Key points.
-
•
Paediatric regional anaesthesia is associated with a low incidence of complications.
-
•
The benefits of using ultrasound guidance are greater in paediatric regional anaesthesia than in adult regional anaesthesia.
-
•
Neuraxial anaesthesia in infants offers significant advantages over general anaesthesia in certain groups of patients.
-
•
Caudal blocks may be superseded by ultrasound-guided peripheral nerve blocks, many of which offer superior block quality and duration.
-
•
Paediatric thoracoabdominal anatomical plane blocks provide satisfactory perioperative analgesia for multiple indications.
Learning objectives.
By reading this article, you should be able to:
-
•
Describe sources of safety data in paediatric regional anaesthesia.
-
•
Explain the alternatives to caudal blocks and their advantages.
-
•
List the considerations of paediatric anatomy, physiology, and pharmacology that influence the conduct of regional anaesthesia.
-
•
Summarise the evidence supporting the use of paediatric thoracoabdominal blocks.
Regional anaesthesia and analgesia have been shown to offer multiple benefits over general anaesthesia and systemic (including opioid) analgesia. Historical concerns over the additional risk posed by needle-related nerve injury have been progressively diluted by the evolution of ultrasound-guided techniques and the publication of big-data studies establishing the low incidence of serious complications of paediatric regional anaesthesia in the modern era.1, 2 These factors, weighed against concerns over the effect of general anaesthesia on the developing brain and the serious adverse events associated with the administration of opioids, mean that paediatric regional anaesthesia remains at the forefront of the perioperative care of children.3 A Cochrane review showed that ultrasound-guided regional anaesthesia improved success rates over non-ultrasound-guided techniques, with improved early postoperative pain scores, prolongation of regional analgesia, shortened block procedure times, and fewer needle passes.2 Furthermore, the effect size was inversely related to the size of the patient (i.e. the smaller the patient and the greater the beneficial effect size).
Preclinical data suggest that general anaesthetics potentially promote neuroapoptosis, and there is conflicting evidence from several cohort studies that young children exposed to volatile anaesthesia might have demonstrable deficits in subtle neurodevelopmental outcomes. It should be noted that the most methodologically robust studies (accounting for the confounding effects of surgery, pathology, and co-morbidity) have failed to substantiate this assertion. However, concerns persist regarding the dose and time-dependent neurodevelopmental consequences from both volatile and i.v. anaesthetic agents during the critical period of synaptogenesis and central myelination. Regional anaesthesia permits the dose reduction or even elimination of general anaesthesia, and the reduction of the adverse effects and events associated with opioid analgesia.
Neuraxial anaesthesia
Concerns regarding general anaesthetic-induced cognitive deficits in patients aged <3 months and the steady prevalence of lung disease of prematurity mean that neuraxial anaesthesia for infants retains its place in the armamentarium of the paediatric anaesthetist. Spinal anaesthesia has been shown to be equivalent to general anaesthesia in terms of neurodevelopmental outcomes in infants, and there are advantages to using the intrathecal technique especially in cases of respiratory impairment.4 In paediatric practice, the neuraxis can be accessed and anaesthetised via two routes: intrathecal or epidural (thoracic, lumbar, or caudal). Advantages of central neuraxial anaesthesia over general anaesthesia include improved postoperative pain outcomes, attenuation of the inflammatory and stress response to surgery, avoidance of airway manipulation and interference with spontaneous ventilation (especially relevant in the event of increased airway reactivity or pre-existing respiratory impairment), cardiovascular stability, reduced need for postoperative ventilation, and reduced paralytic ileus.5
Anatomical and physiological considerations
There are a number of anatomical considerations for neuraxial anaesthesia in infants and children. The thoracic spinous processes are less caudally angulated in infants. The conus medularis will extend to L4 in premature infants, L3 in a term neonate, but by 1 yr of age, it will be nearer its adult location of L1. Similarly, the dural sac extends lower in premature babies and neonates (to around S4), whereas by age 2 yrs it has developed to its adult location at S2. The absence of thoracic kyphosis in infants means greater cephalad spread of injected drugs, extending the dermatomal coverage of a central neuraxial block. The neonatal cerebrospinal fluid volume (4 ml kg−1) is twice that of adults (2 ml kg−1). The vascularity of the pia mater combined with greater cardiac output leads to faster local anaesthetic (LA) reabsorption, and thus, shorter block duration. The immaturity of the sympathetic autonomic nervous system in infants means that the chemical sympathectomy of central neuraxial anaesthesia does not result in the vasoplegia, hypotension, or cardiovascular instability sometimes seen in adults.
Neuraxial adjuvants
There is robust evidence that clonidine added to the LA injectate in both paediatric neuraxial and peripheral nerve blocks extends the quality and duration of the analgesia conferred, although concern still exists regarding systemic adverse effects, such as arterial hypotension, bradycardia, sedation, and delayed discharge.6
Dexmedetomidine, another selective α2 agonist, is being used with increasing frequency for multiple indications in paediatric anaesthesia, including regional anaesthesia, and has been shown in many situations to extend the quality and duration of regional anaesthetic blocks. Dexmedetomidine does not have the decades-long safety track record of clonidine, and we are currently unable to assert the safety of central neuraxial dexmedetomidine in children.
Neonatal spinal anaesthesia
Spinal anaesthesia, as the sole anaesthetic technique, can be used for inguinal, urological, and lower limb surgery. Spinal blocks with plain bupivacaine in neonates and infants will give only 45–60 min of surgical anaesthesia. With the addition of an adjunct or in combination with a caudal epidural injection of an LA, the block duration can be extended to accommodate longer surgeries. Contraindications are rare, and include localised infection, degenerative axonal diseases, and suspected raised non-communicating intracranial pressure.
Ultrasound is useful for the estimation of depth (transverse scan of neonatal vertebral canal with cord visible), estimation of level, or to rule out an abnormally low-lying cord (paramedian scanning). In neonates and infants, incomplete skeletal calcification means that almost all components of spinal anatomy (bone and soft tissue) are visible with ultrasound.
Technique
It is key to ensure that the infant is in a correct and stable position for awake intrathecal injection. The assistant should be coached that the necessary brief restraint will be no longer in duration than for an inhalation induction, after which the infant will rapidly settle. The procedure will be technically challenging and extended in duration if the assistant is tentative and fails to fully flex the patient's lumbar spine. Sucrose on a pacifier may provide some sedation, and topical local anaesthesia (such as eutectic mixture of LAs) may be placed at the intended puncture site before the patient arriving within the operating room to minimise reactivity at time of needle introduction. Lateral decubitus positioning is typical, although the sitting position is also described.
Needle
Paediatric spinal needles (25G, 25 mm) are preferred to adult needles because of lower dead space, smaller bevel profile, and superior precision and accuracy when manipulated. Use of needle introducers from i.v. catheters or hypodermic needles carries the risk of epidermoid body injection to the subarachnoid space. Lumbar puncture is performed at the L4/L5 or L5/S1 interspace, as identified by Tuffier's line or pre-procedure ultrasound scan.
Local
The authors use bupivacaine 1 mg kg−1 for spinal anaesthesia in neonates. Bupivacaine, hyperbaric bupivacaine, levobupivacaine, or ropivacaine are all suitable. No difference between hyperbaric and ‘plain’ bupivacaine has been seen. Suggested dosage regimens for hyperbaric bupivacaine 0.5% are 1 mg kg−1 (children weighing <5 kg); 0.4 mg kg−1 (5–15 kg); and 0.3 mg kg−1 (>15 kg).7 High-quality evidence comparing various agents is lacking. Fentanyl (0.2 μg kg−1), clonidine (1 μg kg−1), and adrenaline (2–4 μg kg−1) have all been shown to prolong spinal analgesia with bupivacaine. Additives should be preservative free, and although the theoretical risk of neurotoxicity persists, there is a long-established track record that supports the safety profile of these three additives. Some centres report success rates over 95%, although elsewhere a failure rate of 17% is reported as a consequence of technical failure, inadequate block, or conversion to general anaesthesia.8 The use of combined spinal and caudal block technique is justified by some practitioners to improve neuraxial block quality and duration. However, evidence to date has not shown superior block success, and this practice in neonates (depending on the regimen chosen) may exceed total LA dosage recommendations.
Deafferentation means that often the infant will fall asleep once the spinal block is established, but it is necessary to prepare for additional sedation or general anaesthesia in the event of agitation or block failure. An incremental approach would be to move from sucrose on a pacifier, through i.v. dexmedetomidine to i.v. propofol or volatile anaesthesia, if necessary. If the original motivation was to avoid airway instrumentation or mechanical ventilation, face mask anaesthesia may still suffice.
Postoperative considerations
Apnoea
The risk of postoperative apnoea is greatest in premature neonates, ex-premature infants, and those with anaemia. The largest study looking at general vs regional anaesthesia and postoperative apnoea showed fewer early postoperative apnoea and fewer severe apnoea events with regional anaesthesia, but no overall difference in the incidence of risk of any apnoea event within 12 h. Consequently, the claimed benefits of awake regional anaesthesia in neonates cannot be extended to the prevention of postoperative apnoea. If extended duration is not necessary, adjuvant fentanyl and clonidine are omitted to reduce the risk of postoperative apnoea.
Post-dural puncture headache
Best practice to minimise the incidence of post-dural puncture headache (PDPH) should include the use of atraumatic needles for dural puncture (25 or 27G). The diagnosis of PDPH in infants is more difficult because of the infant's inability to localise symptoms. The headache generally resolves after 3–5 days, and non-opioid analgesia or caffeine at 10 mg kg−1 24 h−1 in two to three divided doses may be required. The use of an epidural blood patch is reserved for refractory cases. A blood patch involves aseptic injection of the patient's blood (0.1–0.2 ml kg−1) into the epidural space at the level of the dural leak, with or without the addition of iodinated contrast to visualise fluoroscopically the appropriate spread of the patch.
Epidural anaesthesia
There are insufficient data in children to determine if the technique of loss of resistance to saline (as opposed to air) results in clinically significant differences with respect to safety, accuracy, and subsequent efficacy of epidural block.6 The American Society of Regional Anesthesia and Pain Medicine/European Society of Regional Anaesthesia and Pain Therapy (ASRA/ESRA) made a series of recommendations based on retrospective and prospective observational data in a recent joint statement regarding paediatric regional anaesthesia.6 Performing paediatric blocks under general anaesthesia is considered safe and the standard of care. Based on four large-scale retrospective database analyses, the overall risk for all complications (including transient ones) is 0.66% (95% confidence interval [CI]: 0.6–0.7%), and the risk of paralysis is estimated at 0 (95% CI: 0–0.004%), although one case series that preceded the Pediatric Regional Anesthesia Network (PRAN) registry described four cases of neurological injury with epidural catheters. In one series, the most frequent complications were local skin infection and drug error: both are potentially avoidable or reversible. Data from neonates showed an increased complication rate of 13.3% (95% CI: 9.8–17.4%), yet none of these led to any long-term sequelae and virtually all were managed by adjusting the infusion or removing the catheter.
Technique
Depth to the epidural space can be estimated in two ways. A general ‘rule of thumb’ from skin to lumbar epidural space is 1 mm kg−1 body weight and approximately 10 mm in neonates. Depth to space may also be measured ultrasonographically pre-procedure. This topic has been extensively covered elsewhere in this journal.9
The epidural space contains less fat and fibrous tissue in children, and thus, fewer obstructions to catheters threaded several vertebral levels. There are a number of methods for optimising catheter tip positing: epidurography, ECG guidance, nerve stimulation, and ultrasound. Data from the PRAN show one or more of these are used in 47% of neonatal neuraxial catheters. Epidurography is typically performed post hoc for confirmation of catheter position using 0.5–1.5 ml of radiopaque dye (concentration: 120–240 mg kg−1). ECG guidance relies on matching output from ECG electrodes attached to a bespoke epidural set to that of the surface ECG to guide the thoracic epidural placement. The nerve stimulation method (‘Tsui method’) relies on stepwise increments of 1–15 mA through a saline-flushed epidural catheter observing for myotome-specific activity. This method has not been shown to improve placement assessment compared with traditional methods.10
There is a significant rate of false negatives with the use of epidural test doses. Thus, any epidural injection of an LA solution should be incremental and slow with continuous observation of the electrocardiogram (i.e. true to the mantra that ‘every dose is a test dose’). Loading with bupivacaine/ropivacaine at a dose of 1.5–2 mg kg−1 followed by an infusion dose of 0.2 mg kg−1 h−1 is suitable for postoperative analgesia in abdominal surgeries.
The vertebral column is mainly cartilaginous until 6 months of age, which facilitates an ultrasonographic view of spinal structures. Catheter tip position is often not directly visualised via ultrasound and is assumed to be the most cephalad point at which the catheter can be seen. Confirmation of epidural placement is verified by expansion of the epidural space with ventral displacement of the dura upon injection.
Postoperative care
Patients with epidural catheters should be followed daily by an acute pain service for the duration of the epidural infusion and through the transition to systemic analgesia. Epidural catheters are more likely to migrate and displace in smaller patients, with those <10 kg at the highest risk (mean: 1.1 vertebral levels inward). The acute pain service should monitor for sustained block efficacy and remain vigilant to avoid insidious LA toxicity. The template transition formula used at the authors' institution consists of administration of an oral opioid 6 h before the cessation of the epidural infusion and catheter removal, again 3 h before catheter removal, removal of the epidural catheter followed by oral opioid dosing 4-hourly thereafter with breakthrough oral doses available 2-hourly in the event of moderate to severe pain. A member of the anaesthesia or pain team should be available around the clock to troubleshoot issues arising.
Caudal block
Caudal blocks represent the most common form of regional anaesthesia in children with a low failure rate (1%); low complication rate (1.9%); and excellent safety profile consisting of cardiovascular or central nervous toxicity of 0.02%, with one registry of nearly 20,000 blocks showing no long-term sequelae.11 The ASRA/ESRA recommends ropivacaine (0.2%) or levobupivacaine/bupivacaine (0.25%) in volumes not exceeding 1 ml kg−1.6
The classic Armitage regimen of caudal dosing of bupivacaine 0.25% at 0.5 ml kg−1 for sacro-lumbar block, 1 ml kg−1 for abdominal block, and 1.2 ml kg−1 for mid-thoracic block is poorly predictive for cranial extension of LA, and the ‘thoracic’ dose of 1.2 ml kg−1 bupivacaine 0.25% exceeds the usual recommended maximum dose (1 ml kg−1). At our institution, we do not exceed bupivacaine 2.5 mg kg−1 in a single administration. In recent years, the paediatric anaesthesia community has begun to move away from caudal blocks. In virtually every surgery for which a caudal block might historically have been used, there are alternative regional anaesthesia techniques that have been shown to offer equivalent or superior analgesia (quality and duration) that do not require needling the central neuraxis and associated motor block of the lower limbs.
Pudendal nerve block
Indications for pudendal nerve block include most of the same perineal surgeries for which caudal blocks would have historically been used. These include operations for hypospadias, phimosis, perineal incisions, and inguinoscrotal incisions, including orchidopexies. The pudendal nerve block confers greater quality and duration of analgesia (compared to caudal blocks) for circumcisions and penile and hypospadias surgeries.12 Pudendal-nerve-block success rates are reported >85% amongst consultants and non-consultants; however, this compares poorly to caudal success rates of 99%.11
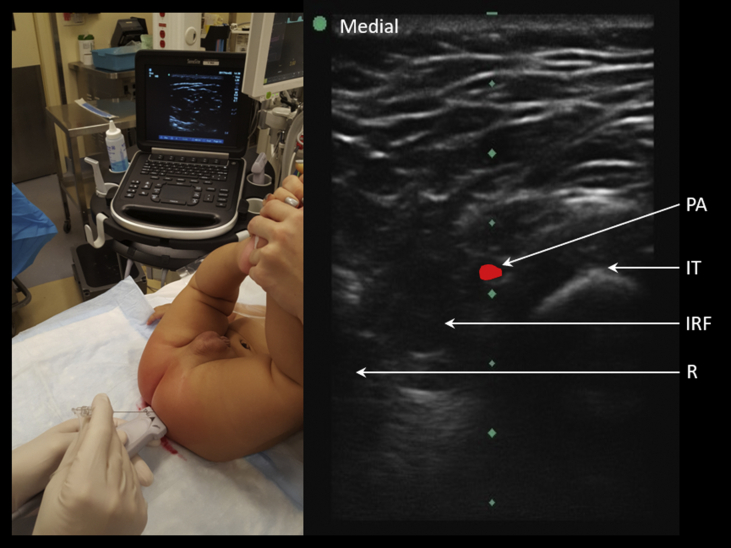
The pudendal nerve originates from the spinal nerve roots S2 to S4; travels with the pudendal artery and veins within the Alcock's canal; and then divides terminally into the dorsal nerve of the penis/clitoris, the perineal nerve, and the inferior rectal nerve. The block is performed with ultrasound guidance with the hips fully abducted (‘frog leg position’) or with an assistant suspending both legs fully extended in the air at 90° angle to the trunk (‘diaper/nappy position’) (see Fig 1). Two entry points at 3 and 9 o'clock, 2–2.5 cm lateral to the anus, are identified (further for a larger child). The ultrasound probe is placed long axis along an imaginary line connecting the ischial tuberosity to the anus. Both in- and out-of-plane techniques are described. Colour Doppler aids in identifying the pulsating pudendal artery. The pudendal nerve can be assumed to be in the neurovascular bundle adjacent to this artery. A 22G 50 mm short-bevelled needle is advanced under real-time ultrasound guidance into the ischiorectal fossa. A distinct ‘pop’ may be appreciated as the needle punctures the sacrotuberous ligament. Infiltration of 0.3–0.4 ml kg−1 bupivacaine 0.25% is adequate. A nerve stimulator may be used to elicit a motor response (contraction of the anal sphincter, bulbospongiosus, or transverse perineal muscle), and thus, facilitate accurate needle tip positioning. The typical duration of analgesia conferred by a pudendal nerve block is around 8 h, significantly longer with the addition of dexmedetomidine 0.5 μg kg−1 in total to the injectate.
Fig 1.
Left pudendal nerve block. Probe placed transversely on imaginary line joining anus to ischial tuberosity. Out-of-plane approach. Nerve adjacent to pulsatile pudendal artery. IRF, ischiorectal fossa; IT, ischial tuberosity; PA, pudendal artery; R, rectum.
Thoracic wall blocks
Paravertebral block
The thoracic paravertebral space is shaped like an isosceles prism and contains structures, including spinal nerves, white and grey rami communicantes, the sympathetic chain, vasculature, and connective tissue. Paravertebral anaesthesia is frequently used for thoracotomy, laparotomy, and pectus repair, but also with increasing frequencies for more minimally invasive surgeries, such as laparoscopy and thoracoscopy. Paravertebral anaesthesia provides ipsilateral somatic anaesthesia of thoraco-abdominal dermatomes and transient chemical sympatholysis. Bilateral or unilateral paravertebral catheters are useful for extended duration of analgesia.
Similar postoperative pain scores are seen when comparing paravertebral catheters to caudal-to-thoracic catheters in infants undergoing major upper abdominal surgery.13 For pectus excavatum repair (Nuss/Ravitch) procedures, patients with bilateral paravertebral blocks have lower pain scores, less opioid use, fewer behavioural disturbances, and shorter length of stay compared to opioid analgesia.14 Unilateral paravertebral catheter placement for thoracotomy for cardiac procedures (coarctation repair and patent ductus arteriosus ligation) provides equivalent analgesia (with decreased complication rates) compared with thoracic epidural analgesia.
Ultrasound-guided approaches are superseding landmark techniques. Common techniques include placing the patient in the lateral decubitus position and accessing the paravertebral space laterally to medially in an in-plane approach with the ultrasound probe placed parasagittally or transversely. However, at least nine different ultrasound-guided techniques are described.15 Single-shot techniques may be single site, but often multisite injections are used to achieve a broader dermatomal spread. In one series, major complications arose with an incidence of 0.04% (95% CI: 0.006–0.3%), and the general complication rate was more similar to peripheral nerve block than epidural anaesthesia.16 No pneumothoraces have been reported in paediatric literature.
Other chest wall blocks
The serratus anterior block and Pecs 1/2 blocks offer the benefit of analgesia of the lateral and anterior thoracic intercostal nerves, respectively, thereby avoiding the potential complications from more proximal paravertebral and thoracic epidural blocks. The Pecs 1 and serratus anterior plane blocks target two different fascial planes. The term Pecs 2 block is reserved for instances when both fascial planes (Pecs 1 and serratus anterior) are injected with a single-needle pass.
Serratus anterior plane block
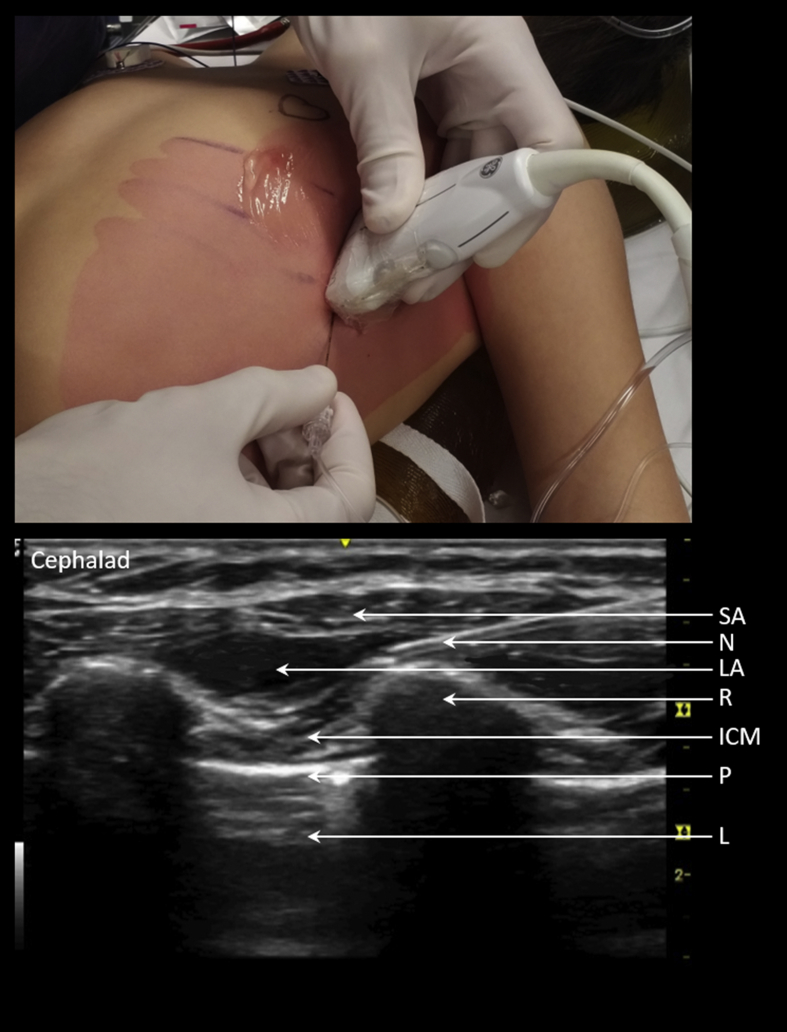
Indications for the serratus anterior plane block continue to widen, including coarctation repair, Nuss procedures, thoracoscopic surgery, and other lateral chest wall procedures from T2 to T9.17 It is a fascial plane block, targeting the lateral intercostal thoracic cutaneous nerves as they pierce laterally through the intercostal muscles and serratus anterior, that is performed at a point further lateral and posterior to the Pecs 1 and 2 blocks. Local anaesthesia (with or without adjuvants) is deposited within the facial plane as a single shot either superficial or deep to the serratus anterior muscle, with no high-quality evidence showing superiority of one over another. Advantages of superficial injection include lower risk of inadvertent arterial puncture or pneumothorax, whereas deep injection within the less distensible facial plane may lead to greater LA dermatomal spread with the additional benefit of sparing the long thoracic nerve. We abduct the arm to 90° and place the probe in a craniocaudal fashion along the mid-axillary line (see Fig 2). We use an in-plane approach with the intended injection site overlying a rib to protect the deep structures should the needle tip pass deep to the deep serratus plane. Bupivacaine 0.125% (0.4 ml kg−1) ( plus optional with or without dexmedetomidine 0.5 μg kg−1) is infiltrated.
Fig 2.
Left serratus anterior block. Probe placed to view ribs in cross section in the mid-axillary line at the level of proposed incision. In-plane approach. Injectate deposited and viewed to spread under the serratus anterior muscle. ICM, intercostal muscles; L, lung; LA, local anaesthetic; N, needle; P, pleura; R, rib; SA, serratus anterior.
Pecs 1/2 block
Other anterior trunk fascial place blocks include Pecs 1 (targets: lateral and medial pectoral nerves located deep to the pectoralis major and superficial to the pectoralis minor muscles) and Pecs 2 (targets: intercostobrachial nerve, intercostal nerves 3–6, and long thoracic nerve between the pectoralis minor and serratus anterior) with observational studies supporting use in intra-cardiac device placement and breast reduction surgery.18 One suggested dosing regimen using bupivacaine 0.25% is 0.15 ml kg−1 (up to 10 ml) for Pecs 1 and 0.3 ml kg−1 (up to 20 ml) for Pecs 2 blocks. Adult data have shown promising results for all three blocks, although high-quality paediatric data are lacking for all three chest wall blocks.
Erector spinae block
The erector spinae plane (ESP) block is a novel interfascial plane block that is being used in place of paravertebral analgesia. The erector spinae muscle is a series of paired muscle bundles and tendons that run along the vertical parasagittal vertebral plane. Preclinical and clinical evidence suggests that a deposition of LA along the fascial plane deep to the erector spinae muscle leads to both craniocaudal and paravertebral spread (the latter via the costotransverse foramen), blocking both the dorsal and ventral rami of the thoraco-abdominal spinal nerves. The block is performed by placing the probe craniocaudally and visualising the ESP overlying the transverse processes imaged in short axis. The needle tip is advanced in plane to the transverse processes, and injectate is deposited ventral to the erector spinae muscle. Presently, the literature in paediatrics is limited to case series reporting successes in laparoscopic and open thoracoabdominal surgeries. The exact role of ESP and optimal dosing regimens within paediatric practice has yet to be determined.
Abdominal wall blocks
The technical aspects of performing these blocks have already been described elsewhere in this journal, and the use of ultrasound has been shown to decrease complications and increase block success.19, 20
Transversus abdominis plane block
Indications for transversus abdominis plane (TAP) blocks in paediatric anaesthesia include appendectomy, cholecystectomy, laparoscopic incisions, hernia repair, iliac crest bone graft harvest, and other anterolateral abdominal wall incisions. Cutaneous sensory changes after TAP blocks are inconsistent and conflicting, and studies used evolving terminology resulting in ambiguity.21 TAP blocks can be subcategorised according to where the LA is deposited: lateral/mid-axillary, ilioinguinal, subcostal, and posterior (Quadratus lumborum types 1–3).21 Studies generally suggest that an oblique subcostal technique may provide better analgesia for procedures above the umbilicus with upper rectus sheath injections for very high incisions (above T8/T9).20 In paediatric patients, a posterior TAP/Quadratus lumborum 1 block with a dose of 0.5 ml kg−1 ropivacaine 0.2% leads to ipsilateral (midline to mid-axillary line) cutaneous sensory deficit from T9 to L1 dermatomes.21 Of note, for hernia surgery, innervation of the spermatic cord derives from L2 and is not covered by TAP blocks.
Complications are rare, with an upper incidence of overall complications of 0.3%, all of which were immediately identified with no resultant morbidity.22 An almost five-fold weight-adjusted dosing variation in LA is seen in paediatric TAP blocks.22 Maximising LA dose (whilst still in the non-toxic range) provides prolonged analgesia compared to lower doses. Typically, we use bupivacaine 0.25% 0.3–0.4 ml kg−1 per side for paediatric TAP blocks, depending on the location and extent of surgical incision. Despite the promise of the block, various studies of TAP blocks in children have failed to show convincing advantage over control.
Dexmedetomidine may be added at a dose from 0.5 to 2 μg kg−1. For paediatric inguinal hernia repair, the addition of dexmedetomidine 2 μg kg−1 to TAP blocks confers better postoperative analgesia with lower LA concentrations of bupivacaine.23 In our experience, however, the systemic absorption of the higher dexmedetomidine doses results in an excessively somnolent child after day-case surgery, so we used 0.5–1 μg kg−1 (total dose) mixed in the injectate.
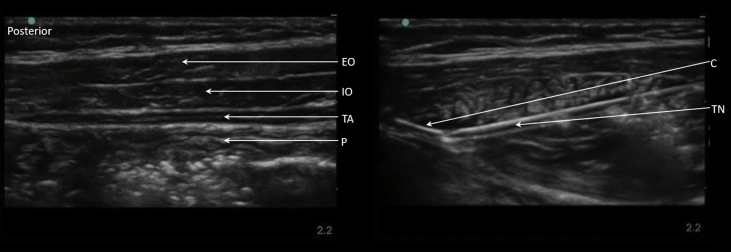
TAP plane catheters may be placed before surgery via a Tuohy needle (see Fig 3), with a peripheral nerve block catheter, or via open placement by the surgeon. Bilateral catheter placement may be a useful option where neuraxial analgesia is contraindicated (e.g. post-kidney/liver transplant and Kasai procedures). Passing catheters in <15 kg children poses technical challenges, as advancing the large Tuohy needle risks inadvertent deep penetration when crossing fascial planes. Successfully introducing a spinal needle to hydro-dissect out the TAP before piercing fascial layer deep to the internal oblique with the Tuohy needle is described. At our institution, we use infusion rates of bupivacaine 0.125% 0.1–0.3 mg kg−1 h−1.
Fig 3.
TAP catheter between the internal oblique and transversus abdominal muscle. The catheter is inserted via a Tuohy needle after hydro-dissection of the plane. C, catheter; EO, external oblique; IO, internal oblique; P, peritoneum; TA, transversus abdominis; TN, Tuohy needle.
Rectus sheath block
Indications for rectus sheath blocks include anterior abdominal wall incisions as for umbilical and epigastric hernia repair, laparoscopic surgery, and pyloromyotomy. The injection is best performed lateral to the maximal depth of the rectus sheath muscle belly to ensure blockage of the nerve prior to the origin of the anterior cutaneous nerve. Advantages over bilateral TAP blocks are limited to lower total LA administration. Ultrasound guidance is advised, as height and weight are poorly predictive of posterior rectus sheath depth. Bilateral ultrasound-guided rectus sheath blocks provide superior analgesia to local infiltration for umbilical hernia repair, but have not been shown to be superior to intraoperative surgically directly placed rectus sheath blocks.24 For pyloric stenosis, studies have shown lower volatile requirements (approximately 0.5 minimum alveolar concentration) and elimination of postoperative opioids when combined with intraoperative remifentanil or minimisation of postoperative opioids without remifentanil.
Ilioinguinal/iliohypogastric nerve blocks
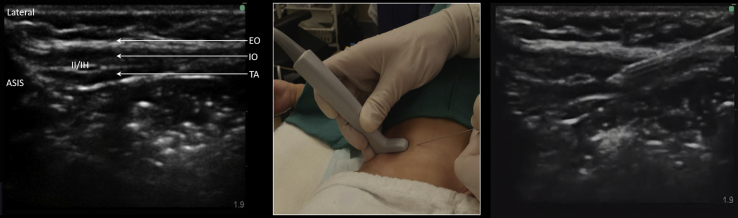
For children undergoing inguinal surgery, an ultrasound-guided ilioinguinal block is superior to TAP block, although the needling time is longer (see Fig 4). Like TAP blocks, the addition of dexmedetomidine improves pain scores, prolongs anaesthesia, and decreases the incidence of emergence delirium.25 Ultrasound-guided ilioinguinal/iliohypogastric nerve blocks for inguinal hernia repair have been shown to be superior to local anaesthesia infiltration, caudal epidural injection, surface-landmark fascial-click ilioinguinal block, and TAP blocks, and should be considered the gold-standard analgesic technique for paediatric inguinal hernia repair.
Fig 4.
Ilioinguinal/iliohypogastric nerve block. Probe placed medial to anterior superior iliac spine (ASIS) on imaginary line joining ASIS to umbilicus. Medial to lateral in-plane approach. EO, external oblique; Il/IH, ilioinguinal/iliohypogastric nerve bundle; IO, internal oblique; TA, transversus abdominis.
Summary
Paediatric regional anaesthesia should be amongst the anaesthetic techniques offered by any paediatric anaesthetist. The benefits conferred have been shown repeatedly to be superior to opioid analgesia and outweigh the risks, historically misconceived to be high, but which are actually, and reassuringly, low. Ancillary techniques to access safely the neuraxial space are increasing both block safety and success. Moreover, the widespread availability of ultrasound is facilitating a range of blocks remote to the neuraxis with acceptable efficacy and safety. The small amount of time added to the induction sequence before the patient is ready for surgery is insignificant compared with the time saved at every other stage of perioperative care, and is entirely justified by the postoperative benefits to patients, staff and carers.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
David Greaney MSc FRCA FCAI FJFICMI is a general and cardiac anaesthesia fellow at The Hospital for Sick Children, Toronto. He has been appointed as a consultant in paediatric anaesthesia in Our Lady's Children's Hospital, Dublin. His research interests include regional anaesthesia for cardiac surgery, medication safety, and human factors in simulation-based medical education.
Dr Tobias Everett MSc EDRA FRCA is an associate professor at the University of Toronto and consultant in paediatric anaesthesia at The Hospital for Sick Children. His research focuses on team and individual optimisation in high stakes and low-frequency events, use of simulation for assessment, and paediatric regional anaesthesia.
Matrix codes: 1D02, 2D02, 2D05, 2E01, 2G02, 2G03, 3A09, 3D00.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2018.12.003.
Supplementary data
The following is the Supplementary data to this article:
References
- 1.Polaner D.M., Taenzer A.H., Walker B.J. Pediatric Regional Anesthesia Network (PRAN): a multi-institutional study of the use and incidence of complications of pediatric regional anesthesia. Anesth Analg. 2012;115:1353–1364. doi: 10.1213/ANE.0b013e31825d9f4b. [DOI] [PubMed] [Google Scholar]
- 2.Guay J., Suresh S., Kopp S. The use of ultrasound guidance for perioperative neuraxial and peripheral nerve blocks in children: a Cochrane review. Anesth Analg. 2017;124:948–958. doi: 10.1213/ANE.0000000000001363. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration . US Food and Drug Administration, Drug Safety Communications; 2016. FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women.https://www.fda.gov/Drugs/DrugSafety/ucm532356.htm [Google Scholar]
- 4.Davidson A.J., Disma N., De Graaff J.C. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosenberg A. Benefits of regional anesthesia in children. Paediatr Anaesth. 2012;22:10–18. doi: 10.1111/j.1460-9592.2011.03691.x. [DOI] [PubMed] [Google Scholar]
- 6.Ivani G., Suresh S., Ecoffey C. The European Society of Regional Anaesthesia and Pain Therapy and the American Society of Regional Anesthesia and Pain Medicine Joint Committee Practice Advisory on controversial topics in pediatric regional anesthesia. Reg Anesth Pain Med. 2015;40:526–532. doi: 10.1097/AAP.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 7.Suresh S., Ecoffey C., Bosenberg A. The European Society of Regional Anaesthesia and Pain Therapy/American Society of Regional Anesthesia and Pain Medicine recommendations on local anesthetics and adjuvants dosage in pediatric regional anesthesia. Reg Anesth Pain Med. 2018;43:211–216. doi: 10.1097/AAP.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 8.Frawley G., Bell G., Disma N. Predictors of failure of awake regional anesthesia for neonatal hernia repair: data from the general anesthesia compared to spinal anesthesia study—comparing Apnea and neurodevelopmental outcomes. Anesthesiology. 2015;123:55–65. doi: 10.1097/ALN.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh S.M., Madjdpour C., Chin K.J. Ultrasound-guided lumbar central neuraxial block. BJA Educ. 2016;16:213–220. [Google Scholar]
- 10.Goobie S.M., Montgomery C.J., Basu R. Confirmation of direct epidural catheter placement using nerve stimulation in pediatric anesthesia. Anesth Analg. 2003;97:984–988. doi: 10.1213/01.ANE.0000080609.05942.38. [DOI] [PubMed] [Google Scholar]
- 11.Suresh S., Long J., Birmingham P. Are caudal blocks for pain control safe in children? An analysis of 18,650 caudal blocks from the Pediatric Regional Anesthesia Network (PRAN) database. Anesth Analg. 2015;120:151–156. doi: 10.1213/ANE.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 12.Kendigelen P., Tutuncu A.C., Emre S. Pudendal versus caudal block in children undergoing hypospadias surgery: a randomized controlled trial. Reg Anesth Pain Med. 2016;41:610–615. doi: 10.1097/AAP.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 13.Sato M., Iida T., Kikuchi C. Comparison of caudal ropivacaine-morphine and paravertebral catheter for major upper abdominal surgery in infants. Paediatr Anaesth. 2017;27:524–530. doi: 10.1111/pan.13104. [DOI] [PubMed] [Google Scholar]
- 14.Loftus P.D., Elder C.T., Russell K.W. Paravertebral regional blocks decrease length of stay following surgery for pectus excavatum in children. J Pediatr Surg. 2016;51:149–153. doi: 10.1016/j.jpedsurg.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Krediet A.C., Moayeri N., Van Geffen G.J. Different approaches to ultrasound-guided thoracic paravertebral block: an illustrated review. Anesthesiology. 2015;123:459–474. doi: 10.1097/ALN.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 16.Vecchione T., Zurakowski D., Boretsky K. Thoracic paravertebral nerve blocks in pediatric patients: safety and clinical experience. Anesth Analg. 2016;123:1588–1590. doi: 10.1213/ANE.0000000000001576. [DOI] [PubMed] [Google Scholar]
- 17.Biswas A., Luginbuehl I., Szabo E. Use of serratus plane block for repair of coarctation of aorta: a report of 3 cases. Reg Anesth Pain Med. 2018;43:641–643. doi: 10.1097/AAP.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 18.Perhac S., Bosenberg A. Poster presented at SPA Winter Meeting 2013, Las Vegas, NV. 2013. The Pecs block—a novel technique for pediatric patients undergoing subcutaneous mastectomy for gynecomastia. [Google Scholar]
- 19.Kaushik V., Philip A., Russell W.C. Ultrasound-guided central neuraxial blocks and peripheral nerve blocks in children. BJA Educ. 2015;15:154–159. [Google Scholar]
- 20.Abrahams M., Derby R., Horn J.L. Update on ultrasound for truncal blocks: a review of the evidence. Reg Anesth Pain Med. 2016;41:275–288. doi: 10.1097/AAP.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez M.A., Vecchione T., Boretsky K. Dermatomal spread following posterior transversus abdominis plane block in pediatric patients: our initial experience. Paediatr Anaesth. 2017;27:300–304. doi: 10.1111/pan.13034. [DOI] [PubMed] [Google Scholar]
- 22.Long J.B., Birmingham P.K., De Oliveira G.S. Transversus abdominis plane block in children: a multicenter safety analysis of 1994 cases from the PRAN (Pediatric Regional Anesthesia Network) database. Anesth Analg. 2014;119:395–399. doi: 10.1213/ANE.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 23.Raof R.A., El Metainy S.A., Alia D.A., Wahab M.A. Dexmedetomidine decreases the required amount of bupivacaine for ultrasound-guided transversus abdominis plane block in pediatrics patients: a randomized study. J Clin Anesth. 2017;37:55–60. doi: 10.1016/j.jclinane.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Litz C.N., Farach S.M., Fernandez A.M. Percutaneous ultrasound-guided vs. intraoperative rectus sheath block for pediatric umbilical hernia repair: a randomized clinical trial. J Pediatr Surg. 2017;52:901–906. doi: 10.1016/j.jpedsurg.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Lundblad M., Marhofer D., Eksborg S., Lönnqvist P.A. Dexmedetomidine as adjunct to ilioinguinal/iliohypogastric nerve blocks for pediatric inguinal hernia repair: an exploratory randomized controlled trial. Paediatr Anaesth. 2015;25:897–905. doi: 10.1111/pan.12704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.