Learning objectives.
By reading this article, you should be able to:
-
•
Describe the epidemiology, genetics, and presentation of Brugada syndrome
-
•
Identify patients at greatest risk
-
•
Recognise a diagnostic Brugada syndrome ECG
-
•
Discuss the approach to investigation when diagnostic uncertainty remains
-
•
Explain which drugs should be avoided, the available cardiological therapies and perioperative management of a patient with Brugada syndrome
Key points.
-
•
Brugada syndrome is an abnormality of cardiac ion channels that increases the risk of ventricular fibrillation (VF) and sudden cardiac death.
-
•
The classic ECG pattern is cove-type ST-segment elevation and T wave inversion in leads V1 and V2.
-
•
It is best to avoid local anaesthetics, propofol infusions, and manoeuvres increasing vagal tone during the perioperative period.
-
•
Isoprenaline should be available in case of either non-sustained or sustained VF, and is the antiarrhythmic drug of choice for VF storm.
-
•
Postoperative ECG monitoring is required after the administration of some anaesthetic agents known to increase the risk of VF in Brugada syndrome.
The Brugada brothers first described the association of right ventricular conduction delay, right precordial ST-segment elevation along with the predisposition to syncopal episodes and sudden cardiac death (SCD) as a result of ventricular arrhythmias in 1992.1 Since then, Brugada syndrome (BrS) has become increasingly recognised as an important cause of SCD in a structurally normal heart and is thought to be responsible for up to 40% of such cases.2, 3 It can exhibit autosomal dominant inheritance, although 60% of patients with BrS have no affected family member; the likely explanation for this is variable expression and incomplete penetrance.2 Although the genotype is distributed equally between the sexes, the phenotype tends to be exhibited more severely in males, in whom it is eight to 10 times more common.4, 5, 6 Brugada syndrome is typically diagnosed in the fourth decade of life although it has been diagnosed in patients as young as 2 days and as old as 84 yr.4, 7 The true prevalence has been difficult to establish as the diagnostic electrocardiographic pattern may only be present transiently and many patients are asymptomatic. It is thought to range from one to five per 10 000 people in Europe to as high as 20 per 10 000 people in South East Asia.3, 4, 5, 8 If present, symptoms may include palpitations, chest discomfort, syncope and nocturnal agonal respiration. However, as many patients are asymptomatic, BrS is frequently an incidental diagnosis on ECG, at family screening or after presentation with aborted SCD secondary to polymorphic ventricular tachycardia (resembling a rapid form of torsades de pointes) or ventricular fibrillation.4 Monomorphic ventricular tachycardia is rare but is more often seen in infants and children.5, 8 Events typically occur during sleep with increased vagal tone, with fever, or can be precipitated by drugs, alcohol, and electrolyte disorders; these potential triggers can all accentuate the ST elevation noted in a Brugada ECG.2, 3, 4, 5 Between 20 and 30% of patients experience supraventricular tachycardias (SVTs) such as atrial flutter, atrioventricular nodal re-entry, and pre-excitation syndromes such as Wolff–Parkinson–White syndrome, although atrial fibrillation is seen most commonly.3, 4, 6 Patients with apparent supraventricular involvement have potentially more advanced disease as this correlates with increased ventricular irritability and a higher risk of ventricular tachycardia or fibrillation.2, 4
In critical care, the most common presentation will be a patient with aborted SCD. The anaesthetist may encounter it as an incidental finding in a patient on an elective or emergency list, or alternatively as a known comorbidity in a patient booked for an elective procedure. A thorough knowledge of BrS management and drugs to be avoided is vital.
Diagnosis
Diagnosis is based on fulfilling the type I BrS ECG morphological criteria from the most recent Expert Consensus Recommendations5: a cove-shaped (i.e. with T-wave inversion) ST-segment elevation ≥2 mm in at least one right precordial leads (V1 or V2) when placed in a standard or superior position (i.e. up to the second intercostal space), either spontaneously or after the administration of a sodium channel blocking agent (e.g. ajmaline/flecainide).
Additional ECG morphologies described are type 2 [saddleback-shaped (i.e. with a positive T-wave) ST-segment ≥1 mm in at least one right precordial lead] and type 3 (saddleback or cove-shaped ST-segment elevation <1 mm in at least one right precordial lead), but neither are diagnostic.
Importantly, the ECG morphology can change with time and an individual with true BrS can manifest all three different morphologies at different times.4, 8
Neither genetic testing nor electrophysiological studies are required for diagnosis.
Differential diagnosis
Many conditions can reproduce a type I Brugada ECG (i.e. so-called Brugada phenocopy), some of which may produce the same clinical presentation; these should be considered and excluded before arriving at the diagnosis of BrS. Causes include early repolarisation, athlete's heart, acute coronary events, pulmonary embolism, electrolyte disturbance, pericarditis, myocarditis, dissecting aortic aneurysm, and arrhythmogenic right ventricular cardiomyopathy.8 Right ventricular conduction delay is well recognised in BrS and differentiating it from right bundle branch block can be difficult. Necessary investigations in this setting include serum electrolytes, a 12 lead ECG performed with high precordial lead placement (i.e. including second and third intercostal space recordings above the standard V1 and V2 positions) and a transthoracic echocardiogram.
Should diagnostic uncertainty remain, a cardiac magnetic resonance imaging can be considered to detect more subtle cardiac structural abnormalities not detected at transthoracic echocardiography. If the clinical situation warrants it, then further investigations such as contrast computed tomography of the chest to rule out pulmonary embolism or aortic dissection may be required urgently.
As the ECG pattern can vary with time or circumstances, serial 12 lead ECGs or an ambulatory monitor may be useful.9 If the diagnostic ECG is not present but BrS is suspected then a pharmacological provocation test can be performed using a class 1 antiarrhythmic agent. This is typically ajmaline, which is well tolerated and, with a very short half-life, has a low likelihood of inducing sustained ventricular arrhythmias. Furthermore, ajmaline has been shown to be more effective at provoking type I ECG changes than flecainide and procainamide.4, 10 Around 20% of patients with a positive provocation test are subsequently found to have a spontaneous type 1 pattern. Unfortunately, a negative drug provocation test does not fully exclude BrS.2, 4
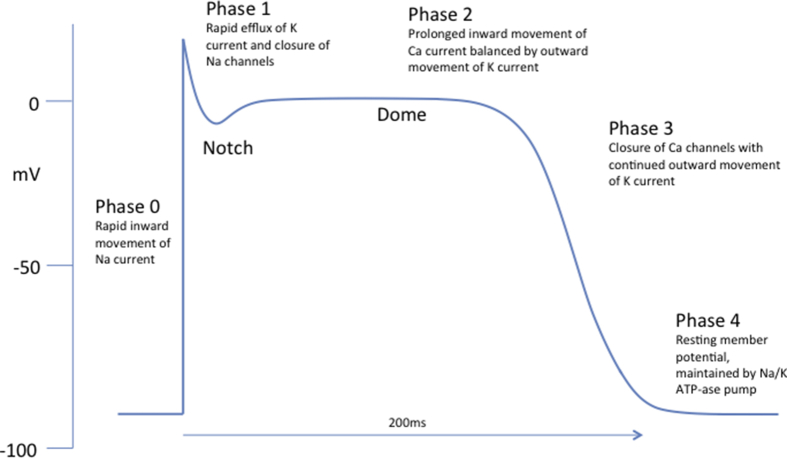
Normal cardiac action potential
Normal cardiac ventricular cells have a resting membrane potential of around –90 mV. This is maintained by the Na+/K+ATP-ase ion pump and the relative differences in permeability of the membrane to the various ions on either side, with the greatest selective permeability for K+ causing the resting negative potential (Phase 4). In Phase 0 depolarisation is initiated by rapid inwards movement of Na+ current through INa channels causing the potential increase to +30 mV. This depolarisation is propagated from cell to cell. Rapid activation followed by inactivation of transient outward K+ channels (Ito) in the late period of Phase 0 combined with closure of INa channels are responsible for Phase 1. The action potential is prolonged (Phase 2) by inward movement of Ca2+ through L-type channels (ICa-L), which is balanced by outward movement of K+ through slow delayed rectifier K+ currents (IKs). This is the period in which the myocyte is refractory. The action potential usually lasts between 200 and 300 ms and is terminated in Phase 3 as the ICa-L close and the IKs and rapid delayed rectifier K+ currents (IKr) remain open. In vitro studies demonstrate that action potentials are not uniform throughout the heart; epicardial cells have a shorter action potential than those in the endocardium. The epicardium has a deep Phase 1 notch (leading to a Phase 2 dome) which is absent in the endocardium. A deeper notch is also evident in the right ventricle, and towards the base of the heart. This difference results from higher density of Ito (so a greater outward K+ current) in the epicardium. The deepest Phase 1 notch is in the right ventricular outflow tract (RVOT).7, 11 It remains to be seen whether these differences are present in the heart in vivo.
Pathogenesis
The exact mechanism behind BrS remains unclear. It is possible that multiple different mechanisms result in similar phenotypes in different patients.2 To date, BrS is linked to 19 genetic mutations that encode for sodium, calcium, or potassium channels and result in either increase or decrease in their activity.4 The commonest mutations are in the SCN5A gene that encodes the α-subunit of the voltage-gated INa channel resulting in a loss of function. There are >300 identified mutations to this gene and together they account for up to 30% of cases of BrS.7 The severity of the ion channel dysfunction caused by the mutation appears to be related to the clinical severity of the condition.12 It is beyond the scope of this article to discuss all mutations associated with BrS, some of which are extremely rare. An excellent summary can be found in the paper by Antzelevitch and Patocskai.4 In up to 80% of patients no causative genetic mutation can be found, suggesting either further unidentified mutations or other mechanisms for the phenotype.7 It is possible for mutations in the same gene to lead to other syndromes, such as long QT syndrome 3.7
Two principal hypotheses have been advanced as the mechanism for BrS and its arrhythmogenicity and resting ECG characteristics. It is possible that both are correct in different patients (see Fig 1, Fig 2, Fig 3, Fig 4).
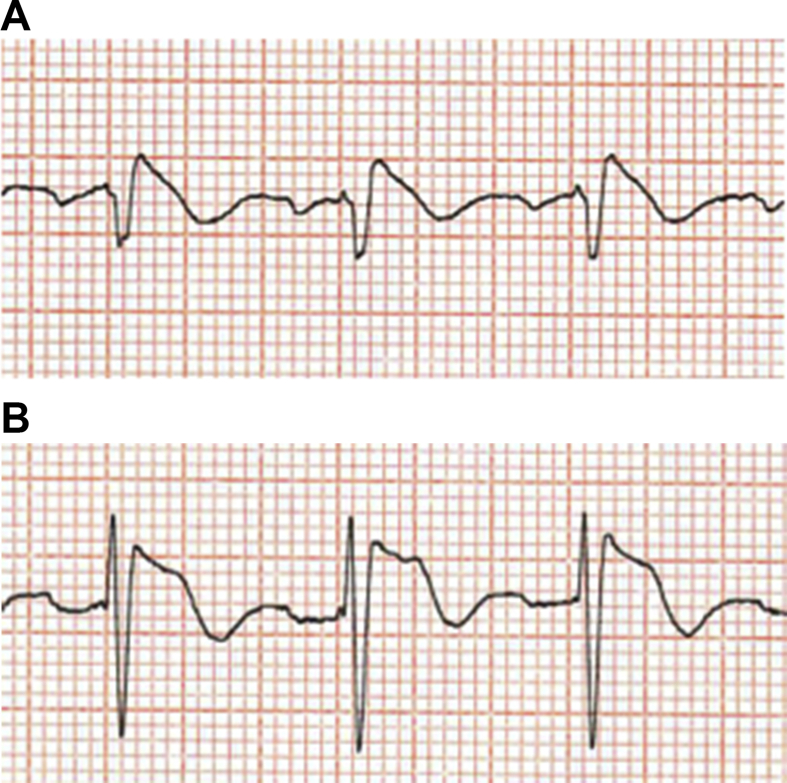
Fig 1.
An ECG pattern (A) very suspicious but non-diagnostic for a type I Brugada ECG in lead V1 and (B) less pronounced changes in lead V2 before an ajmaline provocation test (images courtesy of David Tomlinson).
Fig 2.
Postajmaline provocation in the same patient, with (A) a type 1 Brugada ECG pattern induced in lead V1 (complexes now have subtle convex/‘coved’ ST shape) and (B) more accentuated but still not type I ECG change in lead V2 (images courtesy of David Tomlinson).
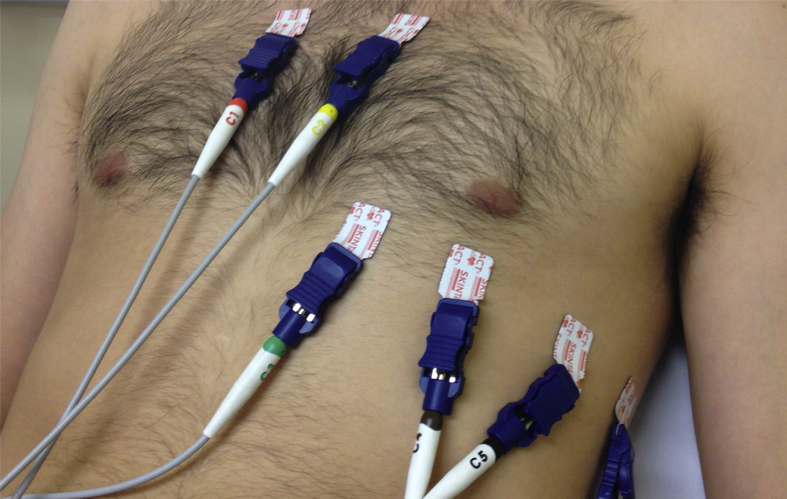
Fig 3.
High precordial lead placement (note V1 and V2 are placed in the second intercostal space). Alternatively, leads V5 and V6 can be moved to the superior position whilst leads V1 and V2 are maintained in their standard position (fourth intercostal space).
Fig 4.
The normal cardiac ventricular cell action potential.
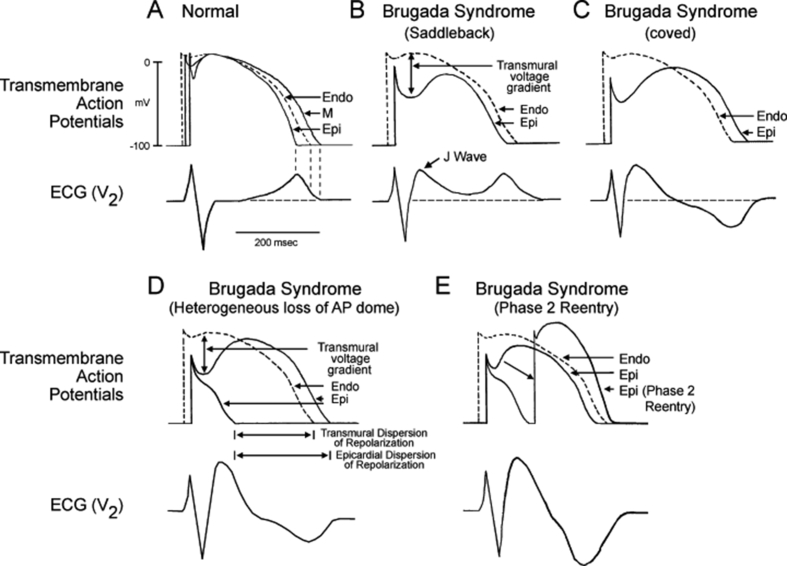
Repolarisation theory
As explained above, the myocardial action potential is not uniform across the heart. There is spread in the notch, dome, and refractory periods from endocardium to epicardium. In the repolarisation theory, dysfunction of the cardiac ion channels results in enhancement of the Phase 1 notch. This can lead to deactivation of ICa-L and so loss of the action potential dome at some epicardial sites (most commonly within the right ventricular outflow tract). These sites lose their refractory period and become vulnerable to re-entrant currents. This is termed Phase 2 re-entry. The currents originate from myocardial sites that have retained their Phase 2 dome (Fig. 5).4, 7 The Phase 2 re-entry generates closely coupled premature beats that can induce VT or VF.
Fig 5.
The repolarisation theory for Brugada syndrome [Endo: Endocardial, M: M region, Epi: Epicardial, AP: Action Potential] (reproduced with permission from Antzelevitch C. Brugada Syndrome. Pacing Clin Electrophysiol 2006; 29: 1130–59).13
The deep Phase 1 notch combined with loss of the Phase 2 dome creates a large transmural voltage gradient. This transmural voltage gradient, which is most pronounced in the RVOT, can explain the characteristic ST-segment elevation seen in the precordial leads on the resting ECG.2 T wave inversion, and so the classic coved appearance, will be present when preserved epicardial Phase 2 domes are longer than that of the adjacent endocardium.4
Depolarisation theory
This hypothesises that there are worse conduction delays as a result of the INa abnormalities (for example). The RVOT depolarises later than the rest of the right ventricle creating a potential difference between them. This will result in the characteristic ECG morphology and preponderance to arrhythmias.2 This theory is supported by evidence that the conduction delay at the RVOT is as a result of loss of the gap junction signalling protein connexin-43 and epicardial and intramyocardial fibrosis replacement, not evident macroscopically or on imaging.14
Management strategies
Targeted patient management will depend on clinical context and risk. Broad clinical categories include: (i) assessment of the need for non-immediate cardiology interventions weighing up lifetime arrhythmia risks and complications of therapy; (ii) perioperative management of patient with likely or confirmed BrS; and (iii) urgent management of an electrical storm.
Assessment of risk
It is generally easy to identify those at highest risk of cardiac arrhythmic events but lower risk groups, who can still present with arrhythmias, are more difficult to stratify and there is no validated algorithm.2, 5, 6 Commonly accepted risk factors are listed in Table 1. A positive family history and the use of electrophysiological studies (EPS) to induce VT/VF for risk stratification remains controversial.4, 5 The presence of symptoms or previous aborted SCD significantly increases the chances of further arrhythmias. The annual event rate in patients with a spontaneous type 1 pattern who are asymptomatic, experience syncopal episodes and who have had previous aborted SCD is 0.5%, 1.9%, and 7.7%, respectively.15 No characteristics have yet been found allowing identification of patients most at risk of arrhythmic storms.16
Table 1.
| Spontaneous type 1 ECG pattern |
| Male sex |
| Syncopal episodes |
| Nocturnal agonal respiration |
| Previous ventricular tachycardia/ventricular fibrillation |
| Fragmented QRS complex (i.e. increased number of deflections) |
| Prolonged QRS |
| T-wave alternans |
| Ventricular refractory period <200 ms (identified at invasive electrophysiology study) |
| Early repolarisation pattern in inferolateral leads |
Drugs to avoid
An exhaustive list of drugs to avoid in BrS can be found at www.Brugadadrugs.org.17 Class 1A and 1C antiarrhythmic drugs, lithium, and tricyclic antidepressants are known to increase the degree of ST elevation in BrS, thereby provoking arrhythmias and should be avoided. Other antiarrhythmics such as amiodarone and verapamil, α adrenergic agonists, β adrenergic blockers, vagotonic agents, selective serotonin reuptake inhibitors, or tetracyclic antidepressants and certain antiepileptics such as carbamazepine, phenytoin, and lamotrigine should preferably be avoided; there is currently no substantial evidence that these drugs can cause malignant arrhythmias, although they will provoke the ECG pattern.4, 18 Glucose in combination with insulin should be used with caution because of the risk of hypokalaemia.4
Cardiological therapies
No large-scale trials have been conducted in this rare condition, so management is based on expert consensus.5, 6 Simple lifestyle changes are recommended to all patients with BrS: avoidance of certain drugs (see above), avoidance of excessive alcohol, and immediate treatment of fever, with hospitalisation recommended for continuous arrhythmia monitoring in the event of sustained pyrexia.
ICD therapy is the only proved prophylactic treatment option for SCD.4, 5 However, there are short- and long-term risks of a transvenous ICD, with the potential requirement for multiple device replacements as this patient group is generally young at presentation. An analysis of ICD implantation in young patients with inherited arrhythmias revealed up to 20% of patients received inappropriate shocks, 10% suffered lead malfunction and was associated with an annual infection rate of up to 0.53% and mortality rate of 0.08%18 As such, many younger patients with an indication for an ICD yet not requiring back-up bradycardia pacing (i.e. as in BrS) would be considered suitable candidates for a subcutaneous ICD. ICD implantation is recommended in any patient who has survived a cardiac arrest or had a documented spontaneous sustained episode of VF/VT. Furthermore, an ICD should be considered in any patient with a spontaneous type 1 ECG who has a history of syncope probably because of ventricular arrhythmia.5 The role of EPS in risk stratification lacks consensus but should VF be inducible an ICD may be considered in this subgroup.2, 5 ICDs are not presently indicated for asymptomatic patients, regardless of family history.5, 8
A number of drugs have been advocated for use in long-term prevention of VF and VT including bepridil, cilostazol, milrinone, quinidine, and the Chinese herb extract Wenxin Keli of which only the class 1a antiarrhythmic quinidine is recommended by the expert consensus group.4, 5, 8, 16 Although quinidine is thought to be an effective alternative to ICD implantation, it does not fully suppress the potential for VF.2, 16 It can be useful in patients who have a history of VF storms, those who qualify for an ICD but in whom it is contraindicated or refused, and in those who have a history of SVTs requiring treatment.5 Quinidine can be considered in asymptomatic patients with a spontaneous type 1 pattern although evidence for its efficacy in this subgroup is lacking.5 Quinidine inhibits Ito reducing efflux of K+, and so restoring the epicardial Phase 2 action potential dome and normalising the ECG.4
Some specialist centres have undertaken radiofrequency ablation of selected epicardial areas of the RVOT for VF storm, targeting sites of electrical conduction delay. The results in the publishing centres are promising with a good short-term success rates and a low incidence of complications.2, 6, 14 Furthermore, when all abnormal substrate has been ablated there are reports of loss of the type I Brugada ECG pattern. Its use can be considered in patients with a history of arrhythmic storms or repeated appropriate ICD shocks.5
An increase in heart rate decreases the ST-segment elevation and susceptibility to arrhythmias suggesting a possible role for the use of cardiac pacing for long term arrhythmia suppression.19 Although not included in the consensus recommendations, there are case reports of the use of DDD pacing to successfully inhibit VF and ventricular burst pacing to treat monomorphic VT.19, 20 Heart transplantation can be considered for patients in whom all other options have been exhausted who continue to experience repeated appropriate shocks.4
Perioperative management
Anaesthesia is generally well tolerated in patients with BrS.21 However, because of the increased risk of provoking malignant arrhythmias, care must be taken throughout the perioperative period. This should extend until at least 36 h after surgery as arrhythmias are most likely to occur in the postoperative period.3 A thorough history should be taken, specifically asking about symptoms, a previous history of SVTs, aborted SCD, and if an ICD is present, whether shocks have been delivered and how many. A 12 lead ECG may help to stratify risk, although again it must be emphasised that the ECG pattern can vary greatly in the same individual. Electrolytes should be checked and corrected with specific attention to the potassium and calcium, as hyperkalaemia, hypokalaemia, and hypercalcaemia have all been known to unmask the Brugada ECG pattern.4 Serum magnesium concentrations should be kept within the normal range. Communication with the patient's cardiologist will ensure adequate optimisation. If an ICD is present, it should be disabled because of the risk of delivery of an inappropriate shock. From this point until reactivation of the ICD, continuous ECG monitoring should be applied. Means for external defibrillation should be immediately available and, if possible, pads should be pre-emptively applied. At the preoperative brief, all team members should be made aware of the underlying diagnosis, the possibility for malignant arrhythmias and the plans for treatment should this occur. The surgical team should be aware of the risk of precipitating arrhythmias with vagotonic manoeuvres (e.g. pneumoperitoneum) and if these are required they should be performed cautiously whilst monitoring the ECG.17
An increased chance of arrhythmias as ST-segment elevation worsens has been suggested and so vigilance to changes in the right precordial leads is necessary throughout.21 The temperature should be continuously monitored and normothermia maintained. Hyperthermia should be treated aggressively. Isoprenaline and, if possible, quinidine (although this can only be administered enterally) should be available (see section on electrical storm).
The safety of specific anaesthetic drugs in BrS is largely drawn from case series. Propofol has been used safely in BrS for bolus dosing at induction of anaesthesia. However, multiple reports of a Brugada-like ECG pattern during propofol infusion in previously healthy patients have raised concerns. When used as maintenance for anaesthesia or sedation in the intensive care unit, the risks and benefits must be considered carefully.3, 21, 22 Thiopental has been used without issue although ketamine and etomidate have both been associated with ST-segment elevation.17, 21, 22 All opioids can be used safely, although data is limited on the use of remifentanil, and tramadol should be used with caution.17 Local anaesthetics should be used with extreme caution because of their mechanism of action. When they are used, the dose should be minimised and areas of rapid absorption avoided.21 Where possible, use lidocaine with adrenaline in preference to bupivacaine as the former limits the potential for systemic absorption and has shorter half-life.17, 21 Application of topical cocaine for nasal surgery should be avoided.4, 17
Alpha-2 agonists such as clonidine should be avoided because of their mechanism of action, although there are no data to suggest arrhythmogenicity.22 No adverse events have occurred with the use of volatile anaesthetics, depolarising and non-depolarising neuromuscular blockers, midazolam, paracetamol, non-steroidal anti-inflammatories, or most commonly used antiemetics (preferably avoid metoclopramide).17, 21, 22 Ephedrine, phenylephrine, atropine, and glycopyrrolate have all been used without complications but data on the use of metaraminol are limited. Noradrenaline has been reported to increase ST-segment elevation.21 Neostigmine in combination with glycopyrrolate has been used without incident although it is suggested it should be given slowly whilst monitoring the ECG because of the possibility of increased vagal tone.3
The drugs that may provoke arrhythmias such as propofol infusions and local anaesthetics have all been used in patients with BrS without adverse events.21 Similarly, regional anaesthesia and central neuraxial blockade have also been used successfully in patients with BrS. The growing use of i.v. lidocaine infusion for treatment of acute pain warrants education of staff on the typical features of BrS, as its use may increase the risk of cardiac events in previously undiagnosed patients.
As much of the literature remains ambiguous with regards to the safe use of anaesthetic drugs we would advocate a pragmatic approach. Should a patient be deemed high risk because of multiple previous arrhythmias it is safest to avoid the use of propofol and use a midazolam, volatile, and opioid anaesthetic. Perform careful cardiac monitoring for ST elevation and for vagal events in relevant surgery (e.g. neck surgery) and avoid pneumoperitoneum if possible. If local anaesthetic is essential, then use small doses and volumes of lidocaine with adrenaline. After the operation, the patient should be monitored for at least 36 hr or 5 half-lives of the anaesthetic drugs given.3, 17 If an ICD is present it should be reactivated.
Management of an electrical (arrhythmic) storm
An arrhythmic storm is defined as more than two episodes of VT or VF in a 24 hr period.5, 6 Immediate management of VT or VF should include defibrillation. The only recommended acute pharmacological option is isoprenaline (isoprotenerol), which has been successfully used to reverse ST-segment elevation and suppress arrhythmic storms2, 5, 16; amiodarone should be avoided. An isoprenaline dose of 0.003 (sd 0.003) μg kg−1 min−1 aiming for a 20% increase in heart rate has been found to be successful in fully suppressing repetitive VF. Pyrexia should be treated aggressively with antipyretics and active cooling, electrolytes optimised and any provocative agents discontinued.17 Once stability has been achieved (with the isoprenaline infusion on-going), longer-term therapies can be considered. Quinidine, which can only be administered enterally, could be introduced to replace the isoprenaline, but this would largely depend on whether VF had been triggered by a reversible cause. A cardiac electrophysiologist may recommend a combination of further treatments, including ICD with or without quinidine or, rarely, pacing or ablation.
If there has been no return of consciousness after the electrical storm, or there are on-going arrhythmias requiring defibrillation, standard postarrest management should be instituted in intensive care with particular attention to the targeted temperature management, electrolytes, and pH. As stated in the perioperative section, several anaesthetic drugs have been linked with worsened ST elevation. Again, because of lack of robust evidence, a pragmatic approach should be taken. If there are ongoing defibrillation attempts it would be sensible to avoid all implicated drugs and use midazolam and morphine as sedative agents, awaiting cardiological treatment, even though this will hamper future neurological prognostication. Once stability has been achieved, further consideration can be given to the most appropriate drugs to use for ICU therapies.
Conclusion
BrS is an important cause of SCD, principally in previously apparently healthy young males. Patients who suffer from recurrent episodes of syncope or documented VT/VF are highly likely to experience further potentially fatal arrhythmic episodes. Implantation of an ICD is highly effective at preventing SCD, whilst quinidine is the only available effective medical alternative currently recommended. Isoprenaline can effectively terminate VF storm. Novel approaches to radiofrequency ablation may complement these treatments in future, but there is only very limited experience of this approach to treatment at present. During the perioperative period or in intensive care, an awareness of which drugs can precipitate worsening ST-segment elevation and arrhythmias, and knowledge of how to manage this situation is imperative.
Declaration of interest
None declared.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
David Levy MRCP FRCA is a specialty trainee in anaesthesia at Torbay Hospital who has completed fellowships in perioperative medicine and regional anaesthesia.
Colin Bigham MRCP FRCA FFICM is a consultant in anaesthesia and Intensive Care medicine at Derriford Hospital, Plymouth. His special interests include echocardiography and ultrasound.
David Tomlinson MD MRCP is a consultant cardiologist and electrophysiologist at Derriford Hospital whose special interests include treatments for heart rhythm abnormalities (electrophysiology and ablation), complex pacing, and implantable cardioverter defibrillator.
Matrix codes: 1A01, 2A03, 3C00
References
- 1.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death; a distinct clinical and electrocardiographic syndrome. A Multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Sieira J., Dendramis G., Brugada P. Pathogenesis and management of Brugada syndrome. Nat Rev Cardiol. 2016;13:744–756. doi: 10.1038/nrcardio.2016.143. [DOI] [PubMed] [Google Scholar]
- 3.Smith D., Martz D.G. Brugada syndrome: a review of perioperative management for the anesthesiologist. Int J Clin Anesthesiol. 2014;2:1019. [Google Scholar]
- 4.Antzelevitch C., Patocskai B. Brugada syndrome: clinical, genetic, molecular, cellular and ionic aspects. Curr Probl Cardiol. 2016;41:7–57. doi: 10.1016/j.cpcardiol.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priori S.G., Wilde A.A., Horie M. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace. 2013;15:1389–1406. doi: 10.1093/europace/eut272. [DOI] [PubMed] [Google Scholar]
- 6.Steinfurt J., Biermann J., Bode C., Odening K.E. The diagnosis, risk stratification, and treatment of Brugada syndrome. Dtsch Arztebl Int. 2015;112:394–401. doi: 10.3238/arztebl.2015.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morita H., Zipes D.P., Wu J. Brugada syndrome: insights of ST elevation, arrhythmogenicity, and risk stratification from experimental observations. Heart Rhythm. 2009;6:S34–S43. doi: 10.1016/j.hrthm.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Vohra J., Rajagopalan S., CSANZ Genetics Council Writing Group Update on the diagnosis and management of Brugada syndrome. Heart Lung Circ. 2015;24:1141–1148. doi: 10.1016/j.hlc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Minoura Y., Kobayashi Y., Antzelevitch C. Drug-induced Brugada syndrome. J Arrhythm. 2013;29:88–95. [Google Scholar]
- 10.Hohmann S., Rudic B., Konrad T. Systematic ajmaline challenge in patients with long QT3 syndrome caused by the most common mutation: a multicenter study. Europace. 2017;19:1723–1729. doi: 10.1093/europace/euw214. [DOI] [PubMed] [Google Scholar]
- 11.Antzelevitch C., Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol. 2001;96:517–527. doi: 10.1007/s003950170002. [DOI] [PubMed] [Google Scholar]
- 12.Meregalli P.G., Tan H.L., Probst V. Types of SCN5A mutation determines clinical severity and degree of conduction slowing in loss-of-function sodium channelopathies. Heart Rhythm. 2009;6:341–348. doi: 10.1016/j.hrthm.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Antzelevitch C. Brugada syndrome. Pacing Clin Electrophysiol. 2006;29:1130–1159. doi: 10.1111/j.1540-8159.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nademanee K., Raju H., de Noronha S.V. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015;66:1976–1986. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Probst V., Veltmann C., Eckardt L., Meregalli P.G., Gaita F. Long-term prognosis of patients diagnosed with Brugada syndrome. Circulation. 2009;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 16.Ohgo T., Okamura H., Noda T. Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm. 2007;4:695–700. doi: 10.1016/j.hrthm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Postema P.G., Neville J., de Jong J.S., Romero K., Wilde A.A., Woosley R.L. Safe drug use in long QT syndrome and Brugada syndrome: comparison of website statistics. Europace. 2013;15:1042–1049. doi: 10.1093/europace/eut018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olde Nordkamp L.R., Postema P.G., Reinoud E.K. Implantable cardioverter defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13:443–454. doi: 10.1016/j.hrthm.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Lee K.L., Lau C.P., Tse H.F., Wan S.H., Fan K. Prevention of ventricular fibrillation by pacing a man in Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11 doi: 10.1111/j.1540-8167.2000.tb00076.x. 955–37. [DOI] [PubMed] [Google Scholar]
- 20.Bertomeu-Gonzalez V., Ruiz-Granell R., Garcia-Civera R., Morell-Cabedo S., Ferrero A. Syncopal monomorphic ventricular tachycardia with pleomorphism, sensitive to antitachycardia pacing in a patient with Brugada syndrome. Europace. 2006;8:1048–1050. doi: 10.1093/europace/eul117. [DOI] [PubMed] [Google Scholar]
- 21.Kloesel B., Ackerman M.J., Sprung J., Narr B.J., Weingarten T.N. Anesthetic management of patients with Brugada syndrome: a case series and literature review. Can J Anesth. 2011;58:824–836. doi: 10.1007/s12630-011-9546-y. [DOI] [PubMed] [Google Scholar]
- 22.Staikou C., Chondrogiannis K., Mani A. Perioperative management of hereditary arrhythmogenic syndromes. Br J Anaesth. 2012;108:730–744. doi: 10.1093/bja/aes105. [DOI] [PubMed] [Google Scholar]