Learning objectives.
By reading this article, you should be able to:
-
•
Describe the components of the endocannabinoid system and its potential role in pain mechanisms.
-
•
Discuss the evidence base (preclinical and clinical) that currently exists to support the role of cannabinoids in pain management.
-
•
Recognise key clinical tenets associated with the safe prescribing of cannabis.
Key points.
-
•
The endocannabinoid system acts as a retrograde neurotransmitter system and is found throughout the mammalian pain pathway.
-
•
The cannabis plant contains many phytocannabinoids, with Δ-9-tetrahydrocannabinol (THC) and cannabidiol the most studied.
-
•
Preclinical studies support a role for cannabinoids in non-cancer pain, but robust clinical studies are still lacking.
-
•
There are no validated clinical dosing guidelines, but in general low doses of THC are required for analgesia.
-
•
Long-term use of cannabis may increase risk of psychosis, worsen manic symptoms and is associated with cannabis use disorder in approximately 9% of users.
Cannabis, widely used for recreational purposes, continues to generate controversy over its status as a putative medicinal agent. In November 2018, the UK moved unlicensed cannabis-based products from Schedule 1 to Schedule 2, enabling their use through prescription. In contrast, Canada legalised non-medicinal cannabis use in October 2018, and with 33 and 11 states in the the USA having legalised medicinal and non-medicinal use, respectively. The WHO and United Nations have also considered rescheduling cannabis to a less restrictive class. The WHO estimates that the annual prevalence of cannabis consumption is 181 million people globally, equivalent to 2.5% of the world's population.1 In comparison, prevalence rates are estimated between 28% and 34% in markets, such as Canada and the USA, where medicinal and recreational cannabis use is legal.2 As countries begin to adopt cannabis use regulations, it is likely that a greater number of patients will seek medical guidance on its effectiveness for relief of various ailments. Currently, clinicians may be reticent to broach this topic with patients given the limited clinical research, exuberant promotion of novel products and byzantine cannabinoid vernacular (Table 1). With a long history of social, cultural and medicinal use and a plethora of anecdotal benefits in chronic symptom management, healthcare providers will require a deeper understanding of the research base that supports, and refutes, the therapeutic uses of cannabis. One consistent role of cannabis throughout history has been its use as an analgesic. Even today, pain continues to be the most commonly reported symptom for which patients seek to use cannabis.3 This article reviews the science of cannabinoids; highlights the preclinical, experimental and clinical evidence assessing the efficacy of cannabis in chronic non-cancer pain; and outlines commonly used heuristics to enable safe therapeutic cannabis trials in patients with chronic pain.
Table 1.
Commonly used cannabis and cannabinoid terminology
| Term | Definition | |
|---|---|---|
| Cannabinoids | A class of chemical compounds that interact with the ECS and cannabinoid receptors (CB1 and CB2) | Endocannabinoids: cannabinoids produced naturally in mammals that interact with CB1 and CB2 receptors (e.g. AEA and 2-AG) |
| Phytocannabinoids: cannabinoids found in the cannabis plant (e.g. THC and CBD); note that nabiximol is an oromucosal spray containing the plant extract of THC and CBD with fixed dosing, also labelled as cannabis-based medicine extract | ||
| Synthetic cannabinoids: cannabinoids manufactured artificially (e.g. nabilone [synthetic THC analogue] and dronabinol [synthetic THC]) | ||
| Cannabis | A plant genus within the larger Cannabaceae family known for producing phytocannabinoids and having various species/subspecies | Species/subspecies of cannabis: Cannabis sativa (‘sativa’), Cannabis indica (‘indica’) and Cannabis ruderalis |
| Medical cannabis: cannabis used for the explicit intention to relieve symptoms related to an underlying disease or condition | ||
| Recreational cannabis: cannabis used for the sole purpose of altering consciousness or achieving a ‘high’ |
Endocannabinoid system and phytocannabinoids
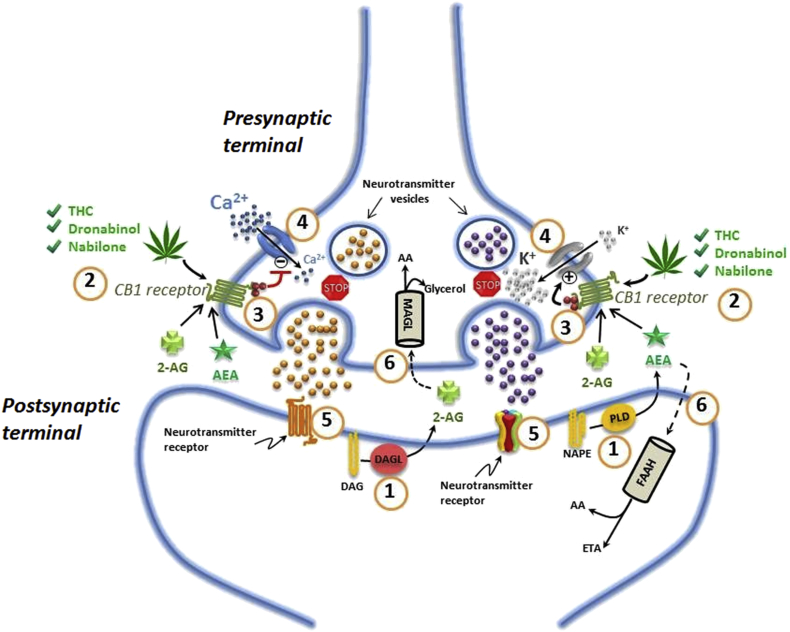
The endocannabinoid system (ECS), found in all mammals, functions as a retrograde neurotransmitter pathway. It primarily consists of two main cannabinoid receptors (CB1 and CB2), endogenous cannabinoid ligands (anandamide [AEA] and 2-arachidonoylglycerol [2-AG]), and degradative enzymes (fatty acid amide hydrolase [FAAH] and monoacylglycerol lipase [MAGL]).4 Endocannabinoid ligands are produced by hydrolysation of membrane phospholipid precursors in the postsynaptic neuronal cell, and then released into the presynaptic space, binding to cannabinoid receptors. Activation of receptors initiates a cascade of downstream events regulating glutamatergic and GABAergic signalling, after which the endocannabinoids are rapidly hydrolysed (Fig. 1). The more prolific CB1 receptor is expressed throughout the body and especially in the CNS. It is found throughout pain-modulating pathways, including primary afferent neurones, dorsal root ganglia, spinal dorsal horn, major descending inhibitory regions, periaqueductal grey and the rostral ventral medulla.5 The less ubiquitous CB2 receptors act by inhibiting proinflammatory responses of immune cells (e.g. macrophages and lymphocytes) and microglia. Whilst emphasis is placed on CB1 and CB2 receptors, preclinical research continues to advance our knowledge of the intricacies of cannabinoid-related G-protein-coupled receptor (GPCR) interactions and novel cannabinoid receptors.6
Fig 1.
Endocannabinoid signalling in the nervous system. 1, Endocannabinoids are manufactured ‘on demand’ in the postsynaptic terminals: AEA is generated from phospholipase-D (PLD)-mediated hydrolysis of the membrane lipid N-arachidonoylphosphatidylethanolamine (NAPE) and 2-AG from diacylglycerol lipase (DAGL)-mediated hydrolysis of the membrane lipid diacylglycerol (DAG); 2, AEA and 2-AG diffuse retrograde towards the presynaptic terminals and bind to activate the presynaptic receptor CB1; 3, binding of phytocannabinoid and endocannabinoid agonists to the CB1 receptor triggers traditional G-protein-coupled signalling leading to decreased formation of adenosine 3ʹ,5ʹ-cyclic monophosphate and activity of protein kinase A; 4, activation of the CB1 receptor also results in an arrest of the release of stored excitatory and inhibitory neurotransmitters; 5, decrease in native neurotransmitters reduces binding to postsynaptic receptors; 6, AEA and 2-AG re-enter the post- or presynaptic nerve terminals where they are catabolised by FAAH or MAGL, respectively, to yield either arachidonic acid (AA) and ethanolamine (ETA), or AA and glycerol.
GPCRs, novel ECS receptors and pain modulation
Cannabinoid ligands have been shown to interact with numerous well-established families of GPCRs, including opioid, serotonin, muscarinic, dopamine and adenosine receptors.7 Allosteric modulation of these receptors by cannabinoid ligands has been proposed, but the exact mechanism of action remains unknown. The orphan receptor GPR55 and its variants have been identified as new endocannabinoid-responsive receptors. GPR55 shares numerous cannabinoid ligands with CB1 and CB2, and could be a potential target for therapeutic use of cannabinoids in the treatment of pain.8 Peroxisome proliferator-activated receptors (PPARs), a family of nuclear transcription factors activated by cannabinoid-like molecules, have also been implicated in modulation of inflammatory and neuropathic pain. Transient receptor potential (TRP) channels, most notably TRP vanilloid-1 (TRPV-1), have a prominent role in pain. Classically, through capsaicin, and also via cannabinoid and cannabinoid-like ligands, activation of TRPV-1 leads to desensitisation (a refractory state) rendering the channel unresponsive to further signalling and produces a paradoxical analgesic effect. Although TRPV-1 is the most well known of the TRP channels, cannabinoids may interact with several different channels in the family [e.g. TRPV-2, transient receptor potential ankyrin 1 (TRPA-1) and transient receptor potential M8 (TRPM8)], signifying the potential for multimodal analgesic and anti-inflammatory effects.9
Phytocannabinoids
In addition to endocannabinoids, ECS receptors are also responsive to various external cannabinoids. The cannabis plant (Cannabis sativa), part of the Cannabaceae family, is known to possess well over 500 unique chemicals classified into distinct groups, such as phytocannabinoids (plant cannabinoids), terpenoids and flavonoids; the latter two compounds are responsible for the smell and colour of the plant, respectively. In 1964, Δ-9-tetrahydrocannabinol (THC) was first isolated and along with cannabidiol (CBD) are the two most studied phytocannabinoids. These chemically neutral compounds are derived from the plant's native acidic form (i.e. δ-9-tetrahydrocannabolic acid and cannabidiolic acid). Conversion of the native acidic cannabinoid to its more biologically active neutral form occurs through decarboxylation, commonly achieved by the application of heat. This process occurs when the dried plant is subjected to smoking, vaporising (‘vaping’) or baking as in edible foods. Smoking exposes cannabis to temperatures above 500°C resulting in production of toxic by-products and 30–50% loss of cannabinoid content through sidestream smoke and pyrolysis. In comparison, vaporising generates temperatures of 200–230°C and 60–70% cannabinoid extraction with minimal amounts of carcinogenic by-products.10
The therapeutic effects of THC, well known for its psychotropic properties, include anti-inflammatory, analgesic, sedative and appetite-stimulating effects. Cannabidiol is less studied compared with THC, and is hypothesised to act primarily as an anti-inflammatory and potentially as an analgesic.11 Co-administration of CBD with THC may counteract the THC-induced neuropsychiatric effects by modulating the extracellular signalling pathway in the ventral tegmental area.12 Whilst there have been over 100 phytocannabinoids identified from cannabis, many, including cannabinol and cannabigerol, are still being investigated for medical use. Often thought of independently, these cannabinoids and others may work synergistically to produce a given effect, a term referred to colloquially as the ‘entourage effect’.13
Cannabinoid effects in preclinical models of pain
Animal models
Cannabinoids have shown effective anti-nociception in numerous animal pain models of chronic inflammatory and neuropathic pain. There is ample evidence to support anti-nociception by dual CB1/CB2 receptor agonists and specific CB1 or specific CB2 agonists.14 Recent evidence supports the presence of neuronal CB2 in midbrain pain circuits, with stimulation increasing dopamine release from neurones of the central tegmental area contributing to pain relief and descending pain control.15 Apart from classical CB1/CB2 agonists, endocannabinoids, such as oleoylethanolamide and palmitoylethanolamide that do not act as direct agonists of CB1 or CB2, provide pain relief in animal models of visceral, inflammatory and neuropathic pain, possibly via PPARs, TRPV-1, downregulation of FAAH and actions on non-neuronal cells.16 In animals, CBD provides anti-inflammatory and anti-nociceptive effects not only in classical nociceptive models, but also in models of visceral pain, multiple sclerosis, Parkinson's-associated pain and intractable cancer pain.17
Genetic animal pain models
Genetic models with deletion of CB1 receptors result in nociceptive hypersensitivity and acute, inflammatory and neuropathic pain.18 In contrast, FAAH knockout mice are protected from nociceptive hypersensitivity and show reduced signs of stress and sleep disturbance.19 CB2 knockout mice were also shown to have reduced sensitivity, but not as profound as with CB1, although a greater role for CB2 was identified with dual agonists in the context of CB1 knockout models.
Human experimental pain models
The effect of cannabinoids in human experimental pain models have mixed outcomes ranging from hyperalgesia to mild analgesic effects.20 For example, several models have demonstrated a reduction in pain threshold when applying electrical stimulation or capsaicin after giving THC. Capsaicin pain models have also demonstrated absent analgesic effects when using THC or its analogues. One study demonstrated no effect on pain intensity, whilst another model documented no alteration of heat threshold, secondary hyperalgesia or spontaneous pain.21
Experimental models using functional MRI have also demonstrated the effects of THC on the structure of the pain matrix in the processing and perception of pain. The unpleasantness of pain was reduced by δ-9-tetrahydrocannabinol, but not the intensity of ongoing hypersensitivity from capsaicin.22 This response was correlated with activity in the amygdala and reduced connectivity between the sensorimotor cortex and the amygdala. The authors concluded that THC was effective by primarily reducing limbic–sensory connectivity, which was directly correlated with unpleasantness and intensity reduction. More recent work using gaseous CO2-induced pain as an experimental model showed THC first interferes with sensory processing that in turn reduces sensory–limbic connectivity resulting in deactivation of affective regions.23
In summary, meta-analysis of human experimental studies reveals cannabinoids may prevent the onset of pain with small increases in pain threshold and pain tolerance (i.e. greater amounts of stimulation were required to induce and withdraw from pain). In contrast, ongoing experimental pain was not associated with reduced intensity after cannabinoid administration, suggesting minimal benefit after the pain threshold had been met. However, a small to medium-sized reduction in perceived unpleasantness of ongoing experimental pain was noted, implying a positive effect on the affective dimension of pain. In these models, cannabinoids were not associated with a reduction in mechanical hyperalgesia, potentially limiting its role in central sensitisation.20
Clinical studies of cannabinoids in pain
Acute pain
Cannabinoids have been assessed in acute pain management, primarily in the postoperative setting. However, the evidence for the efficacy of cannabinoids in this context is weak. A recent systematic review identified seven studies that focused on the effectiveness of cannabinoid medications in the management of postoperative pain.24 Five of the seven studies failed to demonstrate a difference between cannabinoid and placebo with one study documenting worsening pain with cannabinoids. Statistically significant pain relief was demonstrated in only one of these studies (using levonantradol), with the effectiveness deemed equivalent to that of codeine.25
Chronic non-cancer pain
Many systematic reviews have been published assessing the efficacy of cannabis in chronic non-cancer pain (CNCP). Although similar subsets of studies are included across reviews, often different conclusions are reached by the review authors even when using quantitative methodology (Supplementary Table S1, online-only). These discrepancies frequently result from dissimilar analyses, study inclusion criteria and the authors' subjective impressions of the balance of benefits and risks.26
A metareview is beyond the scope of this article, although it is important to appreciate the breadth of primary observational and controlled clinical studies in this domain. To describe the original literature in aggregate, we have retrieved studies found in a more recent, comprehensive systematic review.27 The following sections summarise the general themes and outcomes of these original studies.
Observational studies
There have been 57 observational studies assessing cannabis in CNCP reporting on 5,678 patients.27 Few observational studies included a comparison group with outcome measures consisting primarily of changes in intra-individual pain intensity scores. Patients included those with mixed pain conditions; neuropathic pain (human immunodeficiency virus neuropathy, chemotherapy-induced neuropathy, diabetic peripheral neuropathy, plexus injury,spinal cord injury other mixed central and peripheral pain syndromes); and fibromyalgia. There was a total of 31, 22 and four studies in each group, with 16 (52%), nine (41%) and three (75%) studies reporting statistically positive results, respectively. In this group, the proportion of patients achieving 30% improvement in pain (clinically meaningful pain relief) from their baseline score was 72% across studies that reported this outcome measure. There was poor evidence to support a 50% improvement in pain amongst these studies.
Randomised clinical trials
Randomised clinical trials account for 47 of the published reports in the literature assessing a total of 4,271 patients.27 Studies assessed mixed pain conditions, neuropathic pain, arthritis and fibromyalgia with five/17 (29%), 13/26 (50%), one/one (100%) and two/three (66%) reporting statistically positive results, respectively. Again, RCTs demonstrated a statistical improvement in the prevalence of patients achieving 30% improvement in pain compared with placebo, but effect size was small with a number needed to treat (NNT) of 24 (95% confidence interval [CI]: 15–61).27 The results from these studies were not statistically significant for achieving a 50% improvement in pain.
Summary of clinical studies in chronic pain
Overall, there is not convincing evidence for the use of cannabinoids for pain relief in rheumatic diseases, including fibromyalgia, spinal pain and rheumatoid arthritis.28 However, many authors have noted benefits from cannabinoids as adjuvant therapy in patients with chronic neuropathic pain that is failing to respond adequately to traditional medical management.
Limitations of clinical studies
Several studies have reported positive results, but the number of well-designed, robust studies of cannabinoids in CNCP remains limited. Randomised clinical trials have largely focused on nabilone and nabiximols with a limited number of published RCTs using dried cannabis or whole-plant extracts. The quality of existing studies is also hampered by short trial durations (usually less than 8–12 weeks), small sample sizes and heterogeneous groups of patients that may obscure effects when aggregated. Further, the pooling of results may confound interpretation because of variability across cannabinoid-based medicines and herbal plant content, inconsistent dose ranges and differences in pharmacology linked to different routes of administration. Finally, there has been a paucity of studies assessing global health outcome measures relevant to chronic pain, as recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials, such as disability, psychological health and quality of life.
Long-term consequences of cannabis
Many patients consider cannabis to be a safe substance, despite known contraindications (Table 2) and short-term adverse effects (Table 3). A major concern of cannabis as a therapeutic substance is its potential association with long-term negative effects, such as cannabis use disorder (CUD), risk of psychosis and mood disorders. The estimated lifetime prevalence of CUD is approximately 9%, similar to prescription opioids, but lower than for nicotine (32%), alcohol (15%) and stimulants (11%).29 Whilst psychosis is a contraindication to the medical use of cannabis, data supporting the strength of this association are mixed. A recent meta-analysis documented a dose–response relationship between cannabis use and psychosis, with a four-fold increase in risk for heavy (recreational) cannabis users. The effect of unknown constituents or cannabinoid content in recreational cannabis compared with more balanced cannabinoid ratios in medical cannabis may also confound these results. In short, a direct causal relationship to psychosis or safe dose of cannabis has yet to be defined.30 Finally, cannabis has been linked to increased risk of manic symptoms and worsening bipolar disorder.29 There is little doubt that cannabis is associated with negative mental health outcomes, but similar to gaps in efficacy research, our understanding of this relationship is limited by lack of details on dosing, route of administration and cannabinoid content.
Table 2.
Contraindications to the use of cannabinoid-based medicines
| Contraindications |
|---|
| General |
|
|
|
|
|
| Medical |
|
|
|
|
Table 3.
Common adverse effects of cannabinoid-based medicines and cannabis. Adapted from a report by the Government of Canada34
| Adverse effects | |
|---|---|
| CNS | Dizziness, somnolence, disorientation, dissociation, euphoria, depression, ataxia, visual disturbance, concentration difficulties and headache |
| Cardiovascular system | Hypotension, tachycardia, syncope and palpitations |
| Respiratory system | Dyspnoea, pharyngitis, nasal congestion and sinus headache |
| Gastrointestinal system | Dry mouth, anorexia, increased appetite, vomiting and diarrhoea |
| Genitourinary system | Increased and decreased urination, hot flashes, urinary retention and frequency of micturition |
Translation to clinical practice
The transition from the outcomes of research studies to clinical practice in this area still faces many hurdles. Clinicians have little guidance on the pharmacokinetics of various routes of administration, cannabis dosing, cannabinoid concentrations and drug–drug interactions. Research in these areas is needed to inform validated dosing protocols when prescribing cannabis for patients. For now, the UK-based National Institute for Health and Care Excellence released guidelines in 2019 for cannabis-based medicinal products, which has been endorsed by the British Pain Society (BPS).31,32 They recommend that cannabis-based medicinal products should not be prescribed routinely for managing chronic pain. Rather, those who have demonstrated benefit from medicinal cannabis after participation in a clinical trial should have access to medical cannabis. Patients reporting benefit from the use of recreational cannabis for chronic pain should be considered for clinical studies involving cannabis-based medicinal products.32
In other jurisdictions, where cannabis is legal, clinicians have adopted prescribing ‘protocols’ that focus on harm reduction whilst attempting to maximise efficacy. These heuristics are often summarised by the trite phrase, ‘start low, go slow’, which translates to low-dose THC (i.e. 1–2.5 mg day−1) with gradual titration until symptom relief or onset of adverse effects. It has been suggested that maximal THC doses of 30 mg day−1 suffice for analgesic effect in most patients.33
Conclusions
Pain societies and oversight bodies around the world have adopted various positions on the use of cannabinoids for chronic pain. The International Association for the Study of Pain recommends against the use of cannabinoids based on weak quality of evidence. In contrast, the updated Canadian neuropathic pain guidelines (2014) recommend cannabinoids as a third-line agent ahead of methadone, lidocaine and capsaicin, whilst the BPS takes a more balanced and cautious approach. The current literature is far from definitive or complete, but a few key trends are emerging:
-
(i)
Outcomes of cannabinoids witnessed at the bench side are unlikely to be mirrored at the bedside.
-
(ii)
Cannabinoids, in as yet to be defined ‘well-chosen patients’, may be a useful adjuvant to ameliorate pain and improve quality of life.
-
(iii)
Most important, our understanding of cannabis and cannabinoids continues to evolve compelling pain clinicians to remain open and informed.
Cannabis and cannabinoids will not be a panacea for CNCP, but those clinicians willing to learn more about their potential role in pain medicine will be able to provide patients with yet one more option to manage an often frustrating and disabling condition.
Declaration of interests
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) are accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Amol Deshpande MD MBA is assistant professor of Quality and Innovation at the University of Toronto. He completed a fellowship in pain management at University Health Network and currently is active staff in the comprehensive interdisciplinary pain programme at Toronto Rehabilitation Institute. His clinical and research interests include cannabis in chronic non-cancer pain with an interest in complex regional pain syndrome and inflammatory bowel disease. He is a member of the research committee of the Toronto Academic Pain Medicine Institute.
Howard Meng MD FRCPC is a resident in pain medicine at the University of Toronto and a consultant anaesthesiologist. His major research interests are cannabis and cannabinoids for chronic and perioperative pain.
Matrix codes: 1A02, 2E03, 3E00
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2020.05.002.
Supplementary material. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hall W., Renstrom M., Poznyak V. World Health Organization; Geneva: 2016. The health and social effects of nonmedical cannabis use. [Google Scholar]
- 2.Goodman S., Wadsworth E., Leos-Toro C., Hammond D. Prevalence and forms of cannabis use in legal vs. illegal recreational cannabis markets. Int J Drug Policy. 2020;76:102658. doi: 10.1016/j.drugpo.2019.102658. [DOI] [PubMed] [Google Scholar]
- 3.Sexton M., Cuttler C., Finnell J.S., Mischley L.K. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016;1:131–138. doi: 10.1089/can.2016.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou S., Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19:833. doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grotenhermen F. Cannabinoids. Curr Drug Targets CNS Neurol Disord. 2005;4:507–530. doi: 10.2174/156800705774322111. [DOI] [PubMed] [Google Scholar]
- 6.Di Marzo V., Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015;12:692–698. doi: 10.1007/s13311-015-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales P., Reggio P.H. An update on non-CB1, non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res. 2017;2:265–273. doi: 10.1089/can.2017.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marichal-Cancino B.A., Fajardo-Valdez A., Ruiz-Contreras A.E., Mendez-Diaz M., Prospero-Garcia O. Advances in the physiology of GPR55 in the central nervous system. Curr Neuropharmacol. 2017;15:771–778. doi: 10.2174/1570159X14666160729155441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Petrocellis L., Ligresti A., Moriello A.S. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pomahacova B., Van der Kooy F., Verpoorte R. Cannabis smoke condensate III: the cannabinoid content of vaporised Cannabis sativa. Inhal Toxicol. 2009;21:1108–1112. doi: 10.3109/08958370902748559. [DOI] [PubMed] [Google Scholar]
- 11.De Gregorio D., McLaughlin R.J., Posa L. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160:136–150. doi: 10.1097/j.pain.0000000000001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson R., Renard J., Norris C., Rushlow W.J., Laviolette S.R. Cannabidiol counteracts the psychotropic side-effects of δ-9-tetrahydrocannabinol in the ventral hippocampus through bi-directional control of ERK1-2 phosphorylation. J Neurosci. 2019;39:8762–8777. doi: 10.1523/JNEUROSCI.0708-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo E.B. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pertwee R.G. Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philos Trans R Soc Lond B Biol Sci. 2012;367:3353–3363. doi: 10.1098/rstb.2011.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navratilova E., Atcherley C.W., Porreca F. Brain circuits encoding reward from pain relief. Trends Neurosci. 2015;38:741–750. doi: 10.1016/j.tins.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suardiaz M., Estivill-Torrus G., Goicoechea C., Bilbao A., Rodriguez de Fonseca F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain. 2007;133:99–110. doi: 10.1016/j.pain.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Lotsch J., Weyer-Menkhoff I., Tegeder I. Current evidence of cannabinoid-based analgesia obtained in preclinical and human experimental settings. Eur J Pain. 2018;22:471–484. doi: 10.1002/ejp.1148. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal N., Pacher P., Tegeder I. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lichtman A.H., Shelton C.C., Advani T., Cravatt B.F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 20.De Vita M.J., Moskal D., Maisto S.A., Ansell E.B. Association of cannabinoid administration with experimental pain in healthy adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:1118–1127. doi: 10.1001/jamapsychiatry.2018.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraft B., Frickey N.A., Kaufmann R.M. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology. 2008;109:101–110. doi: 10.1097/ALN.0b013e31817881e1. [DOI] [PubMed] [Google Scholar]
- 22.Lee M.C., Ploner M., Wiech K. Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain. 2013;154:124–134. doi: 10.1016/j.pain.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walter C., Oertel B.G., Felden L. Brain mapping-based model of delta(9)-tetrahydrocannabinol effects on connectivity in the pain matrix. Neuropsychopharmacology. 2016;41:1659–1669. doi: 10.1038/npp.2015.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens A.J., Higgins M.D. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaesthesiol Scand. 2017;61:268–280. doi: 10.1111/aas.12851. [DOI] [PubMed] [Google Scholar]
- 25.Jain A.K., Ryan J.R., McMahon F.G., Smith G. Evaluation of intramuscular levonantradol and placebo in acute postoperative pain. J Clin Pharmacol. 1981;21:320S–326S. doi: 10.1002/j.1552-4604.1981.tb02610.x. [DOI] [PubMed] [Google Scholar]
- 26.Hauser W., Petzke F., Fitzcharles M.A. Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management—an overview of systematic reviews. Eur J Pain. 2018;22:455–470. doi: 10.1002/ejp.1118. [DOI] [PubMed] [Google Scholar]
- 27.Stockings E., Campbell G., Hall W.D. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159:1932–1954. doi: 10.1097/j.pain.0000000000001293. [DOI] [PubMed] [Google Scholar]
- 28.Fitzcharles M.A., Baerwald C., Ablin J., Hauser W. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): a systematic review of randomized controlled trials. Schmerz. 2016;30:47–61. doi: 10.1007/s00482-015-0084-3. [DOI] [PubMed] [Google Scholar]
- 29.Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110:19–35. doi: 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- 30.Marconi A., Di Forti M., Lewis C.M., Murray R.M., Vassos E. Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophr Bull. 2016;42:1262–1269. doi: 10.1093/schbul/sbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute for Health and Care Excellence Cannabis-based medicinal products 2019. https://www.nice.org.uk/guidance/ng144 Available from: [PubMed]
- 32.British Pain Society The British Pain Society position statement on the use of medical cannabis and cannabis-based products in the management of chronic pain 2019. https://www.britishpainsociety.org/static/uploads/resources/files/BPS_position_statement_25_11_19.pdf Available from:
- 33.MacCallum C.A., Russo E.B. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–19. doi: 10.1016/j.ejim.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Government of Canada Cesamet (nabilone) and Sativex product monograph and health Canada information for health professionals: cannabis and cannabinoids. October 2018. https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids-eng.pdf Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.