Key points.
-
•
The perioperative period is a crucial stage in the care of a patient with cancer.
-
•
Perioperative interventions may affect long-term cancer outcomes.
-
•
Surgical stress produces an environment that favours tumour growth and metastasis.
-
•
Current evidence suggests that inhalational agents and opioid-based analgesia may have a deleterious effect on cancer outcomes; whilst propofol-TIVA, local anaesthetics, and regional anaesthetic techniques may be protective.
-
•
Decisions on the best perioperative approach should be patient-specific and based on assessment of the relative risks and benefits of each intervention.
Learning objectives.
After reading this article the reader should be able to:
-
•
Explain that their chosen anaesthetic technique may have an impact upon the disease progression of cancer patients beyond the perioperative period.
-
•
Discuss current research findings and controversies related to anaesthetic interventions and cancer biology.
-
•
Summarise the key immunological factors involved in cancer progression and recurrence.
-
•
Recognise that additional research is required to provide definitive evidence in this field.
One in three people in the UK will be diagnosed with cancer in their lifetime and currently one in four will die from it. There are more than 350,000 new patients diagnosed with cancer in the UK each year, 45% of whom undergo surgery to remove their tumour as part of their primary treatment, and huge numbers of patients with cancer undergoing surgery for other reasons. The perioperative care of cancer patients forms a significant part of the routine work of most anaesthetists.
The perioperative period represents a ‘perfect storm’ of stress-induced immunosuppression at a time when tumour cells may be disseminated. There is also evidence to suggest that the perioperative environment, including the physiological stress of the surgical insult and perioperative medications, can result in the activation of molecular mechanisms that alter gene transcription.1 These epigenetic changes may have long-lasting effects, which persist well beyond the acute surgical period. The exact nature of the interplay between surgical stress, its sequelae, and the vastly heterogeneous tumour pheno- and genotypes is still poorly understood, but as our knowledge of oncological processes and tumour biology has grown, the focus on the potential impact of anaesthesia has intensified.
Recently, initiatives such as enhanced recovery after surgery (ERAS), based on the minimisation of surgical stress and facilitation of rapid return to normal function, have produced improved short-term outcomes (reduced length of stay and complications) across a variety of surgical procedures. Shortened post-surgical recovery times facilitate earlier return to intended oncological therapy (RIOT), maximising the chance of successful treatment. It is also postulated that the reduction of surgical stress and its consequent immunosuppression may reduce the likelihood of both local tumour recurrence and distant metastases. Indeed, with evidence emerging for a positive correlation between RIOT-rate and long-term oncological outcome in some cancers, it has been suggested that RIOT-rate should be used as a quality metric for the surgical management of cancer.2 It is important to note, however, that whilst evidence for the benefit of ERAS is growing, the impact on long-term mortality and, in cancer surgery, disease-free survival, is yet to be shown conclusively.
Laboratory and (predominantly retrospective) clinical studies also suggest there may be a direct effect of peri-anaesthesia drugs and interventions on the tendency of individual cancers to proliferate, metastasise, and recur in the longer term. Although unproven, there is significant potential for benefit, with more than 17 million annual cancer surgeries predicted worldwide by 2030.
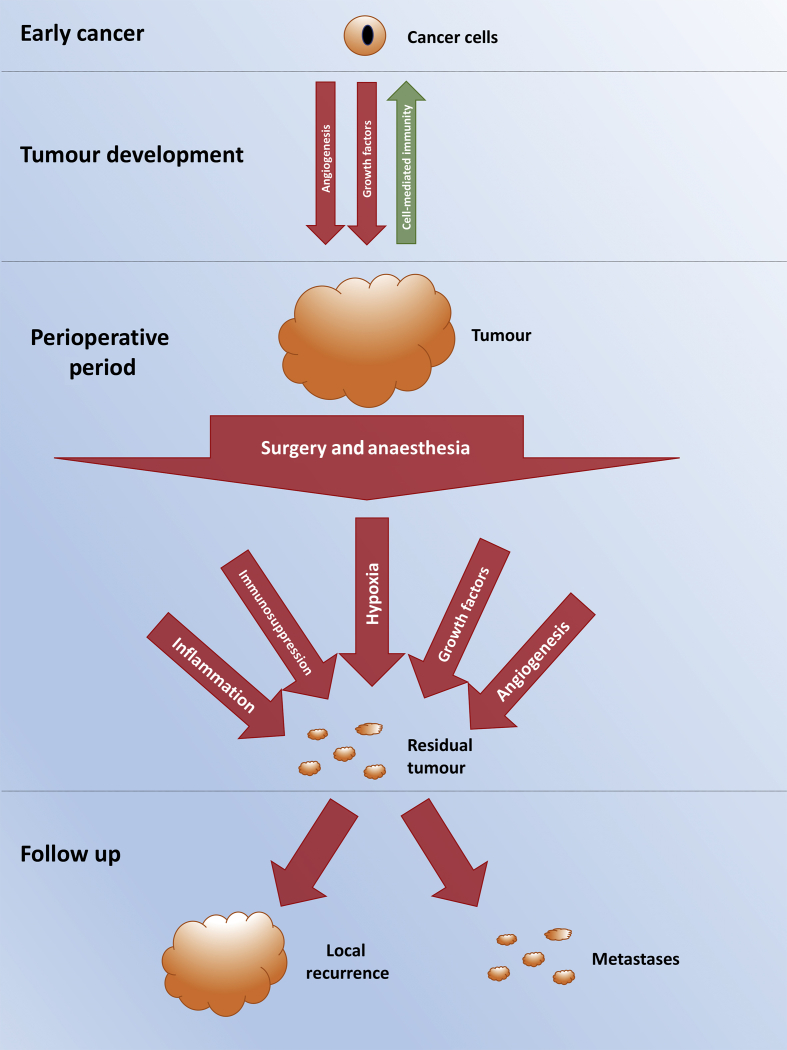
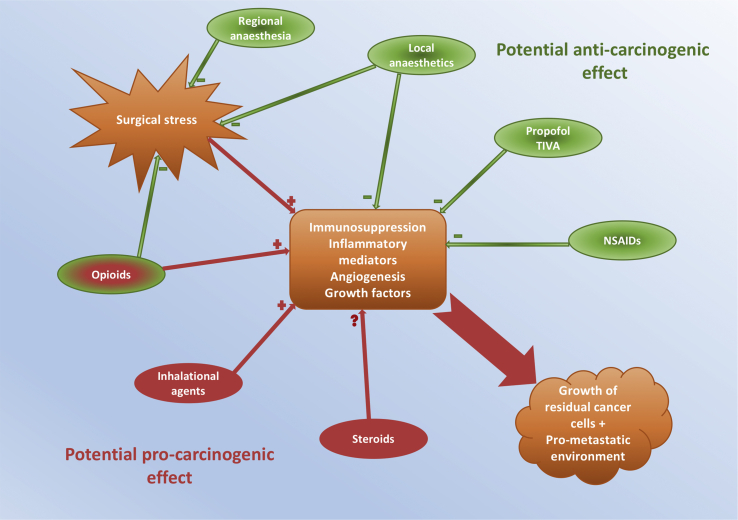
Whilst the surgical episode may be short in the context of the patient's overall experience with cancer, it is biologically plausible that the care they receive during the perioperative period might have a great bearing on successful tumour resection, disease recurrence, and overall outcome (see Fig 1, Fig 2). Within this article we consider some of the current controversies in this area and present a review of the available evidence and expert opinion.
Fig 1.
Overview of the development and progression of cancer in the perioperative period.
Fig 2.
Summary of the potential impact of commonly used anaesthetic agents upon cancer progression, metastasis and recurrence.
Inhalational anaesthesia compared with TIVA
There have been in vitro and in vivo studies suggesting that there may be a contrasting effect of inhalational and i.v. anaesthesia on the long-term growth and metastatic potential of tumours. This seems to stem from differential impacts directly on growth factors, immune system activity, and inflammation. Inhalational anaesthetics have been reported to promote tumorigenic growth factors, including hypoxia-inducible factors (HIFs) and insulin-like growth factor (IGF). The former are a family of transcription factors that mediate the response to hypoxia, resulting in angiogenesis and glycolysis, and promoting cell proliferation. They are overexpressed in many cancers, resulting in tumour growth, mitosis, and metastasis. Isoflurane has been shown to increase HIF-1α by an amount that is dependent upon concentration and time of exposure; the higher the concentration and the longer the exposure, the greater the stimulatory effect. Sevoflurane and desflurane have similarly been shown to increase HIF-1α, and indeed, this is the basis of their effects on ischaemic preconditioning. In contrast, propofol has been shown to abolish ischaemic preconditioning in rat models,3 with further data suggesting that propofol use results in a reduction in HIF-1α expression in a concentration- and time-dependent manner. The implication is that the administration of propofol could potentially lead to inhibition of of tumour cell growth. IGF is similarly overexpressed by solid tumours and results in cell proliferation and suppression of apoptosis (programmed cell death) and has been shown to be stimulated by inhalational agents.4
Surgery commonly leads to a reduction in circulating natural killer (NK) cells, cytotoxic T lymphocytes (CTLs) and in the ratio of T-helper1 to T-helper2 (Th1:Th2) cells. CTLs can directly eliminate tumour cells and Th1 cells (which produce interferon gamma and tumour necrosis factor-α) are responsible for activating and inducing CTL production. Th2 cells, in contrast, produce interleukin (IL)-4, IL-5, IL-6, and IL-10 and their action leads to immunosuppression and tumour promotion. Inhalational anaesthetics (notably isoflurane and sevoflurane) have been shown to decrease NK cells and CTLs and to augment the decrease in the Th1/Th2 ratio commonly seen in the perioperative phase. However, propofol neither inhibits NK cell activity nor the Th1/Th2 ratio, thus preserving the immune response to tumour cells during surgery.5
Clinically relevant concentrations of inhalational agents have been found to promote tumour growth, invasiveness, and migration in numerous cancer cell lines in vitro (including prostate, ovarian, breast, lung, and neuroblastoma). Propofol, in contrast, has been found to increase apoptosis and decrease invasion, migration, and proliferation across multiple cell lines.6 In addition, in serum collected from patients undergoing surgery for breast cancer randomised to propofol-based TIVA or inhalational anaesthesia, patients who had TIVA had better preserved NK function against the breast cancer cells and apoptosis than that from patients who had inhalational anaesthesia.7
Clinical outcome data for cancer patients undergoing surgery with inhalational vs TIVA-based anaesthesia are restricted to retrospective cohort studies, although there are randomised controlled studies underway. The largest series looked at more than 7000 patients anaesthetised over a 3-yr period for elective unselected cancer surgery in a single cancer centre in the UK. Approximately 50% of patients received propofol-based TIVA with the remainder having inhalational anaesthesia. After propensity matching, the hazard ratio (HR) for death in the inhalational group compared with the TIVA group after a median follow-up of 2.6 yr was 1.46 [95% confidence interval (CI) 1.29–1.66, P<0.001], with 87.9% of patients in the inhalational group surviving at 1 yr compared with 94.1% in the TIVA-based group. This mortality disadvantage in the inhalational group was preserved across ASA grading, severity of surgery, and whether or not the patient had known metastases at the time of surgery.5
Three other studies have reported similar findings. A retrospective analysis of 2838 patients undergoing surgery for breast and colorectal tumours in Sweden showed that survival for patients was 4.7% and 5% higher at 1 and 5 yr, respectively, in the propofol group, although after adjustment for confounders, the differences were not significant.5
A retrospective study of 383 patients undergoing mastectomy showed a significant reduction in breast cancer recurrence in the group of patients receiving propofol maintenance anaesthesia, with an estimated HR of 0.55 (95% CI 0.311-0.973).8 Another retrospective study, of 922 patients undergoing oesophagectomy, reported that inhalational anaesthesia was associated with both worse overall survival (HR 1.58; 95% CI 1.24-2.01; P<0.001) and worse recurrence-free survival (HR 1.42; 95% CI 1.12-1.79; P=0.003) after multivariable analysis adjustment.9
However, despite biological plausibility and whilst the published work so far supports the notion that propofol-TIVA may confer a long-term survival benefit compared with inhalational anaesthesia, the evidence is low quality and we await the results of randomised controlled trials (RCTs).
Regional anaesthesia and local anaesthetics
There has been increased interest recently in the use of regional anaesthesia and local anaesthetic infusions in an attempt to modulate cancer progression and recurrence. Several theoretical benefits for the use of regional techniques are postulated.
Firstly, the provision of regional anaesthesia may simply lower the perioperative requirement for high-dose systemic opioids, avoiding their potential effects on cancer progression, as described below.
Secondly, neuraxial techniques and the resultant sympathetic blockade have been shown to attenuate the stress response, better maintaining host immunity and minimising the impact that immunosuppression has upon the oncological disease process.10, 11 This effect has been demonstrated in laboratory studies using rats inoculated with adenocarcinoma cells that underwent surgery with either volatile anaesthesia or combined volatile and spinal anaesthesia. Animals receiving volatile and anaesthesia had both a reduced number and volume of lung metastases.11
Thirdly, local anaesthetics may themselves have an immunomodulatory effect and potentially a direct effect upon the malignancy itself. Local anaesthesia has been shown to inhibit the activity of the epidermal growth factor receptor,11 and downregulate nuclear factor κB (NF-κB),10 both of which would contribute to an anticancer effect. In vitro studies have shown encouraging results, with one demonstrating local anaesthetic-induced cytotoxicity in human lymphoma cells11 and another observing a concentration-dependent inhibition of colon adenocarcinoma cell proliferation.10
Following on from these laboratory findings, early clinical research (mainly retrospective cohort studies) indicates a positive impact with the use of regional anaesthesia, including prolonged overall and disease-free survival for breast, prostate, colorectal, ovarian, and head and neck cancers.10, 11 A meta-analysis examining the data from five prospective trials and 13 retrospective studies concluded that there was an overall survival benefit with epidural anaesthesia, especially in colorectal surgery, although they did not find any impact upon cancer recurrence.12 It should be noted that this meta-analysis was limited by virtue of the significant contribution of heterogeneous, non-randomised, retrospective studies.
Other studies, both retrospective and prospective, have been undertaken in recent years yielding mixed results. Many of these have concluded that regional techniques are of no benefit in terms of cancer recurrence or overall survival.10, 11
A recent systematic review looked at the potential impact of paravertebral block (PVB) on cancer recurrence and long-term survival in patients presenting for breast cancer surgery.13 Because of the number of low quality studies and heterogeneity of biochemical measurements, meta-analysis was not possible, and they concluded that current evidence neither supports nor refutes the oncological benefit of PVB. In addition, another systematic review and meta-analysis comprising 28 studies, including three RCTs and 67,577 patients undergoing surgery for a range of oncological subspecialties, concluded that regional anaesthesia showed no benefit in either long-term survival or cancer recurrence.10
Despite the apparently negative findings, both of the aforementioned reviews noted the relatively low quality of available data and that further prospective, randomised studies are required to evaluate the use of regional anaesthesia in this arena. To this end a multicentre RCT (NCT00418457) investigating the use of regional anaesthesia in breast cancer surgery is underway and due for completion in 2019.
Opioids
Whilst opioid analgesics have long been the mainstay of perioperative pain relief, recently a drive toward adopting a multimodal approach with a focus on opioid-sparing techniques has led to improved patient outcomes, through a reduction in opioid-related adverse effects such as postoperative nausea and vomiting, reduced gut motility and drowsiness. Additionally, in the context of oncological surgery, there is some evidence that reducing opioid usage may reduce angiogenesis, invasive potential, and cancer recurrence.
The μ opioid receptor (MOR) is expressed in a wide range of cancer cells, with significantly increased levels of MOR proteins being found in breast, colon, and lung cancer.14, 15 A study in a murine lung cancer model demonstrated a 65% reduction in metastasis when comparing MOR knockout mice to wild-type, and that treatment with methylnaltrexone (MNTX), a peripheral MOR antagonist, reduced both the development of metastasis and local cancer invasion.11 These findings are supported by clinical evidence from human studies with a recent post hoc RCT analysis of 229 advanced cancer patients demonstrating a longer median survival time with MNTX.16
In laboratory studies, agonism of the MOR with morphine has been shown to promote the release of vascular endothelial growth factor, implicating the receptor in tumour angiogenesis and growth.11 In addition, morphine has been shown to increase the release of urokinase plasminogen activator14 and increase the expression of the NET1 gene11 in breast cancer cell lines, which both promote cancer invasion and migration.
An epidemiological study following 2039 breast cancer patients over a 9-yr period found that women with one or more copies of the A118G allele, a polymorphism of the MOR gene that leads to reduced receptor transcription, not only had a reduced analgesic response to opioids, but also increased breast cancer survival.17
Differing regimens for the management of chronic cancer pain have also provided interesting results. Evidence suggests that using systemic opioid-sparing techniques including chemical splanchnicectomy18 and intrathecal drug delivery systems19 significantly improves survival in a range of different cancers. A similar tendency, albeit not significant, is also seen with neurolytic coeliac plexus block for pancreatic cancer.20
It should be noted that contradictory evidence exists, and the picture may not be entirely clear cut. A study in a murine metastatic colon cancer model found that morphine reduced the adhesive and invasive potential of the tumour cells by inhibiting the production of matrix metalloproteinases; this resulted in a reduction in both the number and volume of lung metastases in treated animals.11 Another murine study, in lung cancer, found that the chronic use of morphine at clinically relevant doses significantly reduced tumour angiogenesis and growth when compared to placebo.11 The authors also suggested that dose and route of administration may be important, hypothesising that the contrasting procarcinogenic effects seen with morphine in other studies may be the result of bolus doses of morphine inducing an intermittent withdrawal state in subjects, thereby producing an increase in the stress response with a subsequent deleterious impact on their immune system.
Although the exact nature of the impact of opioid use upon cancer progression has not been elucidated, it is clear that a balance must be struck between the resulting stress response and the potential impact of opioid usage. Evidence is mixed and influenced by setting, dosing, and chronicity; and the largest epidemiological study to date (including 34,188 patients) showed no association between use of opioids and breast cancer recurrence, regardless of opioid type, strength, chronicity of use, or cumulative dose. However, we would suggest the use of opioid-sparing techniques where possible, ensuring equipotent analgesia, if not purely for the impact upon cancer progression, then for patient satisfaction and avoidance of the negative effects of high-dose systemic opioids.
Glucocorticoid steroid supplementation/dexamethasone
Whether as an antiemetic, anti-inflammatory, or analgesic adjunct, dexamethasone is given by many anaesthetists as part of routine care. However, in cancer surgery, there continues to be concern over the immunosuppressive effects of perioperative glucocorticoids, at the very time an effective immune system may be most needed to reduce risks of cancer metastasis/recurrence.21 Numerous laboratory studies have shown that dexamethasone inhibits both the innate and cellular immune responses by reducing the number and activity of NK cells and of multiple other T cell subtypes (CD3+, CD4+, CD8+, and CD44+).22 It has also been shown to inhibit IL-12 induced IFN-γ secretion, and studies in animal models have demonstrated a suppressed anti-tumour immune response after dexamethasone.22
Despite these findings, there have been several studies that appear to refute such concerns, with some even suggesting benefit. An in vitro study of pancreatic adenocarcinoma cells found that dexamethasone significantly reduced the invasive potential of tumour cells and secretion of proinflammatory cytokines. In vivo, there was a reduction in both the number and volume of metastatic tumour deposits in mice treated with dexamethasone.23
Whilst definitive evidence from human studies is required, one recent retrospective analysis of 260 women with ovarian cancer compared recurrence rates after primary resection in patients who either did or did not receive dexamethasone. It failed to demonstrate an association between perioperative dexamethasone use and ovarian cancer recurrence.21 This echoes the findings of another retrospective analysis of 245 patients with ovarian cancer undergoing chemotherapy, which found that dexamethasone had no impact on the disease-free interval or survival time.21
NSAIDs
NSAIDs exert their action via inhibition of cyclooxygenase 1 (COX-1) and COX-2. Essential in the production of prostaglandins (PG), COX-1 is the constitutive isoenzyme whereas COX-2 is induced by cytokines and growth factors in an inflammatory process. PGs act via G-protein coupled receptors to bring about a wide range of physiological effects, chief among which is the regulation of the inflammatory response. The impact that PGs have on the immune response favours the development and progression of neoplasia. This includes the upregulation of IL-10, IL-4, and IL-6, and the downregulation of TNFα and IFN-γ, ultimately leading to immunosuppression.11 PGs have also been shown to reduce NK cell cytotoxicity, cytokine secretion, and migration, further enabling malignant cells to evade the host immune response.11 In addition, COX-2-catalysed production of PGE-2 has been shown to promote cancer mutagenesis, mitogenesis, angiogenesis, and metastasis.24 Notably, increased COX-2 expression has been reported in various cancers including colorectal, breast, cervical, bladder, and ovarian malignancies of epithelial origin, creating interest in the clinical use of COX inhibitors in malignancy.11
A systematic review of epidemiological studies that examined over-the-counter NSAID use and the relative risk of a range of cancers (breast, colon, prostate, and lung) found a composite risk reduction of 43% for colon cancer, 25% for breast cancer, 28% for lung cancer, and 27% for prostate cancer associated with NSAIDs.24 Interestingly, a subgroup analysis of the studies that used either rofecoxib or celecoxib (both COX-2 selective inhibitors) demonstrated a composite risk reduction of 69% for colon, 85% for breast, 61% for lung, and 55% for prostate cancers.
A meta-analysis of studies investigating aspirin use and breast cancer risk, comprising a pool of greater than 1 million patients, concluded that aspirin was associated with a reduced risk of breast cancer, giving a pooled odds ratio of 0.86 (95% CI 0.81–0.92).25 Whilst this meta-analysis largely included epidemiological or retrospective studies, a large prospective cohort study of 301,240 patients, investigating NSAID use and the development of colonic adenocarcinoma also concluded that NSAID use was associated with an overall reduction in the risk of colorectal cancer.11 In addition to reducing the risk of developing cancer, celecoxib has also been shown to ameliorate morphine-induced COX-2-mediated angiogenesis and metastasis in a murine model of breast cancer.11
Given these findings, and in the absence of clear contraindications, it is reasonable to consider the use of perioperative NSAIDs or COX-2 specific inhibitors, not only as part of opiate-sparing multimodal analgesia, but also for potential benefit with respect to cancer development and progression.
Conclusion
It is tremendously exciting and biologically plausible that perioperative anaesthesia interventions can affect long-term cancer outcomes, but the outcome of definitive clinical trials is awaited. Whilst some of these trials are already underway, obtaining high quality evidence remains a challenge. There is considerable heterogeneity with regard to the histological typing of cancers, staging of said cancer in individuals, and the efficacy of immunological defences. As a result of this huge genetic and phenotypic variability, single interventions are highly unlikely to be universally effective. Consequently, it is incredibly difficult to design studies with sample populations of sufficient size to yield statistically significant results. In addition, the follow-up required to report meaningful outcomes in cancer can take a prolonged period of time (e.g. 5 year survival), which constitutes a challenge in terms of both trial funding and intervention relevance.
Despite the difficulties, a continued effort is required to build a robust body of scientific evidence of perioperative interventions in cancer surgery. Until one exists, the best that clinicians caring for cancer patients undergoing surgery can do is be cognisant of the fact that our care may have consequences beyond the immediate postoperative period, and assess the evidence available to evaluate the risks and benefits of our interventions on a patient-specific basis.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Matthew Evans BSc (Hons), FRCA is a specialty trainee in anaesthesia in the Kent, Surrey and Sussex deanery who has had experience with complex major oncological surgery at several regional centres.
Timothy Wigmore MA, FRCA, FCICM, FFICM is a consultant in intensive care and anaesthesia at the Royal Marsden Hospital. He has several publications on the anaesthetic and critical care management of patients with cancer, including comparison of long-term survival rates in patients receiving different anaesthetic techniques.
L J S Kelliher BSc (Hons), FRCA, MD is a consultant anaesthetist at the Royal Surrey County Hospital who has published research in the field of cancer surgery, enhanced recovery, and the stress response. His MD thesis was on the impact of an enhanced recovery program on biological markers of the stress response after hepatic resection.
Matrix codes, 1A02, 2A07, 3J03
References
- 1.Lirk P., Fiegl H., Webber N.C., Hollmann M.W. Epigenetics in the perioperative period. Br J Pharmacol. 2015;172:2748–2755. doi: 10.1111/bph.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloia T.A., Zimmitti G., Conrad C., Gottumukalla V., Kopetz S., Vauthey J.N. Return to Intended Oncologic Treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol. 2014;110:107–114. doi: 10.1002/jso.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behmenburg F., van Caster P., Bunte S. Impact of anesthetic regimen on remote ischemic preconditioning in the rat heart in vivo. Anesth Analg. 2018;126:1377–1380. doi: 10.1213/ANE.0000000000002563. [DOI] [PubMed] [Google Scholar]
- 4.Perry N.J.S., Daqing M. Inhalationalal anesthetic agents and their effects on cancer cell biology. Curr Anesthesiol Rep. 2015;5:268–277. [Google Scholar]
- 5.Wigmore T.J., Mohammed K., Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery. A retrospective analysis. Anesthesiology. 2016;124:69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 6.Wei J., Jing L., Xin L. How does the anesthetic agent propofol affect tumors? Int J Clin Exp Med. 2017;10:5995–6003. [Google Scholar]
- 7.Buckley A., McQuaid S., Johnson P., Buggy D.J. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113(Suppl 1):i56–i62. doi: 10.1093/bja/aeu200. [DOI] [PubMed] [Google Scholar]
- 8.Lee J.H., Kang S.H., Kim Y., Kim H.A., Kim B.S. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Kor J Anesthesiol. 2016;69:126–132. doi: 10.4097/kjae.2016.69.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jun I.J., Jo J.Y., Kim J.I. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep. 2017;7:14020. doi: 10.1038/s41598-017-14147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandhi R.K., Lee S., Abd-Elsayed A. The relationship between regional anesthesia and cancer: a metaanalysis. Ochsner J. 2017;17:345–361. [PMC free article] [PubMed] [Google Scholar]
- 11.Heaney Á., Buggy D.J. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth. 2012;109(Suppl 1):i17–i28. doi: 10.1093/bja/aes421. [DOI] [PubMed] [Google Scholar]
- 12.Chen W.K., Miao C.H. The effect of anesthetic technique on survival in human cancers: a meta-analysis of retrospective and prospective studies. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez-González O., Cuéllar-Guzmán L.F., Soliz J., Cata J.P. Impact of regional anesthesia on recurrence, metastasis, and immune response in breast cancer surgery. Reg Anesth Pain Med. 2017;42:751–756. doi: 10.1097/AAP.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 14.Gach K., Szemraj J., Stasikowska-Kanicka O., Danilewicz M., Janecka A. Opioid-receptor gene expression and localization in cancer cells. Cent Eur J Biol. 2010;6:10–15. [Google Scholar]
- 15.Mathew B., Lennon F.E., Siegler J. Novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112:558–567. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janku F., Johnson L.K., Karp D.D., Atkins J.T., Singleton P.A., Moss J. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol. 2016;27:2032–2038. doi: 10.1093/annonc/mdw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bortsov A.V., Millikan R.C., Belfer I., Boortz-Marx R.L., Arora H., McLean S.A. Mu opioid receptor gene A118G polymorphism predicts survival in patients with breast cancer. Anesthesiology. 2012;116:896–902. doi: 10.1097/ALN.0b013e31824b96a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lillemoe K.D., Cameron J.L., Kaufman H.S., Yeo C.J., Pitt H.A., Sauter P.K. Chemical splanchicectomy in patients with unresectable pancreatic cancer: a prospective randomized trial. Ann Surg. 1993;217:447–457. doi: 10.1097/00000658-199305010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith T., Coyne P.J., Staats P.S. An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM) Ann Oncol. 2005;16:825–833. doi: 10.1093/annonc/mdi156. [DOI] [PubMed] [Google Scholar]
- 20.Wong G.Y., Schroeder D.R., Carns P.E. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer. JAMA. 2004;291:1092–1099. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
- 21.Colin B., Gan T.J. Cancer recurrence and hyperglycemia with dexamethasone for postoperative nausea and vomiting prophylaxis: more moot points? Anesth Analg. 2014;118:1154–1156. doi: 10.1213/ANE.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 22.Chen L., Jondal M., Yakimchuk K. Regulatory effects of dexamethasone on NK and T cell immunity. Inflammopharmacology. 2018;26:1331–1338. doi: 10.1007/s10787-017-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egberts J.H., Schniewind B., Patzold M. Dexamethasone reduces tumor recurrence and metastasis after pancreatic tumor resection in SCID mice. Cancer Biol Ther. 2008;7:1044–1050. doi: 10.4161/cbt.7.7.6099. [DOI] [PubMed] [Google Scholar]
- 24.Harris R.E. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 25.Luo T., Yan H.M., He P., Luo Y., Yang Y.F., Zheng H. Aspirin use and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2012;131:581–587. doi: 10.1007/s10549-011-1747-0. [DOI] [PubMed] [Google Scholar]