Learning objectives.
After reading this article you should be able to:
-
•
Outline the complexities of dynamic assessment of fluid-responsiveness in intensive care patients.
-
•
Use more detailed knowledge of transthoracic echocardiography examination and calculations when assessing fluid-responsiveness.
-
•
Discuss the strengths and limitations of the use of transthoracic echocardiography in the critically ill.
Key points.
-
•
Intravascular volume assessment in the critically ill is difficult; only 50% of patients are fluid-responsive.
-
•
Dynamic measures based on heart–lung interactions are the cornerstone for predicting fluid-responsiveness.
-
•
A virtual or actual fluid challenge should be considered the gold standard in assessing fluid-responsiveness.
-
•
Transthoracic echocardiography assesses cardiac structures, function, and haemodynamic indices. It can be used with continuous cardiac monitoring for individualised fluid management in changing clinical circumstances.
The assessment of intravascular volume status in the critically ill and ability to predict those who may benefit from volume expansion is important: half of all patients presenting with shock are not fluid-responsive and are at risk of subsequent fluid overload.1 International guidelines advocate large volumes of fluid in the context of sepsis despite a mounting body of evidence to suggest that inappropriate fluid therapy can increase morbidity and mortality.2, 3, 4 In patients with sepsis, a positive fluid balance and a higher central venous pressure are associated with increased mortality.5 Echocardiography is now a desired standard of care in the intensive care unit (ICU) for haemodynamic monitoring; including assessment of both static and dynamic fluid-responsiveness.6
This article describes the role of transthoracic echocardiography (TTE) in the assessment of dynamic fluid-responsiveness, including its advantages and limitations compared with other modalities.
Why use echocardiography?
Echocardiography offers many advantages over traditional cardiac output monitors:
-
(i)
Advanced non-invasive haemodynamic assessments at the bedside, allowing differentiation of the aetiology of shock, which is often multifactorial in the critically ill.
-
(ii)
Direct close estimates of measurements by an experienced operator, including stroke volume (SV) and cardiac output. Most cardiac output monitors use inferred or calculated values.
-
(iii)
Structural and functional cardiac assessments that are a prerequisite to reliable interpretation of haemodynamic data. The presence of right heart failure invalidates SV variation as a marker of fluid-responsiveness and the presence of valvular lesions (especially in the context of arrhythmias) affects the interpretation of data from traditional cardiac output monitors.
-
(iv)
Assessment of diastolic function and left ventricular end-diastolic pressure (LVEDP), which can mitigate harm from excessive fluid.
-
(v)
Easy integration with other point-of-care ultrasound techniques such as lung ultrasound.
Heart–lung interactions and their implications
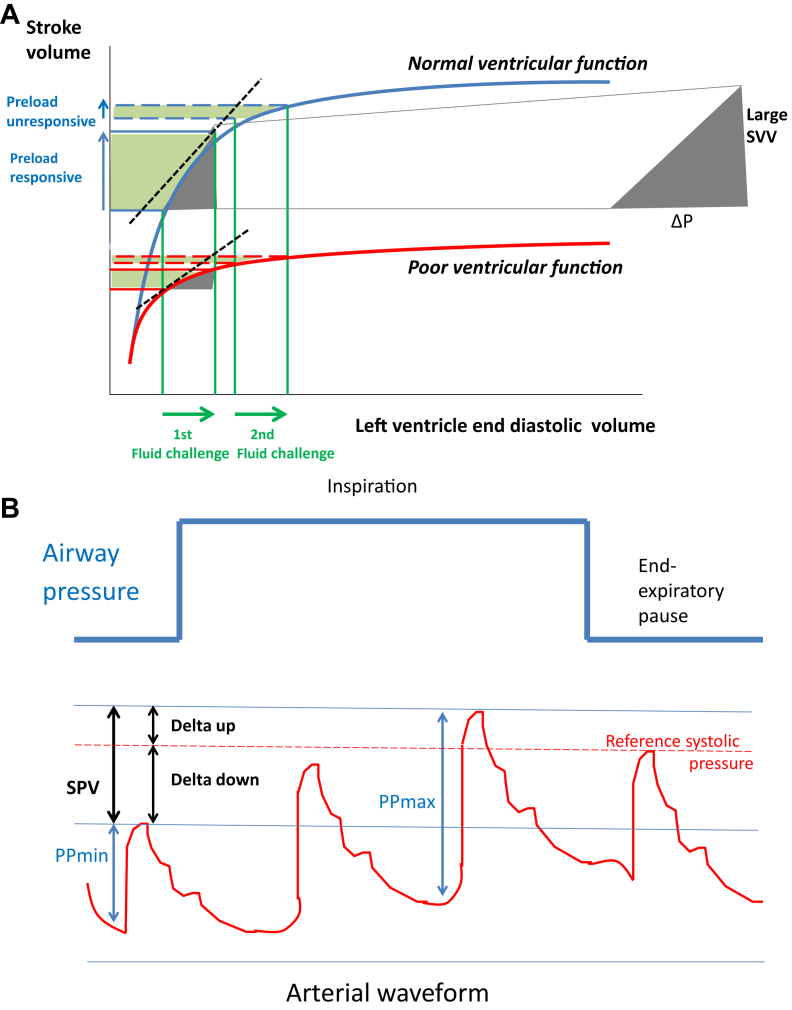
Heart and lung interactions are influenced by the volume status of the mechanically ventilated patient alongside the lung compliance and ventilator settings.7 Intermittent positive pressure mechanical ventilation produces cyclical changes in intrathoracic pressures (ITPs), which change the loading conditions of the ventricles. The systemic return of blood into the right atrium relies on passive flow down a pressure gradient. During the inspiratory phase of a mechanically ventilated breath, the right ventricle (RV) preload decreases because venous return is decreased. This results from an increase in both pleural pressure and RV afterload caused by an increase in trans-pulmonary pressure (alveolar pressure minus the ITP). The decrease in RV preload and increase in RV afterload lead to a decrease in RV SV at the end of the inspiratory period. Ventricular interdependence decreases the left ventricle (LV) filling pressure after a lag phase of a few heartbeats, with a reduction in LV preload (end-diastolic wall tension) and subsequently LV SV during the expiratory phase. This effect is pronounced when intravascular volume is decreased significantly. The magnitude of the effects of respiratory changes on the LV SV indicates the biventricular preload dependence and suggests that the LV is operating on the ascending (fluid-responsive) part of the Frank-Starling curve (Fig. 1A).
Fig 1.
(A) Frank–Starling curve and predicting fluid responsiveness. (B) Delta down and systolic pressure variation (SPV) indices based on the variation in arterial pressure associated with mechanical ventilation. SVV, stroke volume variation.
Left ventricular SV is a major determinant of the systolic arterial pressure. Mechanical ventilation is associated with reversed pulsus paradoxus, where there is a decrease in venous return to the right heart on inspiration and an increase in systolic arterial pressure during inspiration; it is caused by increased ITP, which decreases LV afterload and forces blood into the left atrium with a consequent increase in left ventricular SV. The difference between the maximum and minimum systolic arterial pressure over one respiratory cycle compared with a reference systolic pressure (measured over an end expiratory pause) determines the systolic pressure variation. There are two components: the ‘delta up’ and the ‘delta down’ (Fig. 1B).8 In the context of hypovolaemia, the delta down effect is exaggerated with positive pressure ventilation because of a decrease in right atrial pressure and superior vena cava collapse.
The effects of intermittent positive pressure mechanical ventilation on ITP and SV are the basis for the dynamic measurements that are used to determine fluid-responsiveness.
TTE measures of fluid-responsiveness
In the complex patient in the ICU, shock is often multifactorial: hypovolaemic, cardiogenic, distributive, or obstructive. With basic clinical assessment only 50% of patients are ‘responders’ to a fluid challenge (increase their SV by ≥10–15%).9 In general static volume and pressure measures (Table 1) are unable to predict fluid-responsiveness. A static marker reflects preload at some point on the Frank–Starling curve but does not demonstrate a capacity to move along the curve. Dynamic indices based on heart–lung interactions during mechanical ventilation are likely to be better, with a modest degree of accuracy in predicting fluid-responsiveness. Assessments based on either a virtual (passive leg raise; PLR) or a real actual fluid challenge and are likely to be the gold standard. Current ‘Surviving Sepsis’ guidelines recommend that dynamic measures are used to predict fluid-responsiveness.2 Fluid-responsiveness can be assessed with TTE by either volume expansion (actual or virtual) or respiratory variation.
Table 1.
Measures of fluid responsiveness. IVC, inferior vena cava; LVOT, left-ventricular outflow tract; SVC, superior vena cava; VTI, velocity-time interval. *The SVC can be difficult to visualise with transthoracic echocardiography but can be seen on transoesophageal echocardiography
| Clinical assessment | Echocardiography assessment | |
|---|---|---|
| Static pressure and volume measures | Blood pressure Heart rate Urine output Lactate Mean arterial pressure Central venous pressure Mixed venous oxygen saturation Pulmonary capillary wedge pressure/pulmonary artery occlusion pressure Flow time corrected |
End-diastolic ventricular volumes, area and diameters Size of the IVC Left ventricular end-diastolic pressure |
| Dynamic measures based on heart–lung interactions | Passive leg raise Pulse pressure variation End-expiratory occlusion Pleth variability index Rapid fluid challenge Mini fluid challenge |
LVOT maximum velocity LVOT VTI Stroke volume variation Distensibility index of IVC Collapsibility index of SVC* |
Evaluation of the ventricles
In profound overt hypovolaemia, preload is insufficient to allow adequate cardiac filling and both ventricles appear small and hyperkinetic; this finding is useful in predicting a fluid-responsive state.10 In severe cases of hypovolaemia the LV is seen to collapse in systole, referred to as ‘kissing-ventricles’. Without the presence of profound overt hypovolaemia (which indicates significantly low preload) static measurements of LV size such as LV end diastolic; volumes, areas, and indices have no correlation to fluid loading and fluid-responsiveness.11 Therefore these static measures have limited utility in ICU, where hypovolaemia is often less obvious and optimum fluid status is important. On TTE assessment, LV hypertrophy may mimic hypovolaemia where the thickened heart muscle wall visually gives the illusion of a small LV cavity size.
The inferior vena cava
Central venous pressure has a low predictive value for fluid-responsiveness and in general the absolute size of the inferior vena cava (IVC) does not predict fluid-responsiveness.12 A small IVC diameter (<10 mm) may, however, predict a positive response to a fluid challenge.13
The IVC can be identified in the subcostal view; assessment is either by two-dimensional or M-mode with measurements taken with acquisition of the respiratory trace and over one respiratory cycle. During mandatory mechanical ventilation with no spontaneous effort, the IVC dilates during inspiration (maximum diameter) because of increased ITP (which impedes venous return) and contracts during expiration (minimum diameter). The IVC distensibility index is the percentage variation of the IVC during inspiration verses expiration (Fig. 2G). A distensibility index >18% offers 90% sensitivity and specificity in discriminating fluid responders from non-responders.14
| (1) |
Fig 2.
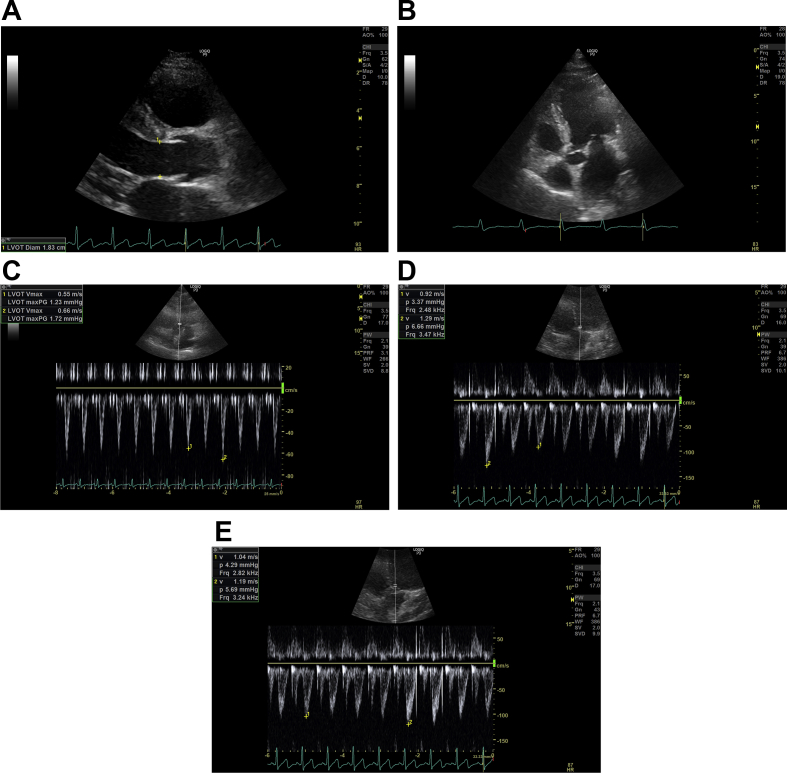
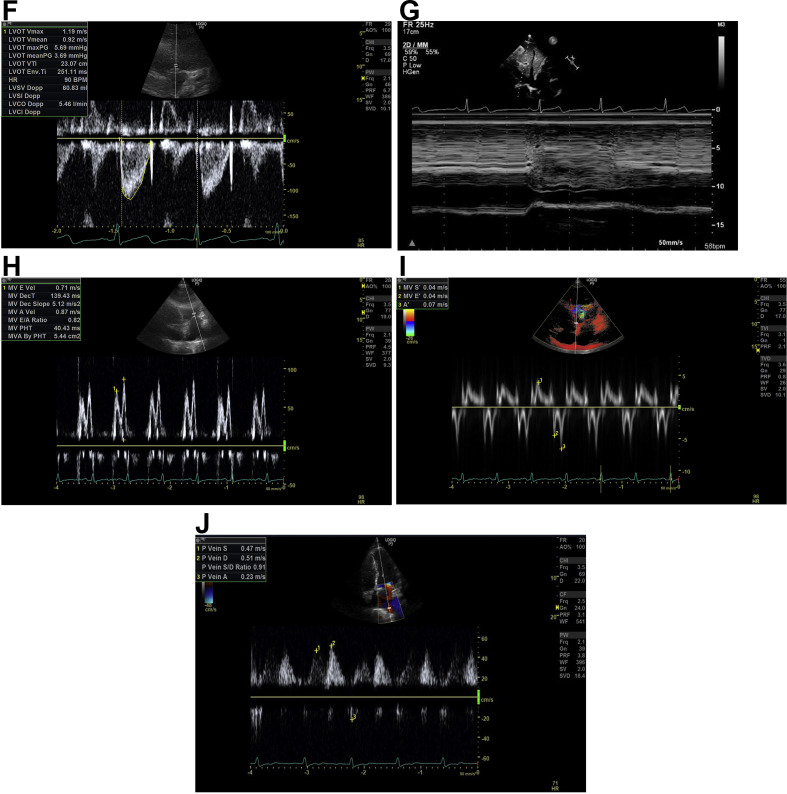
(A) Left ventricular outflow tract (LVOT) diameter. Parasternal long axis view is found by placing the probe to the left of the sternum, perpendicular to the chest, in the third or fourth intercostal space with the indicator of the probe pointing at the 11 o'clock position. By zooming into the LVOT tract, the image is frozen in systole whilst the aortic cusps are fully opened; the LVOT diameter is measured about 0.5 cm back from the aortic valve cusp insertion points on the ventricular side. The LVOT diameter is measured inner edge to inner edge at the widest diameter. (B) LVOT Apical 5 chamber (A5C) view. This view is obtained by placing the probe in the mid-axillary line, fifth intercostal space, with the indicator of the probe pointing at the 3 o'clock position. The probe is tilted up slightly anteriorly to visualise the left ventricular aortic outflow tract. The Doppler cursor with the pulsed wave (PW) Doppler sample volume is placed at the level of the aortic valve annulus within 15° to the LVOT. (C) LVOT velocity: non-fluid-responsive state. Aortic blood flow can be recorded using PW Doppler across the LVOT in the A5C view. The normal horizontal sweep speed is 100 mm s−1, the sweep speed is increased to ensure several respiratory cycles are represented and the image is frozen. LVOT maximum velocity variation during the respiratory cycle on inspiration and expiration can be measured. The LVOT velocity variation with respiration here is 17% (<20%), suggesting a non-fluid-responsive state. (D) LVOT variation: fluid-responsive state. This is the A5C view with the PW Doppler recording across the LVOT. The normal horizontal sweep speed is 100 mm s−1, the sweep speed is increased to ensure several respiratory cycles are represented and the image is frozen. The beat-to-beat variation in LVOT maximum velocity can be measured. Here, before a fluid challenge, the beat-to-beat variation in LVOT maximum velocity is 28% (>12%), suggesting a fluid-responsive state. (E) Effect of a fluid challenge: non-fluid-responsive state. This is the A5C view with the PW Doppler recording across the LVOT. The normal horizontal sweep speed is 100 mm s−1, the sweep speed is increased to ensure several respiratory cycles are represented and the image is frozen. The beat-to-beat variation in LVOT maximum velocity can be measured. Here the PW Doppler across the LVOT after a fluid challenge can be seen and the beat-to-beat variation in LVOT maximum velocity is 12% suggesting a non-fluid-responsive state. (F) LVOT velocity-time integral (VTI). As before, the aortic blood flow can be recorded using PW Doppler trace across the LVOT. The normal horizontal sweep speed is 100 mm s−1, the sweep speed is reduced, the recording frozen and the envelope is traced. The machine computes the area under the curve which is the VTI in cm. The LV stroke volume can be calculated from the VTI. Repeat VTI measurements on three to five consecutive beats to calculate and averaged stroke volume. For stroke volume variation, increase the sweep speed and trace the maximum and minimum VTI over one respiratory cycle. (G) Inferior vena cava (IVC): fluid-responsive state. The subcostal view is obtained, with the patient lying flat. To visualise the IVC vessel in its long axis the probe is rotated counter-clockwise moving the indicator from the 3 to 12 o'clock position. The M mode is activated through the IVC just distal to the hepatic vein and the image frozen. The respiratory waveform (green) can be used to measure the IVC diameter during inspiration and expiration. Here the IVC distensibility index is 60% (i.e. >18%), suggesting a fluid-responsive state. (H) Mitral inflow. In the apical four chamber (A4C) view, PW Doppler can be used to evaluate the MV inflow. The PW Doppler sample volume is placed at the tips of the mitral valve leaflets, and a PW trace is obtained. The Peak E wave velocity (the first peaking of velocity in early diastole) and the Peak A wave velocity (the second peak representing the end-diastolic atrial contraction) are measured. E-A fusion as seen here is often seen with tachycardia. The E/A ratio is automatically calculated by the machine. (I) Tissue Doppler imaging (TDI). In the A4C view, TDI can be activated and the PW Doppler sample volume is placed across the medial mitral annulus to obtain the septal e′. Similarly, a PW Doppler sample volume should be placed across the lateral mitral annulus to obtain the lateral e′. The septal and lateral e′ are averaged to represent the early myocardial velocity (the myocardium moving away from the transducer) which corresponds to early diastolic relaxation. The E wave from Figure 2H is 0.71 m s−1 giving an E/e′ of 18 (>13), suggesting high left ventricular end-diastolic pressure. (J) Pulmonary vein. In the A4C view, the PW Doppler sample volume is placed inside a pulmonary vein to examine pulmonary venous flow. There are three components: the S wave represents forward flow into the left atrium during ventricular systole, the smaller D wave represents forward flow during ventricular diastole and the A wave represents flow reversal in the pulmonary vein during atrial systole. Here, the D wave is bigger than the S wave and the S/D ratio is 0.9, suggesting high left ventricular end-diastolic pressure.
In patients breathing spontaneously the IVC collapses on inspiration because ITP is negative. The IVC collapsibility index is the percentage variation of the IVC during expiration divided by the maximum diameter. Currently there are insufficient data to support its use in this context.15
| (2) |
Potential pitfalls include poor image quality and translational artefacts whereby the liver and IVC are often displaced with changes in respiration. In patients breathing spontaneously, large changes in ITP can cause changes in the IVC diameter.
Maximum velocity in the left ventricular outflow tract
The left ventricular outflow tract (LVOT) is best seen in the apical five chamber (A5C) or apical three chamber view. The pulse-wave (PW) Doppler is positioned in the LVOT within 15° to the outflow tract for the correct flow estimation (Fig. 2B). The PW Doppler allows velocity measurement precisely at the site where the LVOT diameter is measured (see below). The blood flow velocity is measured in the LVOT allowing for the measurement of the maximum and minimum variation in LVOT maximum velocity (Fig. 2C–E).
A beat-to-beat variation in the LVOT maximum velocity of >12% or a beat-to-beat variation in maximum LVOT velocity during the respiratory cycle of >20% predicts fluid-responsiveness to volume expansion.16
Limitations of this method are that breathing and movement artefacts can affect the LVOT velocity trace signal. In patients in atrial fibrillation (AF) a rapid heart rate, loss of atrial contraction and irregular ventricular ejection can affect the haemodynamics, resulting in an intrinsic beat-to-beat variation in SV. Therefore, in patients with AF, several consecutive measures of velocity-time interval (VTI) or LVOT maximum velocity should be averaged. In practice an average of 10 measurements is considered satisfactory. A more pragmatic alternative approach would be to look for three heartbeats with similar R-R intervals but without a preceding long or short heartbeat on the ECG trace, to ensure that the LVOT velocity trace signals look similar and then measure the velocity of one of these beats.
VTI in the LVOT
Firstly, assess the aortic value in the parasternal long axis view to ensure that there is no significant aortic stenosis. The image is frozen in systole, when the aortic cusps are fully open and the LVOT diameter is measured by placing the callipers just proximal at the insertion of the cusps, inner edge to inner edge usually about 2 cm in adults (Fig. 2A).
A PW Doppler recording in the LVOT (as above) can be traced manually; the area under the trace is the VTI, which is the stroke distance or the displacement of red blood cells during systole (Fig. 2F). The VTI is a summation of all the velocities per heartbeat and is represented by the area under the curve for each heartbeat. Respiratory variations in LVOT VTI predict fluid-responsiveness in ventilated patients, at a threshold of 20%.17
SV variation
SV can be calculated by estimating volumetric flow by Doppler analysis. SV is estimated from the LVOT velocity. This is because the geometry of the LVOT is almost circular and because the flow profile through the LVOT is laminar and flat.
The SV can be calculated by calculating the cross-sectional area (CSA) of the LVOT.
| SV=VTI×CSA | (3) |
| CSA=πr2 |
| CSA=3.14×(Diameter/2)2 |
| CSA =3.14×(Diameter2/4) |
| CSA=3.14/4×Diameter2 |
| CSA=0.795×(LVOT Diameter)2 |
The VTI measurements can be repeated on three to five consecutive beats (one respiratory cycle) and an averaged SV can be calculated (Fig. 2F).
| CO ml min−1=Averaged SV (ml beat−1)×heart rate (beat min−1). | (4) |
Further still, by tracing the largest and smallest VTI over a respiratory cycle, the averaged SV variation (SVV) can be calculated:
| (5) |
If accurately measured and calculated, the cardiac output is comparable to that measured by thermodilution using a pulmonary artery catheter.18 SVV of >12% accurately predicts fluid-responsiveness with values >14% having a very high positive predictive value and <10% a high negative predictive value.9 There is a grey zone between 12 and 14% where other markers of fluid responsiveness should be reviewed.
There are limitations to calculating SV and cardiac output from the LVOT VTI. The main inaccuracy occurs in measuring the LVOT diameter. Any error in its measurement will be squared when calculating the CSA. Aortic valve stenosis and subaortic obstruction limit the use of LVOT VTI where it would represent the maximum velocity of the stenosis rather than stroke distance. Also, this method does not predict fluid-responsiveness in spontaneous breathing patients where changes in inspiratory effort may alter the ITP.19 Another important consideration is dynamic LVOT obstruction, which occurs classically with hypertrophic cardiomyopathy but can occur with dehydration, excessive use of positive inotropic drugs, hypertensive heart disease and exercise. Dynamic LVOT obstruction is now more common, with an aging population and increasing incidence of hypertensive heart disease. The gradient in the LVOT is affected and affects the assessment of fluid-responsiveness using LVOT VTI.
LVEDP
LVEDP as a static marker is not useful in predicting fluid-responsiveness but can help diagnose and guide management of cardiogenic (hydrostatic) pulmonary oedema.13 Hydrostatic pulmonary oedema can exist with normal LV systolic function in the context of valve disease, volume overload, and diastolic dysfunction. High LVEDP consistent with increased filling pressures helps to identify those patients who are at risk of volume overload. TTE can be used to estimate LV filling pressures in the critically ill, which may help in evaluating fluid tolerance and deciding when further fluid administration may lead to harm:20
-
(i)
Mitral inflow by placing the PW Doppler across the tips of the open mitral valve leaflets in the A4C (Fig. 2H), measuring the E wave/A wave ratio. The E wave velocity m/s represents the transmitral passive LV filling and the A wave velocity m/s represents active atrial contraction. A restrictive mitral flow pattern (an increase in E/A>2) would suggest a high LVEDP.
-
(ii)
Tissue Doppler imaging with PW Doppler positioned at the basal part of the LV wall either medially or laterally where the mitral annulus inserts in the A4C view (Fig. 2I) can be used to measure E-prime (e′) velocity (m/s). Unlike the trans-mitral E wave, e′ is less susceptible to changes in LV loading and preload, making it a load independent marker of early active LV relaxation. Usually the lateral e′ velocity is higher than the septal e′ velocity and both can be affected by regional wall motion abnormalities. It is therefore best to use an average of the septal and lateral e′ velocities. The E/e′ ratio corrects for the effects of early relaxation to give the most accurate determination of LV filling pressures and can be thought of as how much pressure is needed to move blood. An E/e′<8 would suggest normal LVEDP, whereas an E/e′>13 indicates a raised LVEDP.
-
(iii)
Pulmonary venous flow velocity pattern can also be helpful in identifying raised filling pressures. The PW Doppler sample volume is placed at the orifice of the pulmonary veins, usually a triphasic pattern is seen consisting of a systolic S wave representing flow into the pulmonary veins, a diastolic D wave representing passive flow and a reversed atrial A wave representing atrial contraction (Fig. 2J). Normal S/D ratio is ≥1. As the LVEDP increases the D wave becomes more dominant and an S/D ratio of <1 would suggest high LVEDP.
One of the limiting factors is that the pulmonary veins can be incredibly difficult to identify on TTE. Firstly, identify the A4C view, increase the depth so that you can see beyond the left atrium and use colour Doppler to find the red pulsing venous flow.
In patients in AF these measurements can be limited, a short diastole leads to a decreased LVEDP, the A waves of the mitral and pulmonary inflows disappear, and the S wave of the pulmonary inflow decreases. Inevitably, patients with AF will have diastolic dysfunction. E/e′ ratio has not been formally evaluated in AF; however, even in the context of AF it may still be a useful way of evaluating LVEDP.
Fluid-responsiveness: actual and virtual fluid challenges
Actual fluid challenge
The normal physiological state of a healthy individual is to be a fluid responder in the ascending part of the Frank–Starling curve. Therefore, even if the cardiac output increases with fluid administration (i.e. a positive test for fluid-responsiveness), this does not mean that the patient should automatically be given more fluids. TTE allows an assessment of the capacity to tolerate fluid if clinically indicated, so clinical judgement is paramount in deciding if the patient should receive fluid. Only patients in whom an increase in SV is considered beneficial should be given a fluid challenge.
The standard definition of fluid-responsiveness is an increase in SV by 15% after the patient receives a 500 ml bolus of crystalloid over 10–15 min.21 The Surviving Sepsis Campaign recommends initial fluid resuscitation with 30 ml kg−1 followed by fluid challenges in 250–500 ml boluses.2 There is now increasing interest in the ‘mini fluid challenge’. An infusion of 100 ml of colloid over 1 min predicts the fluid-responsiveness (10% increase in LVOT VTI) of a full fluid challenge.22
Virtual fluid challenge with PLR
PLR is a validated method for temporarily increasing preload without the long-term potential harmful effects of actual fluid administration with an autotransfusion of around 300–500 ml. This technique can be used in patients who are undergoing mechanical ventilation or breathing spontaneously and in the presence of arrhythmias. It is safe and reversible.
The lower limbs are raised to 45° from a semirecumbent position. An increase in LVOT VTI by 12% correlates with fluid-responsiveness regardless of cardiac rhythm or mode of ventilation.23 A PLR induced increase in SV of 12.5% or more predicts an increase in SV of 15% or more after volume expansion with a sensitivity of 77% and a specificity of 100%.24
In profound hypovolaemia or in conditions of high intra-abdominal pressure, the PLR test may give a false negative result. Further still use of compression stockings may mean that there is not enough blood in the legs to cause a change in VTIs.
Limitations of the use of TTE for dynamic fluid-responsiveness
Although dynamic TTE measures are more useful and should be considered gold standard, there are several limitations to their use:
-
(i)
Lack of continuous monitoring ability. Serial TTE monitoring is laborious and time-consuming.
-
(ii)Presence of conditions limiting interpretation of measures of fluid-responsiveness:
-
(a)Heart failure: LV/RV/diastolic
-
(b)Heart failure with preserved left ventricular function
-
(c)Valvular conditions
-
(d)Arrhythmias
-
(e)Pericardial disease
-
(a)
-
(iii)Preconditions or validity criteria to using TTE (or indeed any form of cardiac output monitor) for the assessment of dynamic fluid-responsiveness when using SVV, max LVOT velocity, and VTI variations:
-
(a)The patient must be mechanically ventilated with no spontaneous respiratory effort.
-
(b)The patient must be in sinus rhythm
-
(c)Tidal volumes should be >7 ml kg−1 (lower tidal volumes may give false negatives).
-
(d)Intra-abdominal pressure has to be normal.
-
(e)Thorax cavity has to be intact.
-
(a)
-
(iv)Technical limitations
-
(a)Critically ill patients are difficult to study and optimal positioning can be difficult, although image quality is sufficient in most patients.
-
(b)Image artefacts from mechanical ventilation, chest drains, invasive vascular lines, etc.
-
(c)Variable operator expertise.
-
(d)Varying use of sedation in intensive care causing fluctuations in heart rate and blood pressure.
-
(a)
In some cases, fluid-responsiveness is obvious such as in haemorrhagic shock, clinically obvious hypovolaemic shock and in the early phase of septic shock where fluid has not yet been administered. In these circumstances delaying fluid administration could be harmful and complex tests for fluid-responsiveness may not be suitable.
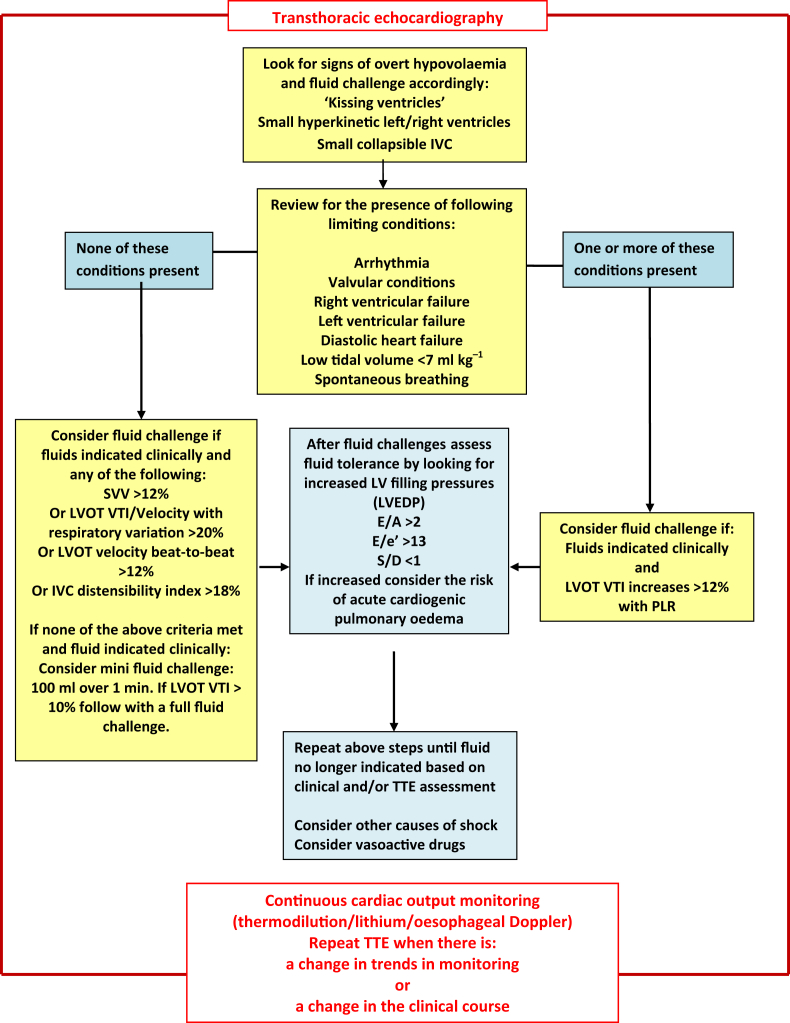
Figure 3 summarises an approach and important considerations for using TTE to assess dynamic fluid-responsiveness in the critically ill patient, where fluid is indicated clinically.
Fig 3.
Summary of an approach and considerations when using transthoracic echocardiography (TTE) for the assessment of dynamic fluid responsiveness in the critically ill patient where fluid may be indicated clinically. IVC, inferior vena cava; LVEDP, left ventricular end-diastolic pressure; LVOT, left-ventricular outflow tract; SVV, stroke volume variation; VTI, velocity-time interval.
The role of cardiac output monitors in the era of TTE
At present there is no other bedside tool that provides a non-invasive detailed evaluation of both the function and structure of the heart. However, it is neither practical nor feasible to use echocardiography continuously 24 h a day. A pragmatic solution would be to perform an initial TTE and repeat it in response to significant or unexpected changes in clinical course. Continuous cardiac monitoring could be used in parallel to create an individualised fluid strategy as the patient and clinical situation evolves over time. The future may include an integrated approach with heart and lung ultrasound-guided fluid-responsiveness assessment for all critically unwell patients aiming to establish and maintain the optimum fluid balanced state.
Summary
TTE is considered a desired standard of care in the critically ill for haemodynamic monitoring including the assessment of fluid-responsiveness and fluid tolerance. Dynamic measures for fluid-responsiveness with a virtual or an actual fluid challenge are considered gold standard and superior to static measures. However, for the correct practical application of TTE in the assessment of fluid-responsiveness one must have an understanding of the technical and physiological intricacies of echocardiography, and an appreciation of the limitations and preconditions to its use in the critically ill.
Declaration of interest
None declared.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Nishita Desai BSc, MRCP is a specialty trainee in Intensive Care medicine at the London North West Healthcare NHS trust. She is a FICE-accredited mentor.
David Garry MD, MRCP, FRCA, FFICM is a consultant in Intensive Care medicine and Anaesthetics at Oxford University Hospitals NHS trust who has British Society of Echocardiography accreditation.
Matrix codes: 1A01, 2A05, 3C00
References
- 1.Michard F., Teboul J.L. Predicting fluid-responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes A., Evans L.E., Alhazzani W. Surviving sepsis Campaign: International guidelines for management of sepsis and septic shock. Intensive Care Med. 2017;43:304–355. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 3.Durairaj L., Schmidt G.A. Fluid therapy in resuscitated sepsis: less is more. Chest. 2008;133:252–263. doi: 10.1378/chest.07-1496. [DOI] [PubMed] [Google Scholar]
- 4.Hjortrup P.B., Haase N., Bundgaard H. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Seven-Day Profile Publication. Intensive Care Med. 2016;42:1695–1705. doi: 10.1007/s00134-016-4500-7. [DOI] [PubMed] [Google Scholar]
- 5.Boyd J.H., Forbes J., Nakada T.A. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 6.Cecconi M., De Backer D., Antonelli M. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of intensive care medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheifetz I.M. Cardiorespiratory interactions: the relationship between mechanical ventilation and hemodynamics. Respir Care. 2014;59:1937–1945. doi: 10.4187/respcare.03486. [DOI] [PubMed] [Google Scholar]
- 8.Perel A., Pizov R., Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded haemorrhage. Anesthesiology. 1987;67:498–502. doi: 10.1097/00000542-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Marik P.E., Cavallazzi R., Vasu T. Dynamic changes in arterial waveform derived variables and fluid-responsiveness in mechanically ventilated patients. A systematic review of the literature. Crit Care Med. 2009;37:2642–2647. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]
- 10.Tavernier B., Makhotine O., Lebuffe G. Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology. 1998;89:1313–1321. doi: 10.1097/00000542-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A., Anel R., Bunnell E. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691–699. doi: 10.1097/01.ccm.0000114996.68110.c9. [DOI] [PubMed] [Google Scholar]
- 12.Marik P.E., Cavallazzi R. Does the central venous pressure predict fluid-responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 13.Feissel M., Michard F., Faller J.P. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30:1834–1837. doi: 10.1007/s00134-004-2233-5. [DOI] [PubMed] [Google Scholar]
- 14.Barbier C., Loubieres Y., Schmit C. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid-responsiveness in ventilated septic patient. Intensive Care Med. 2004;30:1740–1746. doi: 10.1007/s00134-004-2259-8. [DOI] [PubMed] [Google Scholar]
- 15.Airapetian N., Maizel J., Alyamani O. Does inferior vena cava respiratory variability predict fluid-responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400. doi: 10.1186/s13054-015-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feissel M., Michard F., Mangin I. Respiratory changes in aortic blood velocity as an indicator of fluid-responsiveness in ventilated patients with septic shock. Chest. 2001;119:867–873. doi: 10.1378/chest.119.3.867. [DOI] [PubMed] [Google Scholar]
- 17.Charron C., Fessenmeyer C., Cosson C. The influence of tidal volume on the dynamic variables of fluid-responsiveness in critically ill patients. Anesth Analg. 2006;102:1511–1517. doi: 10.1213/01.ane.0000209015.21418.f4. [DOI] [PubMed] [Google Scholar]
- 18.McLean A.S., Needham A., Stewart D. Estimation of cardiac output by non-invasive echocardiographic techniques in the critically ill subject. Anaesth Intensive Care. 1997;25:250–254. doi: 10.1177/0310057X9702500307. [DOI] [PubMed] [Google Scholar]
- 19.Perner A., Faber T. Stroke volume variation does not predict fluid-responsiveness in patients with septic shock on pressure support ventilation. Acta Anaesthesiol Scand. 2006;50:1068–1073. doi: 10.1111/j.1399-6576.2006.01120.x. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh S.F., Appleton C.P., Gillebert T.C. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 21.Messina A., Longhini F., Coppo C. Use of the fluid challenge in critically ill adult patients: a systematic review. Anesthes Analgesia. 2017;125:1532–1543. doi: 10.1213/ANE.0000000000002103. [DOI] [PubMed] [Google Scholar]
- 22.Muller L., Toumi M., Bousquet P.J. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid-responsiveness: the mini-fluid challenge study. Anesthesiology. 2011;115:541–547. doi: 10.1097/ALN.0b013e318229a500. [DOI] [PubMed] [Google Scholar]
- 23.Cavallaro F., Sandroni C., Marano C. Diagnostic accuracy of passive leg raising for prediction of fluid-responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010;36:1475–1483. doi: 10.1007/s00134-010-1929-y. [DOI] [PubMed] [Google Scholar]
- 24.Lamia B., Ochagavia A., Monnet X. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–1132. doi: 10.1007/s00134-007-0646-7. [DOI] [PubMed] [Google Scholar]