär C18 flash column and gradient mobile phase (H2O + 0.1% formic acid → acetonitrile +0.1% formic acid) to yield the product BODIPY FL VH032 (5, 45.4 mg, 64.3% yield, and 95.9% purity) as a brown-red solid. 1H NMR (500 MHz, chloroform-d): δ 8.70 (s, 1H), 7.36 (dd, J = 7.6, 4.2 Hz, 5H), 7.07 (s, 1H), 6.87 (d, J = 4.0 Hz, 1H), 6.45 (s, 1H), 6.29 (d, J = 4.0 Hz, 1H), 6.10 (s, 1H), 4.73 (t, J = 8.0 Hz, 1H), 4.59–4.47 (m, 3H), 4.35 (dd, J = 15.0, 5.4 Hz, 1H), 4.06 (d, J = 11.0 Hz, 1H), 4.01 (d, J = 2.7 Hz, 2H), 3.71–3.54 (m, 13H), 3.50 (t, J = 5.2 Hz, 2H), 3.39 (dq, J = 8.3, 5.2, 4.6 Hz, 2H), 3.28 (t, J = 7.6 Hz, 2H), 2.62 (t, J = 7.6 Hz, 2H), 2.56–2.47 (m, 7H), 2.24 (s, 3H), 2.18–2.10 (m, 1H), 0.94 (s, 9H). 13C NMR (126 MHz, DMSO-d6): δ 170.11, 169.24, 167.47, 166.93, 157.44, 156.20, 149.80, 146.09, 142.39, 137.79, 132.76, 131.31, 129.48, 128.03, 127.26, 127.22, 127.03, 126.48, 125.80, 123.68, 118.59, 114.95, 68.78, 68.18, 68.13, 68.06, 67.94, 67.92, 67.89, 67.45, 67.22, 57.08, 54.92, 54.03, 40.01, 36.95, 36.26, 34.06, 31.97, 24.51, 22.31, 14.26, 12.84, 9.34. ESI-TOF HRMS m/z: [M + H]+ calcd for C46H63BF2N7O9S+, 938.4464; found, 938.4484.
Biology
The Tb-anti-GST antibody, 1,4-dithiothreitol (DTT, 1 M), Tris (1 M, pH 7.5), and DMSO were purchased from Fisher Scientific (Pittsburgh, PA). HEPES (1 M, pH 7.4) was purchased from Teknova, Inc. (Hollister, CA). Triton X-100, Tween-20, and bovine serum albumin were purchased from Sigma-Aldrich (St. Louis, MO). VH-298, VH032-amine, Me-VH032-amine, and VH032-PEG4-NH2 were purchased from MedChemExpress, LLC (Monmouth Junction, NJ). BOC-VH032 was purchased from LabNetwork (Cambridge, MA). VH032 phenol and thalidomide-4′-O-acetamido-alkylC4-amine were purchased from Bio-Techne Corporation (Minneapolis, MN). MZ1, dBET1, and (+)-JQ1 were purchased from Cayman Chemical (Ann Arbor, MI). Echo 384-well low dead volume compound plates were purchased from Labcyte, Inc. (San Jose, CA). 384-well, black low-volume assay plates were purchased from Corning Incorporated Life Sciences (Tewksbury, MA).
GST–VCB Protein Preparation
The pGEX-4T-1-GST–VHL (54-213 aa) plasmid (Figure S7), pCDFDuet-1-flag-elongin-C (17-112 aa)-strep II-elongin-B (1-118 aa) plasmid (Figures S8–S9), and the GST–VCB protein complex were custom-created, expressed, and purified by GenScript USA, Inc. (Piscataway, NJ). Briefly, VHL (54-213 aa) was subcloned into the pGEX-4T-1-GST bacterial expression vector between the BamHI and XhoI restriction sites. Flag-elongin-C (17–112 aa) and strep II-elongin-B (1–118 aa) were, respectively, subcloned between the NcoI and HindIII and NdeI and XhoI restriction sites into the pCDFDuet-1 bacterial expression vector. Escherichia coli BL21(DE3) competent cells were transformed with the recombinant pGEX-4T-1-GST–VHL (54-213 aa) and pCDFDuet-1-flag-elongin-C (17–112 aa)-strep II-elongin-B (1–118 aa) plasmids. A single colony was inoculated into the LB medium containing ampicillin and streptomycin, and the culture was incubated at 37 °C with shaking at 200 rpm. Once the cell density reached the optical density of 0.6–0.8 at 600 nm, 0.5 mM IPTG was introduced for induction at 25 °C for 16 h. The cells were then harvested and lysed. The cell lysate supernatant was subjected to one-step purification by a GST column to provide the GST–VCB protein complex. Aliquots of GST–VCB protein were stored at −80 °C in 50 mM Tris (pH 8.0), 150 mM NaCl, and 10% glycerol. The purity (∼85%) of GST–VCB was determined by SDS-PAGE and Western blot analysis (Figure S10), and the concentration (0.30 mg/mL) of GST–VCB was determined by the Bradford assay with BSA as the standard (Figure S11).
Chemical Stock Solution Preparation and Chemical and Reagent Dispense
Chemicals, including BODIPY FL VH032 (5), were solubilized in DMSO as 1000× stock solutions. Stock chemical DMSO solutions, positive control VH298 (2), and negative control DMSO were all plated in Echo low dead volume compound plates. For the probe or chemicals tested in dilutions, their stock dilutions were prepared in Echo low dead volume compound plates as 1000× DMSO stock solutions for all concentration levels. TR-FRET and FP assay buffers (10 μL/well) were first dispensed. The BODIPY FL VH032 (5) 1000× DMSO stock solution in dilutions or a single concentration was then dispensed (20 nL/well) with an Echo 555 acoustic liquid handler, and the 1000× DMSO stock control and chemical solutions were subsequently transferred (20 nL/well) with an Echo 555 acoustic liquid handler. Protein solutions (2× stocks, 10 μL/well) in corresponding assay buffers were finally dispensed to yield a total of 20 μL/well assay volume. The fluorescent probe and each of the chemical and control solutions (20 nL/well) were dispensed to achieve a final total volume of 20 μL/well at 1–1000× dilution.
General Assay Conditions
The final DMSO concentration was 0.2% in all tests unless specified, with 0.1% DMSO contributed from the stock BODIPY FL VH032 (5) solution (1000× DMSO stock) and 0.1% DMSO contributed from the test compound stock solutions (1000× DMSO stock solutions). If no chemicals were tested under certain conditions, 0.1% DMSO was supplemented to achieve a final 0.2% concentration for these assay conditions. An Echo 555 acoustic liquid handler (Labcyte Inc., San Jose, CA) was used to dispense chemicals. The final assay volume was 20 μL/well, and all assays were performed at room temperature (∼25 °C). All assays were performed three times independently with quadruplicate sample replicates.
General TR-FRET Assay Protocol
The BODIPY FL VH032 (5)-mediated VHL TR-FRET binding assay was performed as previously reported,23 except that an Echo 555 acoustic liquid handler (Labcyte Inc., San Jose, CA) was used to dispense chemicals and that BODIPY FL VH032 (5), GST–VCB, and Tb-anti-GST antibody were used. The VHL TR-FRET binding assay buffer was composed of 50 mM Tris (pH 7.5), 0.01% Triton X-100, 0.01% bovine serum albumin, and 1 mM DTT, which was freshly prepared before each experiment.
Determination of BODIPY FL VH032 (5) Kd of the GST–VCB Protein Complex in the TR-FRET Binding Assay
Dilutions of BODIPY FL VH032 (5, 1 to 2 dilutions, concentration range: 0.06–500 nM) were incubated with 2 nM Tb-anti-GST and 2 nM GST–VCB + DMSO, with 2 nM GST–VCB + VH298 (2, 30 μM), or without GST–VCB + DMSO. The TR-FRET signals were monitored every 30 min from 30 min to 300 min with a PHERAstar FS plate reader equipped with a TR-FRET optic module (excitation: 340 nm; emission 1: 520 nm; emission 2: 490 nm). The TR-FRET signals were fitted into GraphPad Prism 8.4.3 software (GraphPad Software; San Diego, CA) with the equation for a one-site total binding with nonspecific interactions to derive curves for each group. The binding affinity Kd values were derived from the 2 nM Tb-anti-GST and 2 nM GST–VCB + DMSO samples.
Signal Stability of the BODIPY FL VH032 (5)-Mediated VHL TR-FRET Binding Assay
BODIPY FL VH032 (5, 4 nM) and 2 nM Tb-anti-GST were incubated with 2 nM GST–VCB + DMSO (group 1, negative control), with 2 nM GST–VCB + VH298 (2, 30 μM; group 2, positive control), without GST–VCB + DMSO (group 3, background control), or with 2 nM GST–VCB + dilutions of VH298 (2, 1–3 dilutions, concentration range: 2.1 pM to 30 μM, group 4, dose–response positive control). The TR-FRET signals were monitored every 30 min from 30 min to 300 min. The TR-FRET signal of groups 1, 2, or 3 was divided by that of group 2 to derive the TR-FRET signal fold change of each group. The TR-FRET signals or the signal fold changes of groups 1, 2, and 3 were plotted. The dose-dependent TR-FRET signals of the positive control VH298 (2, group 4) were fitted into GraphPad Prism software by using a Sigmoidal dose–response equation to derive the IC50 values.
Binding Inhibitory Activity of Selected VHL Ligands or Nonligands with the BODIPY FL VH032 (5)-Mediated VHL TR-FRET Binding Assay
BODIPY FL VH032 (5, 4 nM) was incubated with the positive control VH298 (2, 30 μM), negative control DMSO, or dilutions of selected VHL ligands or nonligands (1–3 dilutions; concentration range: 2.1 pM to 30 μM), along with 2 nM GST–VCB and 2 nM Tb-anti-GST. The TR-FRET signals were determined at a 90-min incubation time. The percent inhibition (% inhibition) of each tested ligand at its individual concentration was calculated by normalizing it to that of the positive control VH298 (2, 30 μM) and negative control DMSO using eq 1.
| 1 |
When applicable, the normalized % inhibition values for each ligand at various concentrations were fitted to a sigmoidal dose–response equation using GraphPad Prism software to derive the IC50 values. The TR-FRET Ki values were then calculated using eq 2 (i.e., the Cheng–Prusoff equation).35
| 2 |
where IC50 is the concentration of the tested ligand that inhibits 50% of BODIPY FL VH032 (5) binding to GST–VCB, [L] is the BODIPY FL VH032 (5) concentration of 4 nM in the assay mixture, and KL is the Kd value of BODIPY FL VH032 (5) in the assay, which was 3.01 nM. The TR-FRET Ki values were used to compare the relative binding affinities of the test ligands for VHL.
DMSO Tolerance Test of the BODIPY FL VH032 (5)-mediated VHL TR-FRET Binding Assay
In the presence of final total DMSO concentrations of 0.2, 0.5, 1, 2, 5, or 10%, the negative control DMSO, the positive control VH298 (2, 30 μM), or dilutions of positive control VH298 (2, 1–3 dilutions, optimized concentration range: 2.1 pM to 30 μM) were incubated with BODIPY FL VH032 (5, 4 nM), 2 nM GST–VCB, and 2 nM Tb-anti-GST. The TR-FRET signals (RTUs) were determined at a 90 min incubation time. The % RTU to 0.2% DMSO (set as 100%) was calculated as 100% × RTU of the indicated group/RTU of the 0.2% DMSO group. The RTUs, % RTUs to 0.2% DMSO of the negative control DMSO or positive control VH298 (2), or RTUs of dilutions of VH298 (2) in the presence of various concentrations of DMSO were graphed with GraphPad Prism software and IC50 values of VH298 (2) were derived using a sigmoidal dose–response equation.
Pilot Screening with the BODIPY FL VH032 (5)-mediated VHL TR-FRET Binding Assay
A custom-assembled alpha-helix library with 2,011 alpha-helix-mimetic small molecules (10 μL/well of 10 mM stock in DMSO) from ChemDiv, Inc. (San Diego, CA) were plated in seven 384-well Echo LDV plates with chemicals in columns 3 to 12 and columns 15 to 24 (columns: 1, 2, 13, and 14 empty). A control plate with the positive control VH298 (2, 10 μL/well 30 mM stock in DMSO) in column 1 (16 wells) and the negative control DMSO (10 μL/well) in column 13 (16 wells) was prepared with an Echo LDV plate. For screening, 20 nL/well of library chemicals, positive control VH298 (2), or DMSO was transferred with an Echo 555 acoustic liquid handler to black 384-well assay plates with 20 μL/well BODIPY FL VH032 (5, 4 nM), 2 nM GST–VCB, and 2 nM Tb-anti-GST. The final tested chemical concentration was 10 μM and the final positive control VH298 (2) concentration was 30 μM. The final DMSO concentration was 0.2% for all tested wells with 0.1% from tested chemicals, 30 mM positive control VH298 (2) DMSO stock or negative control DMSO, and 0.1% from the BODIPY FL VH032 (5, 4 μM) DMSO stock. The TR-FRET signals (RTUs) were determined at a 90 min incubation time point. The screening plate Z-prime value was calculated using eq 3.43
| 3 |
where σ+ is the standard deviation of the positive control VH298 (2, 30 μM) group, σ– is the standard deviation of the negative control DMSO group, mean+ is the average signal of the positive control VH298 (2, 30 μM) group, and mean– is the average signal of the negative control DMSO group.
The percent inhibition (% inhibition) of each tested chemical was calculated by normalizing it to that of the positive control VH298 (2, 30 μM) and negative control DMSO using eq 4.
| 4 |
General FP Assay Protocol
The BODIPY FL VH032 (5)-mediated VHL FP binding assay was performed according to the general TR-FRET assay protocol, except that the Tb-anti-anti-GST antibody was not added and that a PHERAstar FS plate reader (BMG Labtech; Durham, NC) was equipped with an FP optic module (excitation: 485 nm; emission: 520 nm) to read the FP assay signals. The VHL FP assay buffer was composed of 25 mM HEPES (pH 7.4), 0.01% Tween-20, 0.5 mM DTT, and 0.01% bovine serum albumin, which was freshly prepared before each experiment.
BODIPY FL VH032 (5) Concentration Optimization in a VHL FP Assay
BODIPY FL VH032 (5, 70, 60, 50, 40, 30, 20, 10, 5, 2, or 1 nM) was incubated with dilutions of GST–VCB (1 to 2 dilutions, concentration range: 0.03 nM to 1 μM). The FP signals were monitored with a PHERAstar FS plate reader equipped with an FP optic module (excitation: 485 nm; emission: 520 nm). The representative data collected at the 90-min incubation time point were plotted with GraphPad Prism software.
Determination of BODIPY FL VH032 (5) Binding Kd for the GST–VCB Protein Complex in a FP Binding Assay
BODIPY FL VH032 (5, 10 nM) was incubated with dilutions of GST–VCB (1 to 2 dilutions, in a concentration range of 0.03 nM to 1 μM) along with the DMSO group or VH298 (2, 30 μM) group. In addition, BODIPY FL VH032 (5, 10 nM) was incubated with DMSO only but without GST–VCB. The FP signals were determined with a PHERAstar FS plate reader. The representative data collected at 90-min incubation time were plotted with GraphPad Prism software using the equation for a one-site total binding with nonspecific interactions to derive curves for each group. The binding affinity Kd values were derived from the DMSO with GST–VCB sample.
Binding Activity of VHL Ligands or Non-Ligands in the BODIPY FL VH032 (5)-Mediated VHL FP Binding Assay
BODIPY FL VH032 (5, 10 nM) was incubated with the positive control VH298 (2, 30 μM), negative control DMSO, or dilutions of selected VHL ligands or nonligands (1–3 dilutions; concentration range: 2.1 pM to 30 μM), along with 100 nM GST–VCB. The FP signals were determined at a 90-min incubation time. The FP signal fold change of the DMSO or VH298 (2, 30 μM) group was divided by that of the VH298 (2, 30 μM) group to derive the FP signal fold change. The FP signals or FP signal-fold changes were plotted with GraphPad Prism software. The % inhibition of each tested ligand at its individual concentration was calculated by normalizing it to that of the VH298 (2, 30 μM) and DMSO groups using eq 1. The FP Ki values were calculated by the method developed by Nikolovska-Coleska et al.36 using the Ki calculator available at the following link: http://www.umich.edu/?shaomengwanglab/software/calc_ki/index.html. The FP Ki values were used to compare the relative binding affinities of the test ligands to VHL.
Acknowledgments
The authors thank ALSAC for support, Nisha Badders, PhD, ELS (St. Jude Department of Scientific Editing) for editing the manuscript and other members of the Chen research laboratory for their valuable discussions on the manuscript.
Glossary
Abbreviations
- VHL
von Hippel–Lindau protein
- VCB
VHL–elongin-C–elongin-B
- HIFs
hypoxia-inducible factors
- PROTACs
proteolysis-targeting chimeras
- ITC
isothermal titration calorimetry
- SPR
surface plasmon resonance
- FP
fluorescence polarization
- TR-FRET
time-resolved fluorescence resonance energy transfer
- Kd
dissociation constant
- BET
bromodomain and extra-terminal domain
- HIF1α
hypoxia-inducible factor 1 alpha
- Hyp
hydroxyproline
- PEG
polyethylene glycol
- BRD
bromodomain-containing protein
- Ki
inhibition constant
- IC50
half-maximal inhibitory concentration
- Tb-anti-GST
terbium-labeled anti-GST-tag antibody
- DMSO
dimethyl sulfoxide
- RTU
relative TR-FRET unit
- DTT
1,4-dithiothreitol
- DIPEA
N,N-diisopropylethylamine
- DCM
dichloromethane
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05221.
1H NMR, 13C NMR, and HRMS spectra of VH032 (1) and BODIPY FL VH032 (5); GST–VCB protein sequence information; protein purity determination by SDS-PAGE and Western blot analysis; and protein concentration determination by the Bradford assay (PDF)
The authors declare the following competing financial interest(s): A provisional US patent application that is based on the research reported in the paper has been filed: Chen T, Lin W, Li Y. Provisional US Patent Application No. 63/111,407: Novel VHL small molecule probe. Filing date: November 9, 2020.
Notes
A provisional US patent application that is based on the research reported in the paper has been filed: Chen T, Lin W, Li Y. Provisional US Patent Application no. 63/111,407: Novel VHL small molecule probe. Filing date: November 9, 2020.
Supplementary Material
References
- Kibel A.; Iliopoulos O.; DeCaprio J.; Kaelin W. Jr. Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science 1995, 269, 1444–1446. 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- Stebbins C. E.; Kaelin W. G. Jr.; Pavletich N. P. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science 1999, 284, 455–461. 10.1126/science.284.5413.455. [DOI] [PubMed] [Google Scholar]
- Maxwell P. H.; Wiesener M. S.; Chang G.-W.; Clifford S. C.; Vaux E. C.; Cockman M. E.; Wykoff C. C.; Pugh C. W.; Maher E. R.; Ratcliffe P. J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Buckley D. L.; Van Molle I.; Gareiss P. C.; Tae H. S.; Michel J.; Noblin D. J.; Jorgensen W. L.; Ciulli A.; Crews C. M. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. J. Am. Chem. Soc. 2012, 134, 4465–4468. 10.1021/ja209924v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley D. L.; Gustafson J. L.; Van Molle I.; Roth A. G.; Tae H. S.; Gareiss P. C.; Jorgensen W. L.; Ciulli A.; Crews C. M. Small-molecule inhibitors of the interaction between the E3 ligase VHL and HIF1α. Angew. Chem., Int. Ed. Engl. 2012, 51, 11463–11467. 10.1002/anie.201206231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Molle I.; Thomann A.; Buckley D. L.; So E. C.; Lang S.; Crews C. M.; Ciulli A. Dissecting fragment-based lead discovery at the von Hippel-Lindau protein:hypoxia inducible factor 1α protein-protein interface. Chem. Biol. 2012, 19, 1300–1312. 10.1016/j.chembiol.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdeano C.; Gadd M. S.; Soares P.; Scaffidi S.; Van Molle I.; Birced I.; Hewitt S.; Dias D. M.; Ciulli A. Structure-guided design and optimization of small molecules targeting the protein-protein interaction between the von Hippel-Lindau (VHL) E3 ubiquitin ligase and the hypoxia inducible factor (HIF) alpha subunit with in vitro nanomolar affinities. J. Med. Chem. 2014, 57, 8657–8663. 10.1021/jm5011258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost J.; Galdeano C.; Soares P.; Gadd M. S.; Grzes K. M.; Ellis L.; Epemolu O.; Shimamura S.; Bantscheff M.; Grandi P.; et al. Potent and selective chemical probe of hypoxic signalling downstream of HIF-α hydroxylation via VHL inhibition. Nat. Commun. 2016, 7, 13312. 10.1038/ncomms13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J.-H.; Yang H.; Ivan M.; Gertler F.; Kaelin W. G. Jr.; Pavletich N. P. Structure of an HIF-1alpha -pVHL complex: hydroxyproline recognition in signaling. Science 2002, 296, 1886–1889. 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- Hon W.-C.; Wilson M. I.; Harlos K.; Claridge T. D. W.; Schofield C. J.; Pugh C. W.; Maxwell P. H.; Ratcliffe P. J.; Stuart D. I.; Jones E. Y. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature 2002, 417, 975–978. 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Jiang X.; Feng F.; Liu W.; Sun H. Degradation of proteins by PROTACs and other strategies. Acta Pharm. Sin. B 2020, 10, 207–238. 10.1016/j.apsb.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd M. S.; Testa A.; Lucas X.; Chan K.-H.; Chen W.; Lamont D. J.; Zengerle M.; Ciulli A. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat. Chem. Biol. 2017, 13, 514–521. 10.1038/nchembio.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P.; Qi J.; Picaud S.; Shen Y.; Smith W. B.; Fedorov O.; Morse E. M.; Keates T.; Hickman T. T.; Felletar I.; et al. Selective inhibition of BET bromodomains. Nature 2010, 468, 1067–1073. 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares P.; Gadd M. S.; Frost J.; Galdeano C.; Ellis L.; Epemolu O.; Rocha S.; Read K. D.; Ciulli A. Group-Based Optimization of Potent and Cell-Active Inhibitors of the von Hippel-Lindau (VHL) E3 Ubiquitin Ligase: Structure-Activity Relationships Leading to the Chemical Probe (2S,4R)-1-((S)-2-(1-Cyanocyclopropanecarboxamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide (VH298). J. Med. Chem. 2018, 61, 599–618. 10.1021/acs.jmedchem.7b00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa A.; Lucas X.; Castro G. V.; Chan K. H.; Wright J. E.; Runcie A. C.; Gadd M. S.; Harrison W. T. A.; Ko E. J.; Fletcher D.; et al. 3-Fluoro-4-hydroxyprolines: Synthesis, Conformational Analysis, and Stereoselective Recognition by the VHL E3 Ubiquitin Ligase for Targeted Protein Degradation. J. Am. Chem. Soc. 2018, 140, 9299–9313. 10.1021/jacs.8b05807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew A. P.; Raina K.; Dong H.; Qian Y.; Wang J.; Vigil D.; Serebrenik Y. V.; Hamman B. D.; Morgan A.; Ferraro C.; et al. Identification and Characterization of Von Hippel-Lindau-Recruiting Proteolysis Targeting Chimeras (PROTACs) of TANK-Binding Kinase 1. J. Med. Chem. 2018, 61, 583–598. 10.1021/acs.jmedchem.7b00635. [DOI] [PubMed] [Google Scholar]
- Zoppi V.; Hughes S. J.; Maniaci C.; Testa A.; Gmaschitz T.; Wieshofer C.; Koegl M.; Riching K. M.; Daniels D. L.; Spallarossa A.; et al. Iterative Design and Optimization of Initially Inactive Proteolysis Targeting Chimeras (PROTACs) Identify VZ185 as a Potent, Fast, and Selective von Hippel-Lindau (VHL) Based Dual Degrader Probe of BRD9 and BRD7. J. Med. Chem. 2019, 62, 699–726. 10.1021/acs.jmedchem.8b01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea W. A.; Simeonov A. Fluorescence polarization assays in small molecule screening. Expert Opin. Drug Discovery 2011, 6, 17–32. 10.1517/17460441.2011.537322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. J.; Law T. L.; Lenoch F. J.; Bolger R. E. Development of high throughput screening assays using fluorescence polarization: nuclear receptor-ligand-binding and kinase/phosphatase assays. J. Biomol. Screening 2000, 5, 77–88. 10.1177/108705710000500204. [DOI] [PubMed] [Google Scholar]
- Du Y.; Nikolovska-Coleska Z.; Qui M.; Li L.; Lewis I.; Dingledine R.; Stuckey J. A.; Krajewski K.; Roller P. P.; Wang S.; et al. A Dual-Readout F2Assay That Combines Fluorescence Resonance Energy Transfer and Fluorescence Polarization for Monitoring Bimolecular Interactions. Assay Drug Dev. Technol. 2011, 9, 382–393. 10.1089/adt.2010.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerke N. J. Fluorescence Polarization (FP) Assays for Monitoring Peptide-Protein or Nucleic Acid-Protein Binding. Curr. Protoc. Chem. Biol. 2009, 1, 1–15. 10.1002/9780470559277.ch090102. [DOI] [PubMed] [Google Scholar]
- Lin W.; Chen T. Using TR-FRET to Investigate Protein-Protein Interactions: A Case Study of PXR-Coregulator Interaction. Adv. Protein Chem. Struct. Biol. 2018, 110, 31–63. 10.1016/bs.apcsb.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.; Liu J.; Jeffries C.; Yang L.; Lu Y.; Lee R. E.; Chen T. Development of BODIPY FL vindoline as a novel and high-affinity pregnane X receptor fluorescent probe. Bioconjugate Chem. 2014, 25, 1664–1677. 10.1021/bc5002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.; Chen T. A vinblastine fluorescent probe for pregnane X receptor in a time-resolved fluorescence resonance energy transfer assay. Anal. Biochem. 2013, 443, 252–260. 10.1016/j.ab.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.; Li Y.; Min J.; Liu J.; Yang L.; Lee R. E.; Chen T. Development of BODIPY FL Thalidomide As a High-Affinity Fluorescent Probe for Cereblon in a Time-Resolved Fluorescence Resonance Energy Transfer Assay. Bioconjugate Chem. 2020, 31, 2564–2575. 10.1021/acs.bioconjchem.0c00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N.; Auld D. S.; Inglese J. Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr. Opin. Chem. Biol. 2010, 14, 315–324. 10.1016/j.cbpa.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J. F.; Wu X.; Mercuri R.; Illy C.; Bowen B. R.; He Y.; Sills M. A comparison of ALPHAScreen, TR-FRET, and TRF as assay methods for FXR nuclear receptors. J. Biomol. Screening 2002, 7, 3–10. 10.1177/108705710200700102. [DOI] [PubMed] [Google Scholar]
- Kurt W. V.; Bryan D. M.; Kevin R. K.; Kevin L. V.; Tina M. H. Facile Conversion of FP to TR-FRET Assays using Terbium Chelates:Nuclear Receptor Competitive Binding Assays as Examples. Lett. Drug Des. Discovery 2008, 5, 416–422. 10.2174/157018008785777261. [DOI] [Google Scholar]
- Top K. P. O.; Hatleberg G.; Berggren K. N.; Ryan D.; Kemper C.; Haugland R. P.; Patton W. F. Green/red dual fluorescence detection of total protein and alkaline phosphate-conjugated probes on blotting membranes. Electrophoresis 2001, 22, 896–905. . [DOI] [PubMed] [Google Scholar]
- Lai A. C.; Toure M.; Hellerschmied D.; Salami J.; Jaime-Figueroa S.; Ko E.; Hines J.; Crews C. M. Modular PROTAC Design for the Degradation of Oncogenic BCR-ABL. Angew. Chem., Int. Ed. Engl. 2016, 55, 807–810. 10.1002/anie.201507634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina K.; Lu J.; Qian Y.; Altieri M.; Gordon D.; Rossi A. M. K.; Wang J.; Chen X.; Dong H.; Siu K.; et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, 7124–7129. 10.1073/pnas.1521738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniaci C.; Hughes S. J.; Testa A.; Chen W.; Lamont D. J.; Rocha S.; Alessi D. R.; Romeo R.; Ciulli A. Homo-PROTACs: bivalent small-molecule dimerizers of the VHL E3 ubiquitin ligase to induce self-degradation. Nat. Commun. 2017, 8, 830. 10.1038/s41467-017-00954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G. E.; Buckley D. L.; Paulk J.; Roberts J. M.; Souza A.; Dhe-Paganon S.; Bradner J. E. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 2015, 348, 1376–1381. 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L. C.; Cox B. G. Kinetics of Amide Formation through Carbodiimide/N-Hydroxybenzotriazole (HOBt) Couplings. J. Org. Chem. 2007, 72, 8863–8869. 10.1021/jo701558y. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Nikolovska-Coleska Z.; Wang R.; Fang X.; Pan H.; Tomita Y.; Li P.; Roller P. P.; Krajewski K.; Saito N. G.; Stuckey J. A.; et al. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal. Biochem. 2004, 332, 261–273. 10.1016/j.ab.2004.05.055. [DOI] [PubMed] [Google Scholar]
- Newman M.; Josiah S. Utilization of fluorescence polarization and time resolved fluorescence resonance energy transfer assay formats for SAR studies: Src kinase as a model system. J. Biomol. Screening 2004, 9, 525–532. 10.1177/1087057104264597. [DOI] [PubMed] [Google Scholar]
- Cashman J. R.; MacDonald M.; Ghirmai S.; Okolotowicz K. J.; Sergienko E.; Brown B.; Garcia X.; Zhai D.; Dahl R.; Reed J. C. Inhibition of Bfl-1 with N-aryl maleimides. Bioorg. Med. Chem. Lett. 2010, 20, 6560–6564. 10.1016/j.bmcl.2010.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink T. A.; Kleman-Leyer K. M.; Kopp A.; Westermeyer T. A.; Lowery R. G. Evaluating PI3 kinase isoforms using Transcreener ADP assays. J. Biomol. Screening 2008, 13, 476–485. 10.1177/1087057108319864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucy J. L.; Lasker J. M. Current in vitro high throughput screening approaches to assess nuclear receptor activation. Curr. Drug Metab. 2010, 11, 806–814. 10.2174/138920010794328896. [DOI] [PubMed] [Google Scholar]
- Deacon M.; Singleton D.; Szalkai N.; Pasieczny R.; Peacock C.; Price D.; Boyd J.; Boyd H.; Steidl-Nichols J. V.; Williams C. Early evaluation of compound QT prolongation effects: a predictive 384-well fluorescence polarization binding assay for measuring hERG blockade. J. Pharmacol. Toxicol. Methods 2007, 55, 238–247. 10.1016/j.vascn.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Peterson K. J.; Sadowsky J. D.; Scheef E. A.; Pal S.; Kourentzi K. D.; Willson R. C.; Bresnick E. H.; Sheibani N.; Gellman S. H. A fluorescence polarization assay for identifying ligands that bind to vascular endothelial growth factor. Anal. Biochem. 2008, 378, 8–14. 10.1016/j.ab.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-H.; Chung T. D. Y.; Oldenburg K. R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screening 1999, 4, 67–73. 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Huang X. Fluorescence polarization competition assay: the range of resolvable inhibitor potency is limited by the affinity of the fluorescent ligand. J. Biomol. Screening 2003, 8, 34–38. 10.1177/1087057102239666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.