Abstract
Objective:
Lassa fever (LF), a hemorrhagic fever endemic to West Africa, has an incidence of approximately 500,000 cases per year. This study evaluated hearing loss and other sequelae following LF.
Methods:
This case–control study enrolled laboratory-confirmed LF survivors, non-LF febrile controls and matched-community controls with no history of LF or recent hospitalization for a febrile illness. Study participants completed a symptom questionnaire. Pure-tone audiometry was completed by a subset of participants.
Results:
A total of 147 subjects aged 3–66 years (mean, 23.3) were enrolled. LF survivors were significantly more likely to report balance difficulties (55% vs. 20%, p < 0.001), hair loss (32% vs. 7%, p < 0.001), difficulty speaking (19% vs. 1%, p < 0.001), social isolation (50% vs. 0%, p < 0.001), and hearing loss (17% vs. 1%, p = 0.002) in comparison with matched-community controls. Similar trends were noted in comparison with febrile controls, although these findings were non-significant. Fifty subjects completed audiometry. Audiometry found that LF survivors had significantly more bilateral hearing loss in comparison with matched-community controls (30% vs. 4%, p = 0.029).
Conclusion:
This study characterized the sequelae of LF and highlighted the need for increased access to hearing care in West Africa.
Keywords: Global health, Hearing loss, Lassa fever, Viral sequelae
Introduction
According to data from the global burden of disease study, hearing loss is currently the second leading cause of years lived with a disability. Approximately 1.4 billion people, or 18.7% of the world’s population, are currently living with hearing loss (James et al., 2018; Graydon et al., 2019). This burden is unequally distributed, with >80% of affected individuals residing in low-income and middle-income countries, where access to specialty care is often limited (Fagan and Jacobs, 2009; Graydon et al., 2019). Understanding the etiologies that are unique to these areas is critical to addressing this global disparity.
Viral hemorrhagic fevers represent an etiology of hearing loss, which contributes to this disproportionate burden. Lassa fever, a viral hemorrhagic fever endemic to Sierra Leone and the West African region, was first discovered in 1969 (Richmond and Baglole, 2003; Ogbu et al., 2007). Shortly thereafter, case reports documenting hearing loss associated with infection were published (White,1972). The acute phase of disease is characterized by a non-specific febrile illness, which later evolves to include renal and hepatic dysfunction, conjunctivitis, severe pharyngitis, facial edema, hematemesis, pleural and pericardial effusion, and death (White, 1972; Richmond and Baglole, 2003; Okokhere et al., 2018). The case fatality rate of this disease is approximately 15–20% for the entire West African region. However, notable variations in mortality occur geographically (Shaffer et al., 2014). No incidence studies of Lassa fever currently exist. However, it is estimated that 250,000–500,000 infections occur annually across Sub-Saharan Africa (Richmond and Baglole, 2003; Ogbu et al., 2007; Ibekwe, 2012). Although the majority of infections are asymptomatic or sub-clinical, it has been noted that approximately a third of Lassa fever infections result in chronic hearing loss (Mertens et al., 1972; Cummins et al., 1990; Ibekwe et al., 2011; Ibekwe, 2012; Yun et al., 2015; Okokhere et al., 2018). This associated prevalence is estimated to be 10–300 times greater than other more common viral etiologies of hearing loss (Cohen et al., 2014; Ficenec et al., 2019). Despite the strong association between Lassa fever and hearing loss, this and other sequelae remain poorly understood.
This case control study further characterizes the relationship between Lassa fever, hearing loss and other viral sequelae. Characterization of the full disease course, including convalescent symptoms, may help to identify risk factors for diagnosis, improve predictions of prognosis, and elucidate the mechanisms of disease pathogenesis. An increased understanding of this disease allows for an improved ability to design interventions and treatments.
Methods
This study was conducted in eastern Sierra Leone from May 2007 to June 2009. The Lassa fever ward at Kenema Government Hospital (KGH) provided patient records from which eligible subjects were identified. Subjects were then located throughout the surrounding areas.
To ensure that findings were not merely a consequence of hospitalization and were specific to Lassa fever, three groups of individuals were enrolled: Lassa fever survivors, febrile controls, and matched-community controls. Lassa fever survivors were defined as individuals diagnosed by positive Lassa fever serology, clinical methods and hospitalized at KGH. Febrile controls were individuals who were hospitalized for a febrile illness at KGH and did not meet Lassa fever diagnostic criteria. All Lassa fever survivors and febrile controls were hospitalized and treated at KGH 3 months to 3 years prior to imitation of this study. An additional age-matched and gender-matched community control was recruited for each Lassa fever survivor and febrile control from the same or neighboring household.
Upon enrollment into the study, individuals completed questionnaires assessing constitutional, psychiatric, neurologic, ocular, otologic, vestibular, respiratory, cardiac, gastrointestinal, and musculoskeletal symptoms, as well as social stigma and fatigue. Audiometric exams were added to the study protocol in 2009 and completed by a subset of 50 participating individuals. Pure-tone thresholds were measured across 0.5–4 kHz and were used to calculate a pure-tone average (PTA) for each ear. These mid-frequencies were chosen as they represent the majority of measured frequencies in the human speech spectrum and standard audiometric exams. Bilateral hearing loss was defined according to WHO standards as a PTA >25 dB in both ears. Individuals with a PTA >25 dB in a single ear were classified as having unilateral hearing loss.
All questionnaires and audiometry were completed in the participating individual’s language of choice. The questionnaires that were employed by this study were tested and verified by local volunteers for appropriate translation and cultural competency. Study personnel collecting data were blinded as to the status of study participants. Data were organized into frequency tables and tested for significance by ANOVA and Pearson’s Chi-squared tests, where appropriate. In order to account for the possibility of spurious relationships when making multiple comparisons, the alpha level required for significance was adjusted using Bonferroni corrections, where appropriate. The alpha level required for significance of Lassa fever symptoms and complaints was adjusted to 0.0023. The alpha levels required for significance of socio-economic and psychiatric complaints as well as audiometry data were not adjusted due to the small number of comparisons being made among these families of data. This study was approved by the Tulane University Institutional Review Board and the administrators of Kenema Government Hospital.
Results
A total of 147 subjects were enrolled in this study. Ages ranged from 3 to 66 years, with a median age of 21 years (Table 1). Sixty-six percent of subjects were adolescents and adults (≥15 years), 30% were school-age children (5–14 years), and 4% were aged <5 years. Approximately half (49.3%) of the enrolled subjects were men. The median age of the febrile control group (28 years) was slightly higher than that of the Lassa fever (21 years) and matched-community control (20 years) groups. However, this result was found to be non-significant (p = 0.224).
Table 1.
Baseline demographic characteristics of 147 patients from Kenema Governmental Hospital.
| Characteristics | Overall (n = 147) | Lassa (n = 47) | Fever (n = 29) | Control (n = 71) | p-value |
|---|---|---|---|---|---|
| Median age (IQR) | 21 (11.5–33.0) | 21 (11.2–32.7) | 28 (14.0–40.0) | 20 (11.0–30.0) | 0.224a |
| Age group (%) | 0.472b | ||||
| Preschool <5 years | 6 (4) | 1 (2) | 2 (7) | 3 (4) | |
| School-age 5–14 years | 44 (30) | 17 (36) | 5 (17) | 22 (31) | |
| Adult 15–44 years | 85 (58) | 26 (55) | 18 (62) | 41 (58) | |
| Older adult ≥45 years | 12 (8) | 3 (6.) | 4 (14) | 5 (7) | |
| Female (%) | 76 (52) | 24 (51) | 15 (52) | 37 (52) | 0.713b |
ANOVA.
Pearson’s Chi-squared test.
All 147 subjects enrolled in this study completed a symptom questionnaire (Table 2). A sub-set of 50 individuals completed a socioeconomic and psychiatric symptom questionnaire (Table 3). In comparison with the matched-community control group, Lassa fever survivors were significantly more likely to report difficulties with balance (55.3% vs. 19.7%, p < 0.001), hair loss (32% vs. 7%, p < 0.001), hearing loss (17% vs. 1%, p = 0.002), and difficulty speaking (19% vs. 1%, p < 0.001). Additionally, Lassa fever survivors were also significantly more likely to report social isolation (50.0% vs. 0%, p < 0.001) in comparison with matched-community controls. Likely due to the small sample sizes recruited for this study, Lassa fever survivors were not found to have significantly increased complaints of any symptom in comparison with the febrile controls. However, trends of increased weight loss (57% vs. 34%, p = 0.052), headaches (74% vs. 55%, p = 0.082) and hair loss (32% vs. 14%, p = 0.076) were found. Few differences in socio-economic and psychiatric complaints were noted (Table 3). The only significant difference found between Lassa fever survivors and febrile controls was a decreased prevalence of social embarrassment (30% vs. 73%, p = 0.032), while only a sense of isolation was significantly more common among Lassa fever survivors and controls (50% vs. 0%, p < 0.001).
Table 2.
Comparisons of signs and symptoms of Lassa fever survivors, febrile and matched controls.
| Signs and symptoms | Lassa (n = 47) n, (%) | Fever (n = 29) n, (%) | Control (n = 71) n, (%) | Overall p-valuea,b | p-valuea,c | p-valuea,d |
|---|---|---|---|---|---|---|
| Weight loss | 27 (57) | 10 (34) | 34 (48) | 0.146 | 0.052 | 0.309 |
| Paresthesia | 11 (23) | 5 (17) | 13 (18) | 0.733 | 0.522 | 0.501 |
| Difficulty with balance/walking | 26 (55) | 11 (38) | 14 (20) | <0.001 | 0.141 | <0.001 |
| Tremor | 20 (43) | 9 (31) | 17 (24) | 0.105 | 0.315 | 0.033 |
| Lymphadenopathy | 9 (19) | 2 (7) | 5 (7) | 0.104 | 0.140 | 0.046 |
| Headache | 35 (74) | 16 (55) | 47 (66) | 0.223 | 0.082 | 0.339 |
| Hair loss | 15 (32) | 4 (14) | 5 (7) | 0.002 | 0.076 | <0.001 |
| Lightheadedness | 25 (53) | 13 (45) | 21 (30) | 0.018 | 0.479 | 0.010 |
| Fainting | 2 (4) | 2 (7) | 5 (7) | 0.800 | 0.616 | 0.530 |
| Vertigo | 10 (21) | 8 (28) | 9 (13) | 0.212 | 0.530 | 0.213 |
| Seizures | 1 (2) | 2 (7) | 3 (4) | 0.593 | 0.300 | 0.538 |
| Hallucinations | 8 (17) | 5 (17) | 9 (13) | 0.735 | 0.980 | 0.510 |
| Hearing loss | 8 (17) | 2 (7) | 1 (1) | 0.005 | 0.205 | 0.002 |
| Tinnitus | 13 (28) | 10 (34) | 15 (21) | 0.369 | 0.529 | 0.414 |
| Blurred vision | 9 (19) | 6 (21) | 9 (13) | 0.505 | 0.870 | 0.338 |
| Difficulty speaking | 9 (19) | 6 (21) | 1 (1) | <0.001 | 0.870 | <0.001 |
| Difficulty thinking clearly | 6 (13) | 1 (3) | 5 (7) | 0.319 | 0.172 | 0.295 |
| Difficulty swallowing | 8 (17) | 3 (10) | 12 (17) | 0.658 | 0.422 | 0.986 |
| Change in the taste of food | 18 (38) | 10 (34) | 12 (17) | 0.020 | 0.738 | 0.008 |
| Change in smell | 7 (15) | 6 (21) | 7 (10) | 0.355 | 0.514 | 0.902 |
| Hospitalized since illness | 5 (11) | 8 (28) | 5 (7) | 0.028 | 0.057 | 0.492 |
α level required for significance adjusted through Bonferroni correction to 0.0023.
Overall p-value of logistic regression model using asymptotic Chi-squared test.
Pearson’s Chi-squared test comparing Lassa fever survivors and febrile controls
Pearson’s Chi-squared test comparing Lassa fever survivors and matched controls
Table 3.
Socioeconomic and psychiatric complaints of Lassa fever survivors, febrile and matched controls.
| Socioeconomic symptoms | Lassa (n = 10) n, (%) | Fever (n = 15) n, (%) | Control (n = 25) n, (%) | Overall p-valuea | p-valueb | p-valuec |
|---|---|---|---|---|---|---|
| Depressionc | 3 (30) | 8 (53) | 4 (16) | 0.018 | 0.249 | 0.349 |
| Isolationc | 5 (50) | 5 (33) | 0 (0) | <0.001 | 0.405 | <0.001 |
| Embarrassmentc | 3 (30) | 11 (73) | 7 (28) | <0.001 | 0.032 | 0.906 |
| Economic hardshipc | 6 (60) | 10 (67) | 15 (60) | 0.905 | 0.734 | 1 |
Overall p-value of logistic regression model using asymptotic Chi-squared test.
Pearson’s Chi-squared test comparing Lassa fever survivors and febrile controls.
Pearson’s Chi-squared test comparing Lassa fever survivors and matched controls.
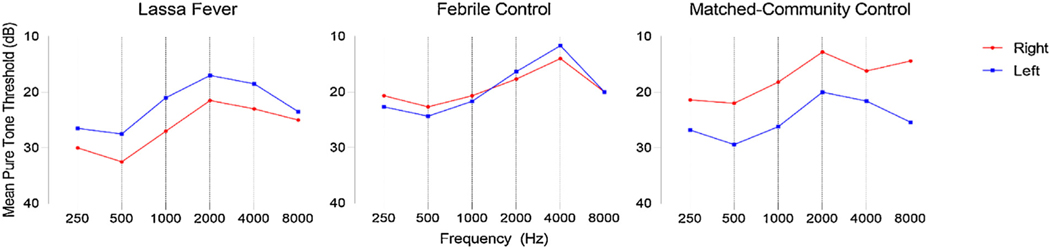
Audiometry was added to the study protocol in 2009 and was performed on a subsample of 50 subjects participating in the study. This sample consisted of 10 Lassa fever survivors, 15 febrile controls, and 25 matched-community controls. Audiometric tracings showed increased pure tone thresholds affecting low-frequency tones in each of the three groups (Figure 1); however, no significant difference was noted between any individually measured frequency (Table 4). Hearing loss in this study was defined as a PTA >25 dB. Overall, 18 subjects were found to have either bilateral or unilateral hearing loss: four (40%) Lassa fever survivors, three (20%) febrile controls, and 11 (44%) matched-community controls (Table 5). No significant differences in the prevalence of hearing loss were found between any of the three groups. However, Lassa fever survivors were more likely to have bilateral hearing loss in comparison with matched-community control subjects (30% vs. 4%, p = 0.029). When hearing loss was categorized by severity, 13 (26%) of 50 individuals had mild hearing loss, four (8%) had moderate hearing loss, none (0%) had severe hearing loss, and one (2%) was found to have profound hearing loss (Table 3). However, no significant differences were found in severity of hearing loss between the three groups. After controlling for age, the results were unchanged.
Figure 1.
Audiometric tracings of Lassa fever survivors and control groups.
Table 4.
Mean pure tone thresholds of Lassa fever survivors and controls.
| Mean pure tone thresholds (dB ± SE) | ||||
|---|---|---|---|---|
| Frequency (kHz) | Lassa (n = 10) | Fever (n = 15) | Control (n = 25) | p-valuea |
| 0.25 | 27 ± 4 | 23 ± 4 | 27 ± 3 | 0.277 |
| 0.5 | 30 ± 5 | 21 ± 5 | 21 ± 2 | |
| 28 ± 3 | 24 ± 3 | 29 ± 2 | 0.237 | |
| 1 | 33 ± 4 | 23 ± 6 | 22 ± 2 | |
| 21 ± 3 | 22 ± 3 | 26 ± 3 | 0.779 | |
| 2 | 27 ± 4 | 21 ± 6 | 18 ± 2 | |
| 17 ± 4 | 17 ± 4 | 20 ± 3 | 0.727 | |
| 22 ± 4 | 22 ± 4 | 13 ± 1 | ||
| 4 | 19 ± 4 | 19 ± 4 | 22 ± 3 | 0.142 |
| 8 | 23 ± 5 | 23 ± 5 | 16 ± 2 | |
| 24 ± 3 | 24 ± 3 | 25 ± 3 | 0.545 | |
| 25 ± 6 | 25 ± 6 | 14 ± 2 | ||
Threshold values displayed with measurements from the left ear above the right ear.
One way ANOVA.
Table 5.
Auditory characteristics of 50 patients from Kenema Governmental Hospital, Eastern Sierra Leone, from May 2007–June 2009.
| Lassa n = 10 n (%) | Fever n = 15 n (%) | Control n = 25 n (%) | Overall p-valuea | p-valueb | p-valuec | |
|---|---|---|---|---|---|---|
| Any hearing loss | 4 (40) | 3 (20) | 11 (44) | 0.276 | 0.566 | 0.445 |
| Bilateral hearing loss | 3 (30) | 1 (7) | 1 (4) | 0.103 | 0.120 | 0.029 |
| Unilateral hearing loss | 1 (10) | 2 (13) | 10 (40) | 0.068 | 1 | 0.183 |
| Hearing loss severity | ||||||
| Mild | 3 (30) | 2 (13) | 8 (32) | 0.697 | 0.438 | 0.930 |
| Moderate | 1 (10) | 0 (0) | 3 (12) | 0.220 | 0.349 | 0.930 |
| Severe | 0 (0) | 0 (0) | 0 (0) | – | – | – |
| Profound | 0 (0) | 1 (7) | 0 (0) | 0.293 | 0.212 | – |
Pearson’s Chi-squared test comparing Lassa fever survivors and febrile controls.
Pearson’s Chi-squared test comparing Lassa fever survivors and matched controls.
Discussion
This case–control study characterizes the long-term sequelae of Lassa fever by comparing the symptoms of survivors with febrile and matched-community control groups. It found that 17% of Lassa fever survivors had subjective hearing loss in comparison with 7% of febrile and 1% of matched-community controls. Subjective complaints have previously been demonstrated as a high sensitivity and specificity method of detecting hearing loss (Clark et al., 1991; Nondahl et al., 1998; Gomez et al., 2001; Sindhusake et al., 2001; Valete-rosalino and Rozenfeld, 2005). Audiometric exams demonstrated that 30% of Lassa fever survivors had bilateral hearing loss, which was significantly greater than the 4% prevalence exhibited by matched-community controls, with a trend of greater prevalence when compared with the 7% of febrile controls. Vertigo and balance difficulties were also commonly noted among Lassa fever survivors, reported by 21% and 55% of the study population, respectively. In addition, Lassa fever survivors were more likely to complain of balance, hair loss, difficulty speaking, and social isolation in comparison with matched-community controls.
Typically, Lassa fever hearing loss is thought to occur during the convalescent stage of infection, arising within 5–22 days following the acute stage of illness. Among survivors of infection, it is estimated that a third may develop chronic hearing loss (Ficenec et al., 2019). This high prevalence would rank Lassa fever as one of the leading causes of viral mediated hearing loss, with a prevalence approximately 10–300 times greater than other viral causes. A 1990 case control study conducted in Sierra Leone examined the seropositivity of 32 individuals known to have hearing difficulties within their respective communities. Of this group, 26 (81.2%) individuals were found to be seropositive for Lassa fever antibodies in comparison with six (18.7%) of those without hearing difficulties (Cummins et al., 1990). Of this group, half of the seropositive individuals reported awareness of any possible Lassa fever exposure. Which suggests that the Lassa fever virus is capable of inducing hearing loss in asymptomatic or mild infections and may represent a cause of an even greater proportion of hearing loss in West Africa.
Ribavirin, the only treatment available for Lassa fever, has been suspected as a possible cause of the associated hearing loss. However, several studies and reviews have found no association between the incidence of hearing loss and administration of ribavirin in Lassa fever patients (Mertens et al., 1972; White, 1972; Cummins et al., 1990; Macher and Wolfe, 2006; Okokhere et al., 2009; Grahn et al., 2016; Mateer et al., 2018). Other mechanisms proposed for the pathogenesis of Lassa fever-associated hearing loss include direct viral damage, an immune-mediated response, and the development of subsequent vasculitis. Although evidence to support any of these theories is limited, past accounts have found gross and histopathology of blood vessels of non-human primates infected with the Lassa fever virus, which subsequently developed hearing loss to resemble that of an autoimmune vasculitis, specifically polyarteritis nodosa (Edington and White, 1972; Cashman et al., 2018). Although there are no specific clinical tests to diagnose polyarteritis nodosa, the American College of Rheumatology has established a set of high specificity criteria (Table 6). Fulfillment of at least three of these criteria is associated with high suspicion of diagnosis (Lightfoot et al., 1990). The Lassa fever survivors participating in the current study were significantly more likely than their matched-community controls to complain of increased prevalence of unexplained weight loss, and polyneuropathies of peripheral and cranial nerves. These symptoms fit the three-criteria threshold establishing high suspicion of vasculitis. Furthermore, the known presence of renal, neurologic and gastrointestinal dysfunction during the acute phase of Lassa fever is in congruence with the symptoms of polyarteritis nodosa (Guillevin et al., 2005; Pagnoux et al., 2010). Additionally, the presence of hearing and hair loss in this study population supports the diagnosis of a systemic autoimmune disease (Parodi and Cozzani, 2014; Ralli et al., 2018). However, additional research is necessary in order to more fully support this hypothesis. An increased understanding of disease pathogenesis will allow for improved treatment and prevention methodologies for Lassa fever sequelae, with the ultimate goal of improving quality of life and reducing stigma in this population.
Table 6.
Vasculitis diagnostic criteria.
| 1. | Unexplained weight loss |
| 2. | Presence of mottled skin or Livedo reticularis |
| 3. | Myalgias of the hip or shoulder girdle |
| 4. | Neuropathy |
| 5. | Hypertension |
| 6. | Elevated BUN or creatinine |
| 7. | Presence of hepatitis B surface antigen |
| 8. | Demonstration of arteriographic abnormality |
| 9. | Biopsy of small or medium sized artery demonstrating polymorphonuclear cells |
The study is the first to present audiometric data of Lassa fever survivors. Although, the presented audiometric data indicate that the majority of hearing loss in these cases is mild, this sequelae should not be discounted. Hearing loss and vestibular symptoms are well known to lead to decreased quality of life and socioeconomic status if left untreated. Previously, hearing loss has been associated with increased odds of under-employment or unemployment, lower socioeconomic status, increased risk of depression, social isolation, and an impairment in the ability to complete activities of daily living (Mulrow et al., 1990; Ruben, 2000; Dalton et al., 2003; Lasisi et al., 2007; Emmett and Francis, 2015). Children with untreated hearing loss have been shown to score lower on tests of cognitive ability and have lower longitudinal educational attainment (Wake et al., 2004; Teasdale and Sorensen, 2007; Lieu, 2012; Emmett et al., 2015). This relationship between children with hearing loss and worse educational outcomes has also been associated with minimal or unilateral hearing loss (Bess et al., 1998; Lieu et al., 2010; Lieu, 2012). Survey data from Sierra Leone have indicated that financial constraints are a major deterrent for seeking care for diseases affecting the head and neck (Van Buren et al., 2014). However, treatment of hearing loss through the use of hearing aids or cochlear implants has been shown to be both cost-effective and mitigate these long-term deficits (Tsakiropoulou et al., 2007; Baltussen and Smith, 2009; Baltussen et al., 2009; Tomblin et al., 2014; Dawes et al., 2015; Magro et al., 2018; Vo et al., 2018). Due to these large quality of life and economic benefits, it is imperative that hearing loss screening and treatment programs for Lassa fever survivors and other individuals be initiated within West Africa. Especially, as modelling efforts predict a rising incidence in Lassa fever in the near future (Redding et al., 2016; Gibb et al., 2017).
Recent Lassa fever forecasting data have shown that due to global climate change, socio-ecological changes in land use and human travel, disease incidence is likely to increase as more rodent species are found to be capable of transmitting the virus and the increased interaction between human and rodent species leads to increased spill-over events and disease transmission (Fichet-Calvet et al., 2007; Redding et al., 2016; Gibb et al., 2017). These predictions serve to strengthen recent calls to action, which establish Lassa fever as a priority disease for the scientific and medical community (Mehand et al., 2018). However, few of these predictive models have included any plan for the long-term management of the chronic sequelae resulting from infection.
Future investigations of Lassa fever should include more detailed examinations of hearing loss and other inner ear disorders. Since the pathogenesis of this chronic sequelae is unknown, studies would benefit from the inclusion of a greater number of specific questions in order to identify symptomatology within organ systems. Understanding the true prevalence and mechanism of these disorders will aid in designing interventions to prevent the life-long effects of hearing loss and increase the quality of life among survivors of this neglected tropical disease. Although the results of this study are limited, due to small sample size and generalized symptom surveys, this data add to the current literature regarding Lassa fever, by defining hearing loss according to the WHO standards, reporting both subjective and objective measurements of hearing loss, and performing symptom surveys, which may aid in the delineation of the pathogenesis of chronic viral sequelae due to Lassa fever.
Acknowledgements
This project was supported by NIH Research Training Grant #D43 TW009340 (SF) funded by the NIH Fogarty International Center, NINDS, NIMH, and NHBLI as well as by 5K12HD043451 and 1R01AI123535 (JS).
Footnotes
Ethical approval
This study was reviewed and given ethical approval by Tulane University IRB and Kenema Government Hospital Administrators.
Conflict of interest
None declared.
References
- Baltussen R, Abraham V, Priya M, Achamma B, Anand J, Gift N, et al. Costs and health effects of screening and delivery of hearing aids in Tamil Nadu, India: an observational study. BMC Public Health 2009;9(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltussen R, Smith A. Cost-effectiveness of selected interventions for hearing impairment in Africa and Asia: a mathematical modelling approach. Int J Audiol 2009;48(3):144–58. [DOI] [PubMed] [Google Scholar]
- Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear 1998;19(5):339–54. [DOI] [PubMed] [Google Scholar]
- Cashman KA, Wilkinson ER, Zeng X, Cardile AP, Facemire PR, Bell TM, et al. Immune-mediated systemic vasculitis as the proposed cause of sudden-onset sensorineural hearing loss following Lassa virus exposure in cynomolgus macaques. mBio 2018;9(5):e01896–e1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K, Sowers M, Wallace RB, Anderson C. Accuracy of self-reported hearing loss in women aged 60–85 years. Am J Epidemiol 1991;134(7):704–8. [DOI] [PubMed] [Google Scholar]
- Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear 2014;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins D, McCormick JB, Bennett D, Samba JA, Farrar B, Machin SJ, et al. Acute sensorineural deafness in Lassa fever. JAMA 1990;264(16):2093–6. [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BEK, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist 2003;43(5):661–8. [DOI] [PubMed] [Google Scholar]
- Dawes P, Emsley R, Cruickshanks KJ, Moore DR, Fortnum H, Edmondson-Jones M, et al. Hearing loss and cognition: the role of hearing aids, social isolation and depression. PLOS ONE 2015;10(3):e0119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edington G, White H. The pathology of lassa fever: a tribute to the late Dr. J. M. Troup. Trans R Soc Trop Med Hyg 1972;66(3):381–9. [DOI] [PubMed] [Google Scholar]
- Emmett SD, Francis HW. The socioeconomic impact of hearing loss in US adults. Otol Neurotol 2015;36(3):545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett SD, Schmitz J, Pillion J, Wu L, Khatry SK, Karna SL, et al. Hearing loss is associated with decreased nonverbal intelligence in rural nepal. Otol Neurotol 2015;36(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J, Jacobs M. Survey of ENT services in Africa: need for a comprehensive intervention. Glob Health Action 2009;2(1):1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficenec SC, Schieffelin JS, Emmett SD. A review of hearing loss associated with Zika, Ebola, and Lassa fever. Am J Trop Med Hyg 2019;101(3):484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichet-Calvet E, Lecompte E, Koivogui L, Soropogui B, Doré A, Kourouma F, et al. Fluctuation of abundance and lassa virus prevalence in Mastomys natalensis in Guinea, West Africa. Vector-Borne Zoonotic Dis 2007;7(2):119–28. [DOI] [PubMed] [Google Scholar]
- Gibb R, Moses LM, Redding DW, Jones KE. Understanding the cryptic nature of Lassa fever in West Africa. Pathog Glob Health 2017;111(6):276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MI, Hwang S-A, Sobotova L, Stark AD, May JJ. A comparison of self-reported hearing loss and audiometry in a cohort of New York farmers. J Speech Lang Hear Res 2001;44(6):1201–8. [DOI] [PubMed] [Google Scholar]
- Grahn A, Bråve A, Lagging M, Dotevall L, Ekqvist D, Hammarström H, et al. Imported case of Lassa fever in sweden with encephalopathy and sensorineural hearing deficit. Open Forum Infect Dis 2016;3(4) [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5152670/ [cited 28 August 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graydon K, Waterworth C, Miller H, Gunasekera H. Global burden of hearing impairment and ear disease. J Laryngol Otol 2019;133(Special Issue 1 (Global Ear Care)):18–25. [DOI] [PubMed] [Google Scholar]
- Guillevin L, Mahr A, Callard P, Godmer P, Pagnoux C, Leray E, et al. Hepatitis B virus-associated polyarteritis nodosa: clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine (Baltimore) 2005;84(5):313–22. [DOI] [PubMed] [Google Scholar]
- Ibekwe T. Lassa fever: the challenges of curtailing a deadly disease. Pan Afr Med J 2012;11: [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3343683/ [cited 28 August 2018]. [PMC free article] [PubMed] [Google Scholar]
- Ibekwe TS, Okokhere PO, Asogun D, Blackie FF, Nwegbu MM, Wahab KW, et al. Early-onset sensorineural hearing loss in Lassa fever. Eur Arch Otorhinolaryngol 2011;268(2):197–201. [DOI] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392 (10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasisi A, Sulaiman O, Afolabi O. Socio-economic status and hearing loss in chronic suppurative otitis media in Nigeria. Ann Trop Paediatr 2007;27(4):2910296. [DOI] [PubMed] [Google Scholar]
- Lieu JEC. Longitudinal study of children with unilateral hearing loss. Laryngoscope 2012;122(9):2088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu JEC, Tye-Murray N, Karzon RK, Piccirillo JF. Unilateral hearing loss is associated with worse speech-language scores in children | articles | pediatrics. Pediatrics 2010;125(6):e1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot RW, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, et al. The American college of rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum 1990;33(8):1088–93. [DOI] [PubMed] [Google Scholar]
- Macher AM, Wolfe MS. Historical Lassa fever reports and 30-year clinical update. Emerg Infect Dis 2006;12(5):835–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro I, Emmett SD, Saunders J. Cost-effectiveness of CI in developing countries. Curr Opin Otolaryngol Head Neck Surg 2018;26(3):190–5. [DOI] [PubMed] [Google Scholar]
- Mateer EJ, Huang C, Shehu NY, Paessler S. Lassa fever-induced sensorineural hearing loss: a neglected public health and social burden. PLoS Negl Trop Dis 2018;12(2) [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5823363/ [cited 18 July 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehand MS, Al-Shorbaji F, Millett P, Murgue B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Res 2018;159:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens PE, Patton R, Baum JJ, Monath TP. Clinical presentation of Lassa fever cases during the hospital epidemic at Zorzor, Liberia, March–April 1972. Am J Trop Med Hyg 1972;22(6):780–4. [DOI] [PubMed] [Google Scholar]
- Mulrow C, Aguilar C, Endicott J, Velez R, Tuley M, Charlip W, et al. Association between hearing impairment and the quality of life of elderly individuals. J Am Geriatr Soc 1990;38(1):45–50. [DOI] [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein R, Klein BEK. Accuracy of self-reported hearing loss. Audiology 1998;37(5):295–301. [DOI] [PubMed] [Google Scholar]
- Ogbu O, Ajuluchukwu E, Uneke CJ. Lassa fever in West African sub-region: an overview. J Vector Borne Dis 2007;44(1):1–11. [PubMed] [Google Scholar]
- Okokhere P, Colubri A, Azubike C, Iruolagbe C, Osazuwa O, Tabrizi S, et al. Clinical and laboratory predictors of Lassa fever outcome in a dedicated treatment facility in Nigeria: a retrospective, observational cohort study. Lancet Infect Dis 2018;18(6):684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okokhere PO, Ibekwe TS, Akpede GO. Sensorineural hearing loss in Lassa fever: two case reports. J Med Case Rep 2009;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnoux C, Seror R, Henegar C, Mahr A, Cohen P, Guern VL, et al. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French vasculitis study group database. Arthritis Rheum 2010;62(2):616–26. [DOI] [PubMed] [Google Scholar]
- Parodi A, Cozzani E. Hair loss in autoimmune systemic diseases. G Ital Dermatol E Venereol Organo Uff Soc Ital Dermatol E Sifilogr 2014;149(1):79–81. [PubMed] [Google Scholar]
- Ralli M, D’Aguanno V, Di Stadio A, De Virgilio A, Croce A, Longo L, et al. Audiovestibular symptoms in systemic autoimmune diseases. J Immunol Res 2018;2018:5798103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding DW, Moses LM, Cunningham AA, Wood J, Jones KE. Environmental-mechanistic modelling of the impact of global change on human zoonotic disease emergence: a case study of Lassa fever. Methods Ecol Evol 2016;7 (6):646–55. [Google Scholar]
- Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ 2003;327(7426):1271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben RJ. Redefining the survival of the fittest: communication disorders in the 21st century. Laryngoscope 2000;110(2 Pt 1):241–5. [DOI] [PubMed] [Google Scholar]
- Shaffer JG, Grant DS, Schieffelin JS, Boisen ML, Goba A, Hartnett JN, et al. Lassa fever in post-conflict Sierra Leone. PLoS Negl Trop Dis 2014;8(3):e2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhusake D, Mitchell P, Smith W, Golding M, Newall P, Hartley D, et al. Validation of self-reported hearing loss. The Blue Mountains Hearing Study. Int J Epidemiol 2001;30(6):1371–8. [DOI] [PubMed] [Google Scholar]
- Teasdale T, Sorensen M. Hearing loss in relation to educational attainment and cognitive abilities: a population study. Int J Audiol 2007;46(4):172–5. [DOI] [PubMed] [Google Scholar]
- Tomblin J, Oleson J, Ambrose S, Walker E, Moeller M. The Influence of Hearing Aids on the Speech and Language Development of Children With Hearing Loss | Child Development | JAMA Otolaryngology–Head & Neck Surgery | JAMA Network. JAMA Otolaryngol-Head Neck Surg 2014;140(5):403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiropoulou E, Konstantinidis I, Vital I, Konstantinidou S, Kotsani A. Hearing aids: quality of life and socio-economic aspects. Hippokratia 2007;11(4):183–6. [PMC free article] [PubMed] [Google Scholar]
- Valete-rosalino C, Rozenfeld S. Auditory screening in the elderly: comparison between self-report and audiometry. Braz J Otorhinolaryngol 2005;71(2):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buren NC, Groen RS, Kushner AL, Samai M, Kamara TB, Ying J, et al. Untreated head and neck surgical disease in Sierra Leone: a cross-sectional, countrywide survey. Otolaryngol Neck Surg 2014;151(4):638–45. [DOI] [PubMed] [Google Scholar]
- Vo QT, Pham D, Choi KJ, Nguyen UTT, Le L, Shanewise T, et al. Solar-powered hearing aids for children with impaired hearing in Vietnam: a pilot study. Paediatr Int Child Health 2018;38(1):40–5. [DOI] [PubMed] [Google Scholar]
- Wake M, Hughes EK, Poulakis Z, Collins C, Rickards FW. Outcomes of children with mild-profound congenital hearing loss at 7 to 8 years: a population study. Ear Hear 2004;25(1):1–8. [DOI] [PubMed] [Google Scholar]
- White HA. Lassa fever A study of 23 hospital cases. Trans R Soc Trop Med Hyg 1972;66(3):390–8. [DOI] [PubMed] [Google Scholar]
- Yun NE, Ronca S, Tamura A, Koma T, Seregin AV, Dineley KT, et al. Animal model of sensorineural hearing loss associated with Lassa virus infection. J Virol 2015;90 (6):2920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]