Key points.
-
•
Tetralogy of Fallot is characterised by the presence of a ventricular septal defect, overriding aorta, right ventricular outflow tract obstruction and right ventricular hypertrophy.
-
•
Tetralogy of Fallot is one of the commonest cyanotic heart defects.
-
•
The severity of cyanosis is determined by the degree of obstruction to pulmonary blood flow.
-
•
Prevention of cyanotic spells is key for safe anaesthesia.
-
•
Late complications include arrhythmias, pulmonary regurgitation and right heart failure.
Learning objectives.
By reading this article, you should be able to:
-
•
Describe the anatomy and pathophysiology of tetralogy of Fallot.
-
•
Create a plan for the prevention and treatment of a cyanotic spell.
-
•
List the common complications seen in later life after tetralogy of Fallot repair.
-
•
State the risks of anaesthesia in the pre- and postoperative periods for a patient with repaired tetralogy of Fallot.
Tetralogy of Fallot (ToF) is one of the commonest cyanotic congenital heart malformations and is characterised by four cardinal features: ventricular septal defect (VSD); right ventricular (RV) outflow tract obstruction (RVOTO), which is often dynamic; an overriding aorta; and RV hypertrophy (RVH). The degree of RVOTO, the relative pressures in the right and left ventricles, and the proportion of the aorta overriding the VSD determine the presentation and severity of this condition. The anaesthetist can encounter ToF in a variety of settings, ranging from the acutely cyanotic infant to an adult with previous ToF repair presenting for unrelated surgery. This article discusses the relevant scientific and clinical aspects of ToF both before and after full surgical repair.
Classical anatomy
Tetralogy of Fallot is one of the commonest forms of cyanotic congenital heart disease, accounting for 7–10% of all congenital cardiac malformations with an incidence of one in 3,500 live births.1 Whilst ToF was recognised as a series of malformations in 1671, it was named after the French physician Etienne-Louis Fallot, who reported the first case series in 1888 with anatomical and pathological descriptions of the four key features:2, 3
-
(i)
VSD
-
(ii)
RVOTO
-
(iii)
Overriding aorta
-
(iv)
RVH
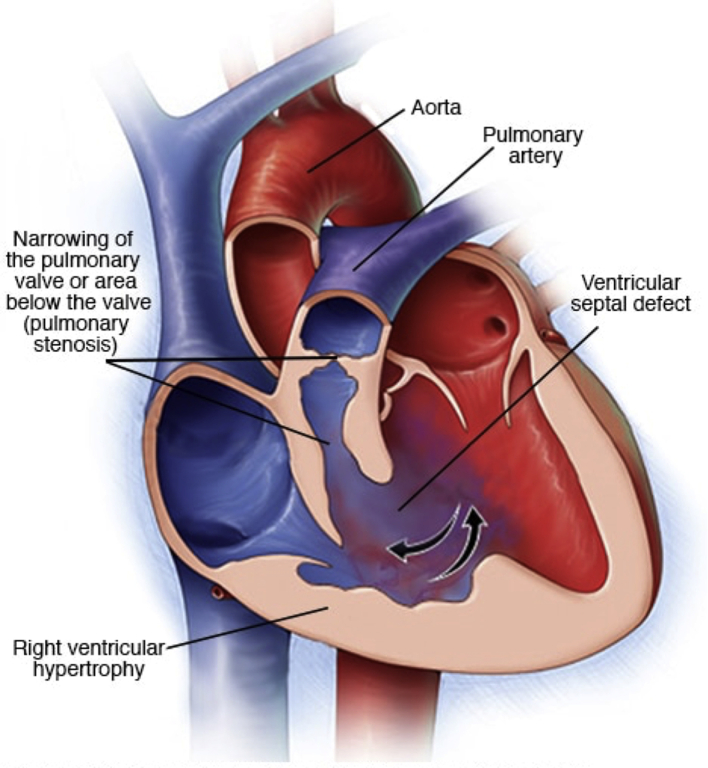
The anatomical appearances of ToF are depicted in Figure 1.4
Fig 1.
Image demonstrating the classical ToF anatomy. Reproduced with permission from the Mayo Foundation for Medical Education and Research.4
Ventricular septal defect
This condition is usually a large non-restrictive defect (i.e. no obstruction to flow across the VSD). It is often located in the perimembranous and muscular regions of the ventricular septum, allowing shunting of blood between the ventricles (right to left in a classical cyanotic ToF).
RV outflow tract obstruction
The obstruction to pulmonary blood flow at the level of the RV outflow tract (RVOT) is a key feature of ToF.3 The RVOTO subsequently causes the RVH (another key feature of ToF). In the presence of an unrestrictive VSD, worsening RVOTO increases the RV pressure, drives a right-to-left shunt through the VSD, reduces pulmonary blood flow, and leads to hypoxaemia. Assuming the patent ductus arteriosus (PDA) is closed and there is no collateral supply, then the more severe the RVOTO, the more hypoxaemic the patient may be on presentation. A key feature is obstruction at the sub-valvular RVOT (in 50% of patients). This condition is usually dynamic and often results from infundibular muscle bundles. However, the obstruction may be at the pulmonary valve (10%), above the pulmonary valve (10%), or a mixture (30%).5 Complete occlusion of the RVOT is known as pulmonary atresia.
Overriding aorta
This condition describes the ventriculoarterial connection where the aorta can override the VSD to varying degrees, because of malalignment of the outlet component of the septum. In ToF, the aorta still arises from the left ventricle, with only some origin from the right ventricle. (This condition is the override.)3, 5 If the aorta arises predominantly from the right ventricle, then this abnormality may be called double-outlet right ventricle (DORV), and the physiology is determined by the VSD position and associated RV or left ventricular (LV ) outflow obstruction.3 This topic is out of the scope of this review. However, it is important to understand DORV because i.v. air in this lesion is more likely to end up in the arterial circulation than in other lesions.
RV hypertrophy
RV hypertrophy develops as a consequence of the RVOTO because increased RV pressure needs to be generated to maintain pulmonary blood flow. This condition also alters the RV cavity size and muscle mass, which are important issues after ToF repair.
Anatomical variants
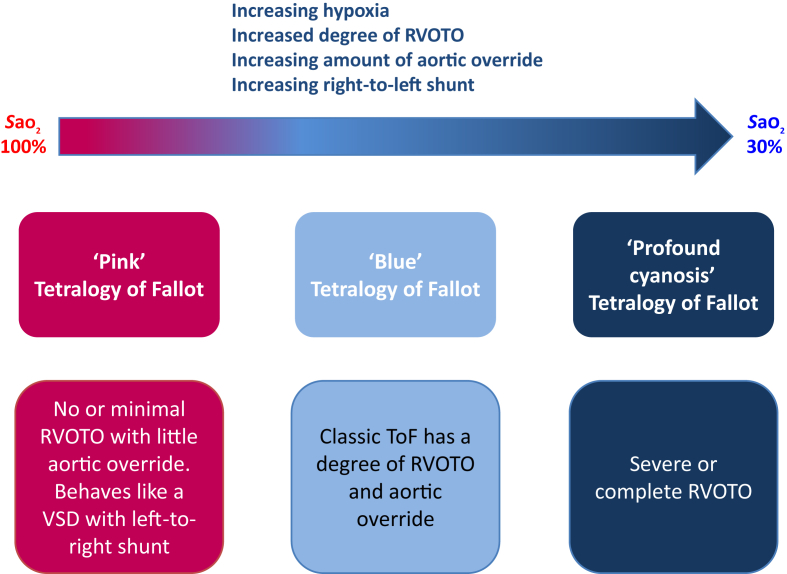
There are a number of subtypes of ToF, largely distinguished by the degree of cyanosis displayed.3 These subtypes are summarised in Figure 2. The following are the key important variants.
Fig 2.
Representation of different anatomical –pathophysiological correlates in ToF.
‘Pink’ Fallot
Children with this variant are acyanotic with normal or near-normal oxygen saturations; there is minimal or no RVOTO. Physiologically, the lesion behaves like a large unrestricted VSD with a left-to-right shunt. Patients can present with heart failure or other features of the left-to-right shunt, although this condition can change with time as the child grows, and features are more like the features of a classical ToF.
Fallot-type pulmonary atresia (15% of ToF)
This type is the severest variant, characterised by complete atresia (i.e. complete occlusion) of the pulmonary valve; therefore, there is no forward flow of blood from the RV into the pulmonary artery. Intracardiac mixing is essential, and all pulmonary blood flow must be supplied from the aorta either by a PDA (‘duct-dependent pulmonary circulation’) or from major collaterals from the aorta to the pulmonary arteries (major aortopulmonary collateral arteries, MAPCAs).6 In the neonatal period, prostaglandin (either alprostadil [prostaglandin E1] or dinoprostone [prostaglandin E2]) may be required acutely to maintain any pulmonary blood flow through the PDA.
ToF with absent pulmonary valve (6% of ToF)
There is no pulmonary valve, but the RVOT is open. These infants are often acyanotic because there is no RVOTO, but the condition is notable for respiratory complications that develop secondary to massive aneurysmal dilatation of the pulmonary arteries caused by absence of the pulmonary valve, with obligatory pulmonary regurgitation.5, 7 These aneurysmal dilatations externally compress the distal trachea and bronchi causing intrathoracic airway obstruction, lung atelectasis, and even pulmonary hypoplasia.5 Tracheobronchomalacia is a major complication of pre- and post-surgical repair, and often requires prolonged ventilatory support.
Other cardiac anatomical variants can coexist with ToF and must be sought. These variations are outside the scope of this article.3, 8
Pathophysiology of ToF
The anatomy of ToF allows mixing of blood between the pulmonary and systemic circulations. This mixing usually occurs at the VSD, with a right-to-left shunt adding deoxygenated blood to the systemic circulation, causing cyanosis. The right-to-left shunt through the VSD is determined by the relative pressure gradient between the RV and LV. The amount of pulmonary blood flow (the RV stroke volume) is determined by the degree of the RVOTO.
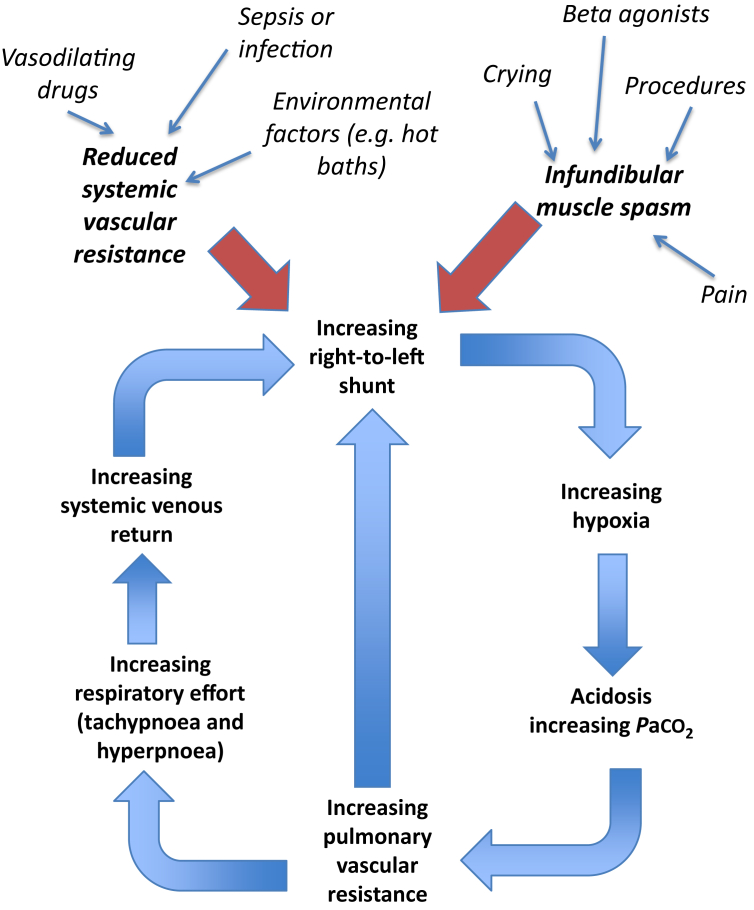
Frequently, the degree of RVOTO has both fixed anatomical and variable physiological components.3, 6 The individual variations in these factors account for the variability in saturations of these patients before repair (Fig. 2). Understanding the dynamic factors that worsen or improve the right-to-left shunt is the key to understanding the management of critically ill or preoperative infants with ToF (Fig. 3). These factors include the following.
Fig 3.
Mechanism and downward spiral of cyanotic spell.
Dynamic RVOTO from sub-valvular muscle bundles (‘spelling’)
Often, RVOTO is caused by sub-pulmonary valve muscle bundles. Infundibular muscle spasm (often during times of distress) causes dynamic RVOTO, adding to any fixed RVOTO, and therefore increasing the right-to-left-shunt fraction. This occurrence leads to profound hypoxaemia. These episodes of sudden oxygen desaturation are often referred to as ‘cyanotic spells’.5
Systemic vascular resistance
A large reduction in systemic vascular resistance (SVR), in the presence of RVOTO, will exacerbate any right-to-left shunt by reducing the LV end-diastolic pressure (LVEDP), thus increasing the right-to-left pressure gradient; an increase in SVR will do the opposite and forms the basis of treatment for spelling episodes.3 Avoidance of reduction of SVR during induction of anaesthesia is vital in patients with an unrepaired ToF.
Pulmonary vascular resistance
In the neonatal period pulmonary vascular resistance (PVR) is higher than in adults and can be fluctuant. Any increase in PVR may further increase the RV afterload adding to any RVOTO, and drive a greater right-to-left shunt.
With any of the physiological changes described previously that cause an increased right-to-left shunt, it is important to remember that cardiac output is often maintained initially because the shunt desaturates systemic blood, but maintains the LV preload and cardiac output, albeit with deoxygenated blood.
In addition to the right-to-left-shunt dynamics, the secondary RVH also has additional implications for the clinical management of these infants.
Diastolic function and HR
Muscle fibre changes in RVH cause RV diastolic impairment, leading to impaired lusitropic function (poor relaxation and diastolic filling). Any additional tachycardia will cause a proportional reduction in diastolic filling, further reducing the stroke volume whilst increasing the oxygen demand.
Increased ventricular diastolic filling pressure (venous return to the right ventricle )
Because of the diastolic dysfunction, these children have an increased RV end-diastolic pressure and are dependent on increased venous return for RV filling. These changes persist post-repair and can cause significant problems in the ICU.
Clinical recognition and evaluation
The majority of cases of ToF are diagnosed on antenatal foetal ultrasound scanning. This procedure allows an appropriate counselling of parents, delivery in a cardiac centre, and planning of postnatal care.
The diagnosis may be made after birth with the time of presentation depending on the severity of the ToF (Table 1). Many neonates will be detected by routine pulse oximetry screening, or the presence of a murmur on a neonatal examination. The classic presentation is of a cyanotic neonate or young infant, who has no features of respiratory distress, fails to respond to oxygen therapy, and has signs of a good cardiac output. Many of these children will have Spo2 of 75–80% as ‘normal’ for their anatomy. Failure to recognise ToF can lead to presentation of a moribund infant with profound hypoxaemia causing myocardial dysfunction with low cardiac output and severe metabolic acidosis when the PDA closes. It is important to remember that the appearance of clinical cyanosis is not only determined by the cardiac lesions, but also by the haemoglobin concentration; anaemic children may not appear cyanosed and children with polycythaemia appear cyanosed more readily.
Table 1.
Clinical features of ToF
| Cyanosis (may be gradual onset and increasing in severity or with age) |
| Hypoxia/low Spo2 with little or no response to oxygen therapy |
| Heart murmurs (pansystolic and ejection systolic) |
| Spelling episode: agitated, distressed, and profound cyanosis |
| Arrhythmias (particularly supraventricular or ventricular) |
| Associated features of other genetic abnormalities |
| Features of late presentation can include poor exercise tolerance, clubbing, polycythaemia, neurodevelopmental delay, failure to thrive, heart failure, recurrent respiratory tract infections, and cerebral abscess and stroke. |
Some other conditions are associated with ToF; 15% are part of syndromes, including Down, DiGeorge, and Alagille syndromes; vertebral defects, anal atresia, cardiac defects, tracheo-oesophageal fistula, renal anomalies, and limb abnormalities (VACTERL association); and velocardiofacial abnormalities.9, 10 Some can be associated with airway abnormalities. Severe lower airway obstructive disease secondary to tracheobronchomalacia is common in ToF with an absent pulmonary valve.3
Investigations that are helpful at the time of diagnosis and before any elective surgery are shown in Table 2.11
Table 2.
Key investigations for the evaluation of a patient with ToF
| Investigation | Key points |
|---|---|
| Chest X-Ray (CXR ) | ‘Boot-shaped heart’ caused by RVH Reduced pulmonary vascular markings because of RVOTO Excludes other causes of hypoxia If acyanotic (i.e. no RVOTO), CXR may be like that for a VSD (high pulmonary blood flow and pulmonary oedema), but pulmonary vascular markings can be normal |
| Blood gas | Low Pao2, which does not increase with oxygen therapy Excludes signs of decompensating (e.g. lactate and bicarbonate) |
| ECG | Right axis deviation Tall R wave in V1: right ventricular hypertrophy Tall P waves (P pulmonale): right atrial enlargement Exclude arrhythmias |
| Echocardiography | Confirms the diagnosis. Key questions:
|
| Cardiac catheterisation | Almost never required unless a therapeutic intervention is planned; can be rarely used in complex cases to delineate coronary anatomy, assess for collaterals, and obtain intracardiac pressure measurements |
Management: before repair
Neonatal presentation
Patients with severe RVOTO may need a PDA to supply enough pulmonary blood flow; such a duct-dependent circulation will require prostaglandin (either alprostadil [prostin E1] or dinoprostone [prostin E2]) infusion until more temporising palliative procedures or a definitive surgical treatment is undertaken. Infants with dynamic RVOTO may require beta-blockade (e.g. propranolol) if spelling is frequent.
Other causes that may mimic or worsen cyanotic spells should be excluded (e.g. upper airway abnormalities). The majority of neonates and infants with ToF do not require any active management and are managed at home with regular outpatient reviews, until they are of a weight suitable to undergo complete corrective surgery. Patients with ToF who are acyanotic and have a large VSD may have a large left-to-right shunt, will need diuretics, and may develop heart failure.
Spelling: emergency resuscitation
An infant with ToF may present with any acute deterioration, and resuscitation should initially follow an ‘ABC’ approach along national paediatric and intensive care guidelines.
Spelling, or a hypercyanotic episode, describes a unique acute desaturation and clinical deterioration in a patient with uncorrected ToF.3 There is an acute reduction in pulmonary blood flow caused by a sudden increase in right-to-left shunt secondary to infundibular spasm or alteration of the SVR:PVR ratio. These are often precipitated by sympathetic stimulation and constitute an emergency, and therefore, indicate an intervention is required. The cyanosis and acidosis that develop worsen the RVOTO/PVR changes, causing further shunt with vicious cycling and deterioration (Fig. 3).5 Spelling can only occur in a patient with ToF that has not undergone surgical repair, but whose PDA has closed.11 Such patients may already be identified as being at high risk and may be taking prophylactic propranolol. Some well-known triggers can precipitate these events (Fig. 3). These triggers include the following:
-
(i)
Sympathetic stimulation, including pain and anxiety (e.g. during venepuncture)
-
(ii)
Exercise
-
(iii)
Breath holding or Valsalva manoeuvre
-
(iv)
Crying, feeding, and defaecation
-
(v)
Vasodilatation and decrease in SVR (e.g. hot baths)
-
(vi)
Hypoxia
-
(vii)
Hypercarbia
-
(viii)
Acidosis
-
(ix)
Induction of anaesthesia
-
(x)
Sympathomimetic drugs
It is important to avoid these triggers, many of which can occur around the time of anaesthesia or clinical procedures.
The clinical features of spelling can be pronounced. Classically, the child becomes restless, agitated, and breathless. Older children may squat into a knee-to-chest position as a learnt mechanism to increase SVR and consequently reduce right-to-left shunt.6 The child will be cyanosed and tachycardic. A reduced murmur may be heard because of reduced blood flow through the RVOT.5 In severe attacks, the conscious level may fall or the patient may have a seizure. As with any right-to-left shunt, there is a low but potential risk of cerebral vascular accident.
The majority of acute hyper-cyanotic attacks are self-limiting, lasting on average 15–30 min.11 If severe, treatment goals are to support airway, breathing and circulation; and reduce right-to-left shunt (breaking the cycle displayed in Fig. 3) by reducing the dynamic RVOTO, decreasing the PVR, and increasing the SVR to raise the LVEDP. Strategies for this management are outlined in Table 3.
Table 3.
Management of an acute hypercyanotic spell
|
Elective or emergency non-cardiac surgery (general anaesthesia)
Whilst stable balanced children with ToF should present no major problem, it would be unusual to operate on these children outside of a regional paediatric cardiac unit. Before transfer, or if this is not possible, close liaison with the local paediatric cardiac centre is mandatory. Parents will give guidance to their usual saturations and state of health; baseline Spo2 readings will naturally be lower than normal, but saturations below 80% should raise questions.
The key principles are to avoid any precipitant that could induce a spell episode, and be prepared to manage profound desaturation whilst under anaesthesia if this happens, as described previously (Table 3).
Any technique that follows the basic principles of anaesthetising infants with critical heart disease, with particular reference to ToF physiology, is acceptable in experienced hands. The principles are to avoid excessive reductions in SVR, increases in PVR, and excessive sympathetic stimulation, whilst maintaining safe ‘ABC’ management. Using ketamine and rocuronium at induction of anaesthesia will provide a balance of all the aforementioned, and is the standard approach in our paediatric ICU (PICU).
Management: surgical repair
The history of surgical repair of ToF has been at the forefront of developments in paediatric cardiac surgery. Early interventions in the face of profound cyanosis were provision of pulmonary blood flow by the use of systemic-to-pulmonary shunts, through evolutions in surgical repair with pulmonary valve-sparing techniques, and corrective operations at earlier and earlier ages, to options of PDA and RVOT stents and more recently complete repair as a neonate or infant with negligible mortality.12 Irrespective of what intervention is used, timely action is needed when adverse symptoms occur to preventing long-term or significant cyanosis to optimise neurodevelopmental outcomes.
Cardiac catheter interventions
Interventional cardiac catheterisation can help improve pulmonary blood flow in neonates and infants whilst waiting growth for a full repair. This procedure is usually in the form of a pulmonary valvotomy or RVOT stenting to reduce the RVOTO and improve pulmonary blood flow.13 The PDA can be stented, reducing the need for i.v. prostaglandin in those smaller duct-dependent neonates. All of these procedures carry their own risks.
Temporising or palliative surgery
The majority of ToF surgical cases involve corrective rather than palliative surgical procedures, with the majority of cases aiming for complete repair in one single operation. In ToF with profound cyanosis unsuitable for early neonatal repair, a temporising measure is the creation of a systemic-to-pulmonary shunt. This measure is most commonly the modified Blalock–Taussig (mBT). This shunt is an extracardiac connection between the subclavian artery and the ipsilateral pulmonary artery using Teflon- or Gore-Tex-coated tube, and acts like a PDA by providing left-to-right continuous flow.14 An mBT shunt will improve pulmonary blood flow irrespective of the RVOTO, improving cyanosis and providing time for the child to grow.
Corrective surgery
Complete corrective ToF surgery is usually performed before 6 months of age as open intracardiac surgery performed under cardiopulmonary bypass conditions in one operation.12, 15 It is performed relatively early in life to reduce the pathophysiological adaptation of the ToF physiology; this shift in timing has led to an observed reduction in mortality by improving pulmonary vasculature development early on, reducing RVH and subsequent fibrosis.16 Neonatal primary ToF repairs are being performed at many centres.15
The repair involves using patch closure of the VSD, and septate the aorta back to the left ventricle, resection of RVOT muscle bundles, and reduction of the degree of RVOT valvular stenosis.6 A transannular patch may be required to sufficiently enlarge the RVOT, but leads to obligatory pulmonary regurgitation. Associated findings (often atrial septal defects and PDA) are also repaired.
Management: after repair
Early postoperative management in paediatric intensive care
The postoperative course is variable depending on the age of the child, duration of cardiopulmonary bypass, the type of ToF repair, and other associated co-morbidities. Performing repairs in infants and neonates has not shortened PICU stays, and patients often present challenging problems. General cardiac surgical complications (e.g. bleeding and systemic inflammatory response) are encountered and managed conventionally. However, key complications particularly associated with ToF repair include:
-
(i)
Arrhythmias: particularly junctional ectopic tachycardia
-
(ii)
Residual lesions: VSD, RVOTO, and pulmonary regurgitation
-
(iii)
Restrictive RV physiology: reduced RV compliance
-
(iv)
Pleural effusions (from high RV end-diastolic pressures)
Long-term sequelae
All children and adults will be under lifelong follow-up with congenital heart disease services. However, it is recognised that compliance with follow-up can be poor, and some patients can be unaware of their diagnosis. On emergency presentation or before elective surgery, consultation should be had with the regional paediatric or adult congenital cardiac centre.
In general, the repaired ToF heart is a biventricular system, but the physiology can be variable and lifelong problems often persist. Problems include pulmonary valve regurgitation, which may be severe; RV dysfunction; and residual lesions (such as tiny VSD or some residual mild RVOTO). Prophylaxis for endocarditis should be considered, depending on the planned surgery and advice of the specialty team. A detailed workup, including ECG (to assess the QRS duration, and hence, risk of arrhythmias); echocardiography; and, perhaps, exercise tolerance testing need to be considered.16, 17
During pregnancy
Although a rare scenario, there is an increased risk to maternal health during pregnancy and delivery if the mother has had previous ToF repair.18 This condition is a specialised area, and a discussion with the combined regional congenital cardiac–obstetric multidisciplinary team must occur. Management will depend on the usual ToF physiology after repair, any residual defects, and cardiac function.19
Prognosis
Unrepaired, the consequence of chronic hypoxia, with the pathophysiological effect of the RVOTO, significantly alters a patient's quality of life, growth and development (including neurodevelopment), and educational achievement.20 The life expectancy, if uncorrected, is between the first and fourth decades; death is caused by cardiac failure, arrhythmia, respiratory infection, or thromboembolic disease.21 Late repairs are often undertaken in low-income countries because of late presentation or lack of access; these cases can be very challenging.
The long-term outcomes of corrected ToF are good, particularly for those without associated syndromes. There remains an increased risk of sudden death, usually from arrhythmias (usually ventricular, but also supraventricular tachycardia); a widened QRS complex may predict risk.16 Patients may require implantable cardioverter defibrillator insertion or cardiac catheter ablation.22 Pulmonary valve incompetence can occur and lead to a progressive decrease in RV function with increasing dilatation, thus increasing the risk of arrhythmias and reducing exercise tolerance.23 This condition is often seen 10–20 yrs after repair, and often requires the placement of a valved homograft, conduit, or percutaneous pulmonary valve. Although these interventions should reverse RV dilatation and the risk of arrhythmias, they have not been shown to increase life expectancy or functional independence.24
Summary
Tetralogy of Fallot is one of the commonest cyanotic congenital heart malformations and is characterised by four cardinal features: VSD; RVOTO, which is often dynamic; an overriding aorta; and RVH. The degree of RVOTO, the relative pressures in the right and left ventricles, and the proportion of the aorta overriding the VSD determine the presentation and severity of this condition. Other variants do exist.
The majority of cases involve single-step corrective surgery, and if corrected early, a good prognosis can be expected. Anaesthetists may encounter this condition before or after repair, and in seeking specialist help early, they should be aware of the relevant anatomy, physiology, and initial management of emergencies associated with the condition.
Declaration of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors would like to thank Dr Harry Phillips (specialist trainee in anaesthesia and intensive care medicine) for his constructive review and comments on this article.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Robin Wilson FRCA is a specialty trainee in anaesthesia in the Wessex Deanery, currently undertaking an advanced fellowship in paediatric anaesthesia at Southampton Children's Hospital.
Ollie Ross DMCC FRCA is a consultant paediatric intensivist and anaesthetist at Southampton Children's Hospital, with a specific interest in bronchoscopy, cardiac critical care, and surgery for scoliosis repair. He has 25 yrs of experience in working overseas on educational partnerships in anaesthesia and paediatric critical care.
Michael Griksaitis MSc MRCPCH FFICM is a consultant paediatric intensivist at Southampton Children's Hospital with a specific interest in cardiac critical care, transport medicine, and point-of-care ultrasound. He is the Chair of the Paediatric Intensive Care Society Acute Transport Group and a member of the Faculty of Medicine at the University of Southampton.
Matrix codes: 1A01, 2D01, 3D00
References
- 1.Villafañe J., Feinstein J.A., Jenkins K.J. Hot topics in tetralogy of Fallot. J Am Coll Cardiol. 2013;62:2155–2166. doi: 10.1016/j.jacc.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 2.Lell W.A., Pearce F.B. Tetralogy of Fallot. In: Lake C.L., Booker P.D., editors. Pediatric cardiac anesthesia. Lippincott Williams & Wilkins; London: 2005. pp. 344–356. [Google Scholar]
- 3.Bailliard F., Anderson R. Tetralogy of Fallot. Orphanet J Rare Dis. 2009;4:2. doi: 10.1186/1750-1172-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tetralogy of Fallot https://www.mayoclinic.org/diseases-conditions/tetralogy-of-fallot/symptoms-causes/syc-20353477 Available from:
- 5.Park M.K. Elsevier; Philadelphia, PA: 2010. pp. 130–140. (The pediatric cardiology handbook). [Google Scholar]
- 6.Sommer R.J., Hijazi Z.M., Rhodes J.F. Pathophysiology of congenital heart disease in the adult; part III: complex congenital heart disease. Circulation. 2008;117:1340–1350. doi: 10.1161/CIRCULATIONAHA.107.714428. [DOI] [PubMed] [Google Scholar]
- 7.Kazim R., Quuagebeur J.M., Sun L.S. The association of tracheal anomalies and tetralogy of Fallot. J Cardiothorac Vasc Anesth. 1996;10:589–592. doi: 10.1016/s1053-0770(96)80134-0. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Soukias N.D., Carvalho J.S., Ho S.Y. Coronary arterial anatomy in tetralogy of Fallot: morphological and clinical correlations. Heart. 1998;80:174–183. doi: 10.1136/hrt.80.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry L.W., Neill C.A., Ferencz C. Perspectives in pediatric cardiology. Futura; Armonk, NY: 1993. EUROCAT working party on congenital heart disease. Epidemiology of congenital heart disease: the baltimore-Washington infant study, 1981–1989; pp. 33–62. [Google Scholar]
- 10.Kramer H.H., Majewski F., Trampisch H.J., Rammos S., Bourgeois M. Malformation patterns in children with congenital heart disease. Am J Dis Child. 1987;141:789–795. doi: 10.1001/archpedi.1987.04460070091033. [DOI] [PubMed] [Google Scholar]
- 11.Gossett J., Kamp A. 2018. Tetralogy of Fallot December.https://bestpractice.bmj.com/topics/en-gb/701 Available from: [Google Scholar]
- 12.Karl T.R., Stocker C. Tetralogy of Fallot and its variants. Pediatr Crit Care Med. 2016;17(Suppl. 1):S330–S336. doi: 10.1097/PCC.0000000000000831. [DOI] [PubMed] [Google Scholar]
- 13.Dohlen G., Chaturvedi R.R., Benson L.N. Stenting of the right ventricular outflow tract in the symptomatic infant in tetralogy of Fallot. Heart. 2009;95:142–147. doi: 10.1136/hrt.2007.135723. [DOI] [PubMed] [Google Scholar]
- 14.Williams W.G., Rubis L., Trulser G.A., Mustard W.T. Palliation of tricuspid atresia: Potts-Smith, Glenn, and Blalock-Taussig shunts. Arch Surg. 1975;110:1383–1386. doi: 10.1001/archsurg.1975.01360170123018. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch J.C., Mosca R.S., Bove E.L. Complete repair of tetralogy of Fallot in the neonate: results of the modern era. Ann Surg. 2000;232:508–514. doi: 10.1097/00000658-200010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatzoulis M.A., Balaji S., Webber S.A. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 17.Valente A.M., Gauvreau K., Assenza G.E. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. doi: 10.1136/heartjnl-2013-304958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelson E., Gatzoulis M., Steer P.J., Lupton M., Johnson M. Tetralogy of Fallot: maternal and neonatal outcomes. BJOG. 2008;115:398–402. doi: 10.1111/j.1471-0528.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 19.Burt C., Durbridge J. Management of cardiac disease in pregnancy. Contin Educ Anaesth Crit Care Pain. 2009;9:44–47. [Google Scholar]
- 20.Hovels-Gurich H.H., Konrad K., Skorzenski D. Long-term neurodevelopmental outcomes and exercise capacity after corrective surgery for tetralogy of Fallot or ventricular septal defect in infancy. Ann Thorac Surg. 2006;81:958–966. doi: 10.1016/j.athoracsur.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Bertranou E.G., Blackstone E.H., Hazelrig J.B. Life expectancy without surgery in tetralogy of Fallot. Am J Cardiol. 1978;42:458–466. doi: 10.1016/0002-9149(78)90941-4. [DOI] [PubMed] [Google Scholar]
- 22.Gatzoulis K., Frogoudaki A., Brili S., Stefanadis C. Implantable defibrillators: from the adult cardiac to the grown up congenital heart disease patient. Int J Cardiol. 2004;97:117–122. doi: 10.1016/j.ijcard.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho J.S., Shinebourne E.A., Busst C., Rigby M.L., Redington A.N. Exercise capacity after complete repair of tetralogy of Fallot: deleterious effects of residual pulmonary regurgitation. Br Heart J. 1992;67:470–473. doi: 10.1136/hrt.67.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bokma J., Geva T., Sleeper L.A. A propensity score-adjusted analysis of clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Heart. 2017;104:738–744. doi: 10.1136/heartjnl-2017-312048. [DOI] [PubMed] [Google Scholar]