Learning objectives.
By reading this article, you should be able to:
-
•
Explain the historical context of spinal anaesthesia in the ambulatory setting.
-
•
Describe the ideal characteristics of an ambulatory spinal anaesthetic.
-
•
Recognise the central role of prilocaine and 2-chloroprocaine in the ambulatory setting.
-
•
Select the right drug for the right patient and the right procedure.
Key points.
-
•
Spinal anaesthesia can provide many of the desired properties of the ideal technique for ambulatory anaesthesia.
-
•
Prilocaine and 2-chloroprocaine are the intrathecal local anaesthetic agents of choice and should be available for ambulatory surgery.
-
•
Spinal anaesthesia provides an alternative approach for patients with comorbidities that predispose them to higher perioperative risk.
-
•
Benefits may include reduced postoperative nausea and vomiting, reduced postoperative pain, early postoperative discharge and lower cost.
-
•
Proficiency with the use of short-acting spinal anaesthetics should be a core competency of anaesthesia specialty training.
Ambulatory surgery places high demands on anaesthetic technique. In this setting, rapid onset and offset of anaesthesia, rapid recovery of protective reflexes, mobility and micturition, and good control of postoperative pain and nausea are required. Since the inception of ambulatory surgery, the favoured anaesthetic technique has been general anaesthesia with short-acting drugs. Concerns about the time to perform spinal anaesthesia and the risks of prolonged motor block and urinary retention have limited its use.
Whilst in the UK, ‘ambulatory surgery’ refers solely to patients being discharged from the hospital shortly after surgery, in the USA this term may also apply to admissions for up to 23 h. In this article, we will consider ambulatory surgery to mean that the patient is discharged home before midnight on the day of surgery.
Spinal anaesthesia has become increasingly popular for inpatient surgery, but, until recently, its use has been limited in ambulatory surgery by the lack of a safe, licensed short-acting local anaesthetic agent. An ideal intrathecal agent for ambulatory surgery should have a rapid onset of motor and sensory blockade, predictable regression within an acceptable time frame, and a low incidence of adverse effects. Historically, lidocaine was the preferred agent in this setting, providing a dense block with rapid recovery, but the identification of a high incidence of transient neurologic symptoms (TNS) has effectively excluded it from use.1, 2 Until recently, the only local anaesthetic preparations licensed for intrathecal use have been hyperbaric bupivacaine alone in the USA, and hyperbaric bupivacaine and plain levobupivacaine in the UK. Both drugs are of limited utility in the ambulatory setting because of their long duration of action.1 Low-dose bupivacaine and ‘unilateral’ blocks have been used in an attempt to reduce block duration, with limited success.1
In 2010, hyperbaric prilocaine 2% was licensed for spinal anaesthesia in the UK, followed in 2013 by plain 2-chloroprocaine 1%. The US Food and Drug Administration (FDA) approved plain 2-chloroprocaine 1% in 2017. These short-acting drugs fulfil the key criteria of an ideal intrathecal agent for ambulatory surgery and have expanded the choices available to the patient and anaesthetist when performing spinal anaesthesia for ambulatory procedures. Spinal anaesthesia with these agents does not necessarily require adjuvants such as intrathecal opioids or the provision of sedation, and may be associated with reduced postoperative analgesic requirements, lower rates of postoperative nausea and vomiting (PONV) and quicker readiness for discharge.3, 4, 5
Intrathecal local anaesthetics
In this article we review the intrathecal local anaesthetic agents relevant for ambulatory surgery. The availability and licensing of these drugs for intrathecal use in the UK and USA are summarised in Table 1.
Table 1.
Summary of local anaesthetic availability and licensing for intrathecal use in the UK and the USA
| Drug | Chemical structure | Medicines and Healthcare products Regulatory Agency (MHRA) licence, UK | FDA approval, USA | Licensed indication | Notes |
|---|---|---|---|---|---|
| Lidocaine | Amide | No | No | Not applicable | High incidence of transient neurologic symptoms.2 Not recommended. |
| Bupivacaine | Amide | Marcain Spinal 0.5% Heavy: Hyperbaric bupivavaine 0.5% (AstraZeneca) Hyperbaric bupivacaine 0.5% (Mercury Pharma) |
Marcaine Spinal: Hyperbaric bupivacaine 0.75% (Hospira) |
UK: ‘urological and lower limb surgery lasting 2–3 hours, abdominal surgery lasting 45–60 minutes’ USA: not specified |
Plain bupivacaine is not licensed for intrathecal use in the UK or USA. |
| Levobupivacaine | Amide | Chirocaine; Levobupivacaine 0.25/0.5/0.75% (Abbvie) Levobupivacaine 0.25/0.5/0.75% (Fresenius Kabi) |
No | UK: ‘surgical anaesthesia’ USA: not applicable |
No licensed hyperbaric levobupivacaine preparation. |
| Ropivacaine | Amide | No | No | Not applicable | No commercial preparation of hyperbaric ropivacaine. Plain ropivacaine used off-label in USA. |
| Mepivacaine | Amide | No | No | Not applicable | May have high incidence of transient neurologic symptoms.2 Used off-label in USA. |
| Articaine | Amide | No | No | Not applicable | No preservative-free preparation. Experimental only. |
| Procaine | Ester | No | No | Not applicable | Concerns about neurotoxicity.14, 15 Experimental only. |
| Prilocaine | Amide | Prilotekal: Hyperbaric prilocaine 2% (Sintetica) |
No | UK: ‘short term surgical procedures’ USA: not applicable |
Appropriate for procedures up to 90 min duration. |
| 2-Chloroprocaine | Ester | Ampres: Plain 2-chloroprocaine 1% (Sintetica) |
Clorotekal: Plain 2-chloroprocaine 1% (B. Braun) |
UK: ‘planned surgical procedure should not exceed 40 minutes’ USA: ‘those suitable for Clorotekal's short duration of action’ |
50 mg may be effective for up to 60 min for lower limb procedures. 60 mg may last up to 90 min; but, doses exceeding 50mg are not approved by MHRA or FDA.17 |
Lidocaine
Lidocaine is an amide local anaesthetic with a rapid onset and fast recovery of motor and sensory block, making it well suited for ambulatory surgery.6 The drug received FDA approval in 1948, and the hyperbaric 5% formulation became available for intrathecal use in 1954. In the early 1990s, neurotoxicity concerns arose after several published case reports of cauda equina syndrome associated with continuous spinal anaesthesia with hyperbaric lidocaine 5%, delivered via intrathecal microcatheters.2
The first report of TNS after single-shot lidocaine spinal anaesthesia was published in 1993.2 Although initially described with lidocaine 5%, the incidence is not dose- or concentration-dependent.2 It is also not exclusively caused by lidocaine, but the relative risk compared with other local anaesthetics (bupivacaine, prilocaine, procaine, levobupivacaine, ropivacaine, and 2-chloroprocaine) is 7.31 (95% confidence interval 4.16–12.86).2
A Cochrane systematic review published in 2009 found that TNS occurs after one in seven spinal anaesthetics with lidocaine.2 The syndrome typically presents as pain in the buttocks and lower extremities after recovery from uncomplicated spinal anaesthesia. Neurological examination, MRI, and electrophysiological tests are typically normal, and in almost all cases, the symptoms resolve within 5 days.2 Although the exact cause is still unclear, the lithotomy position is associated with a higher risk of TNS occurring.2
Lidocaine is no longer licensed for intrathecal use in the UK or USA, and we do not recommend its intrathecal use because of the unacceptably high risk of TNS. It remains a safe and popular choice for epidural anaesthesia.
Bupivacaine
Concerns about the high incidence of TNS with lidocaine and the absence of alternative licensed short-acting agents for ambulatory procedures prompted researchers to evaluate the use of low-dose intrathecal bupivacaine, to mitigate the long duration of action observed with usual doses.7
Bupivacaine is a long-acting amide local anaesthetic, widely used for intermediate- to long-duration surgery. In the UK and USA bupivacaine is currently only licensed for intrathecal use in a hyperbaric formulation although plain bupivacaine is widely administered off-label by the intrathecal route. The onset of block onset occurs within 5–8 min and typically lasts 1.5–3 h. Regression of the block below the level of S2 to allow mobilisation and micturition is slow, ranging from 240 to 380 min.6
Low-dose (<10 mg) intrathecal bupivacaine is associated with a shorter time to voiding and discharge home, although a few patients may still have a long recovery time.6, 8 Strategies aimed at using low doses of bupivacaine for bilateral blockade are associated with an unacceptably high failure rate.7 The addition of fentanyl may facilitate the use of lower doses of intrathecal bupivacaine and reduce postoperative pain scores after knee arthroscopy when compared with bupivacaine alone, but the benefits of adding fentanyl need to be balanced against the potential for opioid-related adverse effects, particularly pruritus, which occurs in up to 75% of patients.7 There is no evidence that the use of intrathecal fentanyl reduces time to discharge.7
Unilateral spinal anaesthesia with low-dose bupivacaine can be effective for knee arthroscopy where a dense motor block is not essential.7 An adequate block may be accomplished with hyperbaric bupivacaine 4–5 mg, administered with the operative side in the dependent position for 10–15 min.7 Time to recovery is reduced to approximately 3–4 h, which is still inferior to 2-chloroprocaine and prilocaine.1, 6, 7
We do not recommend the use of intrathecal bupivacaine for ambulatory surgery in the UK. In the USA, it remains the only FDA-approved local anaesthetic for procedures lasting more than 60 min.
Levobupivacaine
Levobupivacaine is the S(–)-enantiomer of bupivacaine. The speed of onset and quality of the block are comparable with hyperbaric bupivacaine, but isobaric levobupivacaine may have a shorter duration of sensory and motor block than hyperbaric bupivacaine.9 The time to mobilisation still exceeds 5 h, precluding it from routine use in ambulatory surgery.9 Isobaric levobupivacaine is licensed for intrathecal use in the UK but not in the USA.
Ropivacaine
Ropivacaine is the S(–)-enantiomer of propivacaine. It is a long-acting amide local anaesthetic with reduced lipid solubility and lower toxicity than bupivacaine. Compared with bupivacaine, it has a shorter duration of sensory and motor block, a less dense motor block, and a lower incidence of hypotension and bradycardia has been reported.10
Intrathecal hyperbaric ropivacaine is considered superior to isobaric ropivacaine because of its faster onset and offset, and more predictable and higher spread.10 Whiteside and colleagues11 demonstrated that 3 ml hyperbaric ropivacaine 0.5% provided a mean duration of sensory block to T10 of 56.5 min, with a mean time to mobilisation of 253 min, which—although faster than the recovery times observed with hyperbaric bupivacaine—still exceeds the time for block resolution with prilocaine and 2-chloroprocaine1, 6
Ropivacaine is not currently licensed for intrathecal use in the UK or USA. The hyperbaric ropivacaine preparation is not available in the USA, but isobaric ropivacaine is sometimes used intrathecally off-label.
Mepivacaine
Mepivacaine is an amide local anaesthetic that differs from bupivacaine by the absence of a single butyl group on the tertiary amine, making it less lipophilic, less potent, and shorter acting than bupivacaine.12 Mepivacaine is sometimes used off-label intrathecally in the USA as a substitute for lidocaine, and has a similar duration of action. The anaesthetic block produced by 45 mg isobaric mepivacaine 1.5% lasts about 180 min.12 Reducing the dose to 30 mg results in an incomplete anaesthetic block in 28% of patients, making the use of lower doses to decrease block duration inadvisable.12
The frequency of TNS associated with intrathecal mepivacaine may be similar to that of lidocaine.2 Therefore, at present, mepivacaine cannot be recommended for routine intrathecal use. Mepivacaine is not licensed for intrathecal use in the UK or USA.
Articaine
Articaine is an intermediate-potency, short-acting amide local anaesthetic that is rapidly metabolised because of an additional ester group in its structure. It has a fast onset, producing acceptable anaesthesia for procedures lasting up to 1 h and a time to first spontaneous voiding of approximately 3.5 h.13
Articaine is not licensed for intrathecal use in the UK and there is no available preservative-free preparation. In the USA, it is only approved for dental surgery and commercially available only in combination with adrenaline (epinephrine).
Procaine
Procaine is a short-acting ester local anaesthetic with a similar onset and duration of action to lidocaine but with a substantially lower incidence of TNS.2, 14 Concerns have been raised regarding its narrow therapeutic index, neurotoxicity, a high rate of inadequate block, and intraoperative nausea.14, 15 Further research into its pharmacodynamics and adverse effects are required before this drug may be recommended. Procaine is not licensed for intrathecal use in the UK or USA.
Prilocaine
Prilocaine is an amide local anaesthetic with fast onset, intermediate potency and intermediate duration of action. It is associated with a low incidence of TNS.2 The hyperbaric preparation has been shown to have a significantly faster onset and offset and a reduced time to first voiding compared with plain prilocaine.16 Dose-finding studies have concluded that prilocaine doses between 40 and 60 mg are appropriate for lower limb and lower abdominal procedures lasting up to 90 min.1
The time to discharge from hospital after intrathecal prilocaine is dose-dependent, but patients may typically be discharged within approximately 4 h after administration. Doses of up to 60 mg can be used safely and effectively in ambulatory surgery. Perianal procedures can be performed with as little as 10 mg. This dose provides an effective saddle block with little or no haemodynamic disturbance and patients may retain the ability to ambulate throughout.1 The hyperbaric preparation can be manipulated for use for both saddle anaesthesia and periumbilical and laparoscopic ambulatory procedures. Adjuvants such as clonidine and fentanyl are not necessary to achieve adequate anaesthesia, and the risk of increased adverse effects of these agents must be taken into consideration.1, 16
Prilocaine is relatively contraindicated in sickle cell disease because of the risk of methaemoglobinaemia caused by an ortho-toluidine metabolite.1 In healthy adults, the dose required to produce clinically apparent methaemoglobinaemia is 6 mg kg−1, which vastly exceeds the typical intrathecal dose.1
Prilocaine 5% was used via the intrathecal route in the UK from the 1960s until 1978 when it was withdrawn for commercial reasons related to production and stability of the drug.1 Hyperbaric prilocaine 2% was licensed in the UK in 2010. At present, prilocaine is not approved for intrathecal use in the USA.
2-Chloroprocaine
2-Chloroprocaine is an ester local anaesthetic with a very short duration of action that is caused by very low protein binding and rapid metabolism by pseudocholinesterase. It was first used intrathecally in 1952 but was not used routinely for spinal anaesthesia because lidocaine was the established agent for short procedures.17 In the early 1980s there were reports of permanent neurological deficits after inadvertent intrathecal injection of large volumes of 2-chloroprocaine 3% with a sodium bisulphite preservative intended for the epidural space.17 It is unclear whether sodium bisulphite or the high concentration of 2-chloroprocaine was the cause of neurotoxicity.17
A preparation of plain 2-chloroprocaine 1% that is free of both antioxidants and preservatives was introduced in 2004 and has been found to be no more toxic than other local anaesthetics, with a very low risk of TNS.2, 6, 13
Intrathecal 2-chloroprocaine has a rapid onset of sensory block (3–5 min). The duration of block is dose-dependent, with complete resolution of sensory block after 70–150 min with 30–60 mg.6 A dose of 40–50 mg of the 1% solution provides profound motor and sensory block up to T10–12, which is adequate for procedures such as knee arthroscopy or foot surgery. Up to 60 min of adequate surgical anaesthesia may be achieved, despite being licensed for procedures lasting up to 40 min. Although the use of lower doses such as 30 mg have been described, they may be associated with an inadequate duration of anaesthesia.17 It has been our experience that discharge time is not significantly prolonged when 50 mg compared with 40 mg doses are used. A dose of 60 mg typically provides a surgical block lasting in excess of 60 min, but the FDA states that ‘doses above 50 mg have not been adequately tested for efficacy and safety’. Similarly, in the UK, the maximum recommended dose is 50 mg.
Plain 2-chloroprocaine 1% may be used for perineal procedures but a saddle block is difficult to achieve consistently because the preparation is isobaric.17 The addition of adrenaline to prolong the duration of block is not recommended as it can cause flu-like symptoms and back pain.17 Plain 2-chloroprocaine 1% was licensed in the UK in 2013, and in the USA in 2017.
Intrathecal opioids
Fentanyl and sufentanil are widely used off-label in conjunction with local anaesthetics by the intrathecal route, with the goal of improving the quality of intraoperative anaesthesia by reducing visceral pain. These opioids have been reported to provide superior postoperative analgesia, but are associated with higher rates of opioid-induced adverse effects, including pruritus, PONV and postoperative urinary retention (POUR).7 For ambulatory surgery, spinal anaesthesia supplemented by early oral analgesia and locoregional techniques makes the routine addition of intrathecal opioids unnecessary.
Advantages of ambulatory spinal anaesthesia
Choice for the patient and informed consent
Clinicians have an ethical and legal obligation to respect patient autonomy, and have a duty to discuss the options for anaesthesia, taking reasonable care to ensure that the patient is aware of the ‘material risks’ of each option.18 In UK case law, ‘material risks’ have been defined as those to which a reasonable person in the patient's position would be likely to attach significance, or to which the doctor should reasonably be aware that the particular patient would be likely to attach significance.18
The choice of general or spinal anaesthesia should not be presented simply as ‘you will be asleep’ vs ‘you will be awake’. The discussion regarding spinal anaesthesia should include the option of receiving anxiolytic medications and drugs to provide moderate or deep sedation.
Improved patient engagement
Under spinal anaesthesia, patients may choose to observe certain aspects of their procedure and have the opportunity to interact with their surgeon. This can have psychological benefits and improve patient satisfaction.19
Pain
Central neuraxial block is associated with reduced postoperative pain scores and decreased need for analgesia in the PACU.4 The progressive regression of sensory blockade allows the introduction of systemic analgesics, titrated according to the severity of pain, facilitating a smooth transition from anaesthesia to effective analgesia.
Postoperative nausea and vomiting
PONV is unpleasant for the patient and is a common reason for delayed discharge.5 Spinal anaesthesia is likely associated with a reduced incidence of PONV when compared with general anaesthesia, but numerous factors contribute to the risk of PONV with both techniques.5 Hypotension, blocks above the level of T5, and the addition of intrathecal morphine increase the incidence of PONV with spinal anaesthesia.5 Short-acting spinal anaesthesia for ambulatory surgery facilitates the avoidance of systemic opioids, which reduces the risk of PONV. The reduced duration of unopposed vagal activity with short-acting agents may further reduce this risk.5
Comorbidities
Patients presenting for ambulatory surgery are increasingly older, sicker, and more obese. The use of short-acting intrathecal agents enables patients who were previously excluded because of their comorbidities and the risks associated with general anaesthesia, to benefit from ambulatory surgery.
Spinal anaesthesia has minimal effects on pulmonary physiology if the motor block is kept below T6 and is known to reduce postoperative pulmonary complications in patients with chronic obstructive pulmonary disease.20
Obesity is associated with an increased risk of difficult airway, aspiration, and respiratory compromise with general anaesthesia.21 Where appropriate, regional anaesthesia is preferred to general anaesthesia in obese patients.
In patients with significant gastro-oesophageal reflux disease, spinal anaesthesia may reduce the risk of pulmonary aspiration by avoiding the loss of protective airway reflexes that occur with general anaesthesia.
Patients with obstructive sleep apnoea may have increased sensitivity to benzodiazepines, neuroleptics, and opioids, worsening the severity of apnoea through central respiratory depression and pharyngeal muscle relaxation.22 Opioid-free spinal anaesthesia without sedation may therefore be beneficial.
Patients with diabetes may benefit from an opioid-sparing spinal anaesthetic technique that may reduce the risk of PONV.5 Spinal anaesthesia may allow a faster return to oral intake, reducing disruption to their normal anti-hyperglycaemic regimen.
Elderly patients may benefit from avoiding general anaesthesia and sedation, which contributes to postoperative confusion and may play a role in postoperative cognitive decline. Further research is warranted to quantify the benefits of ambulatory spinal anaesthesia to patients with comorbidities and advanced age.
Cost
Ambulatory spinal anaesthesia has the potential to improve operating theatre efficiency and reduce cost. Compared with general anaesthesia, the time to readiness for surgery with spinal anaesthesia is typically only a few minutes longer. Despite this slight delay, the potential ability to bypass PACU may lead to cost savings.
Gebhardt and colleagues compared intrathecal 2-chloroprocaine and total intravenous anaesthesia for knee arthroscopy and reported that patients receiving spinal anaesthesia met discharge criteria earlier and at a lower cost.3
Potential complications of ambulatory spinal anaesthesia
Delayed mobilisation
Delayed mobilisation and discharge have been problematic issues with the use of bupivacaine spinal anaesthesia for ambulatory surgery. Full return of sensory and motor function is required for mobilisation. Prilocaine and 2-chloroprocaine have substantially faster block resolution than bupivacaine.1, 6
We recommend that after the return of normal motor and sensory function patients be mobilised with direct supervision.
Postoperative urinary retention
Spontaneous micturition is the last function to recover after motor block resolution, and requires the regression of sensory block to below the S3 dermatome.1 The incidence of postoperative urinary retention (POUR) depends on risk factors related to the patient, surgery, and anaesthesia.1 Complications include bladder overdistension, which may be accompanied by an autonomic response and may have an adverse effect on urodynamics, as well as risk of infection.
Prilocaine is associated with a lower risk of POUR than bupivacaine, but the exact incidence of urinary retention is not known and depends on dose and other risk factors.1 Reported mean times to micturition with prilocaine range from 218 to 306 min.1 There are no known reported cases of POUR secondary to intrathecal 2-chloroprocaine.6
Avoiding excessive intravenous fluids during surgery may minimise the risk of POUR.1 We recommend giving no more than 500 ml to avoid bladder overdistension, whilst recognising that some patients may require additional volumes of fluids and vasopressor therapy to treat hypotension caused by spinal anaesthesia-induced sympathectomy.
Ambulatory surgery units may consider allowing low-risk patients aged <70 yrs and without a history of voiding difficulty to undergo low-risk procedures (not hernia or urological surgery) with short-acting spinal anaesthetics, particularly 2-chloroprocaine, to be discharged from hospital before voiding.1, 6 High-risk patients should be required to void prior to discharge. Bladder catheterisation should be performed if bladder volume exceeds 600 ml.1 Discharging low-risk patients before voiding requires a robust local support framework and thorough education of patients.
Post-dural puncture headache
Post-dural puncture headache (PDPH) is an unpleasant complication of spinal anaesthesia that may be severely debilitating. The risk of PDPH is very low with modern spinal anaesthesia and is unaffected by the local anaesthetic used.23 Epidural blood patch is effective in 70–98% of patients with PDPH if carried out more than 24 h after the dural puncture, and 72% of cases will resolve within 7 days without treatment.23
We recommend using a 25–27 G non-cutting spinal needle, balancing the increased risk of PDPH with larger needles with the increased likelihood of technical failure with smaller needles.23 All patients must be informed of what to do in the event of a postoperative headache, and there should be a local protocol in place for their management.
Hypotension and bradycardia
Spinal anaesthesia commonly causes both hypotension and bradycardia, primarily through preganglionic sympathetic blockade. General anaesthesia is also commonly associated with hypotension caused by the vasodilatory and negatively inotropic effects of many anaesthetic agents. In both cases, hypotension can be effectively managed with the judicious use of fluids and vasopressors.
Neurotoxicity and nerve injury
Neurotoxicity has been demonstrated with all local anaesthetic agents in studies using animal models.2 In clinical practice the incidence of TNS with prilocaine and 2-chloroprocaine is very low, and comparable with that of bupivacaine.2
The national audit of major complications of central neuraxial block in the UK (3rd National Audit Project [NAP3]) showed that whilst the consequences can be devastating, major complications are extremely rare.24 The risk of permanent nerve damage with spinal anaesthesia is no more than one in 160,000.24 It should be borne in mind that peripheral nerve injury is also common with general anaesthesia, occurring in one in 350 cases.24
Effective ambulatory spinal anaesthesia
Ambulatory surgical procedures at the level of the umbilicus and below are well suited to spinal anaesthesia. The choice of drug and dose must be targeted to the location of the surgical field and the length of surgery. Intravenous access and monitoring should be established before spinal anaesthesia.
If a saddle block is required, 10–20 mg of hyperbaric prilocaine 2% administered in the sitting position is effective, reducing adverse cardiovascular effects and enabling rapid postoperative mobilisation.25 Hyperbaric bupivacaine can be used if prilocaine is not available. Discharge may not be delayed if lower limb motor and sensory functions are not affected.
If a block of T10 or above is required, for example for an epigastric hernia repair, 60 mg hyperbaric 2% prilocaine would be suitable.
If a block at T10 or below is required, the anticipated duration of surgery should guide the decision to use either 40–50 mg plain 2-chloroprocaine 1% for procedures estimated to last up to 40 min, or 40–60 mg hyperbaric prilocaine 2% for procedures lasting up to 90 min. The surgical preparation time must be included in these calculations.
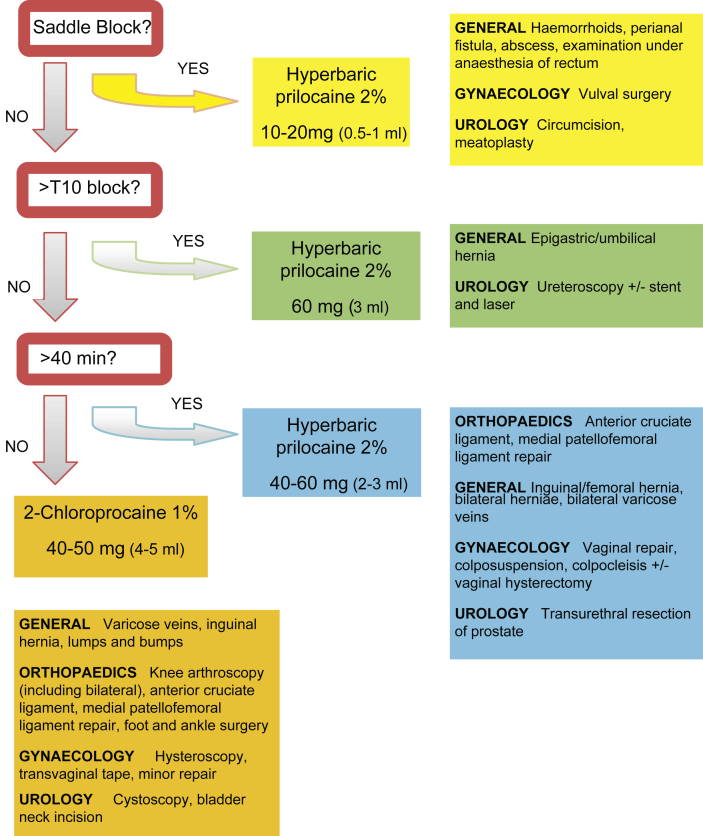
We have designed a simple and pragmatic flowchart to assist colleagues in deciding which drug and at what dose is most appropriate for common ambulatory procedures (Fig. 1).
Fig 1.
Algorithm for procedure targeted spinal anaesthesia for ambulatory surgery (R. Erskine, G. Turner, E. Erskine, 2015). If hyperbaric 2% prilocaine is not available alternative techniques may be effective, including using high-dose (60 mg) plain 1% 2-chloroprocaine, mepivacaine, ropivacaine, or low-dose bupivacaine. The literature to support these techniques is not strong, and they may not be recommended reliably.
Providing an ambulatory spinal anaesthesia service
We recommend that prilocaine, if commercially available, and 2-chloroprocaine should be on the formulary wherever ambulatory surgery is conducted. Education of surgeons and preoperative, operating theatre and recovery staff is fundamental to the success of an ambulatory spinal anaesthesia service. Specialty-specific lists of procedures commonly performed in the ambulatory setting, describing the preferred anaesthetic approach and highlighting all those amenable to short-acting spinal anaesthesia, can be very useful to colleagues usually working in other services. Advice on how to supplement analgesia with locoregional techniques and systemic analgesia should also be provided. Establishing local protocols for patient mobilisation after spinal anaesthesia and the management of POUR and PDPH are recommended.
Conclusions
Ambulatory surgery can be performed effectively with spinal anaesthesia. The availability of intrathecal prilocaine and 2-chloroprocaine have increased the options available to the anaesthetist and provide more effective and predictable anaesthesia than low-dose and unilateral bupivacaine spinal anaesthesia. The adverse effects of ambulatory spinal anaesthesia, including delayed mobilisation and POUR, are reduced with short-acting agents, making spinal anaesthesia a safe, effective and economical alternative to general anaesthesia.
Declaration of interest
R.E. is an advisor to Sintetica UK on day case spinal anaesthesia. A.H. is a consultant for Sintetica (Switzerland) and for B Braun USA.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
William Rattenberry FRCA is a specialty trainee in anaesthesia whose interests include quality improvement and regional anaesthesia.
Arthur Hertling MD FASA is a professor of clinical anaesthesiology at New York University School of Medicine. He is the director of the regional anaesthesia fellowship programme and of ambulatory anaesthesia. Previously an orthopaedic surgeon, his interests include regional anaesthesia and long-term outcomes.
Robbie Erskine FRCA is a consultant anaesthetist at the Royal Derby Hospital with extensive experience of ambulatory spinal anaesthesia. He won the British Association of Day Surgery (BADS) gold award for a presentation of the first use of intrathecal 2-chloroprocaine in day surgery in the UK. He has authored guidelines for BADS.
Matrix codes: 1A02, 2G01, 3A06
References
- 1.Manassero A., Fanelli A. Prilocaine hydrochloride 2% hyperbaric solution for intrathecal injection: a clinical review. Local Reg Anesth. 2017;10:15–24. doi: 10.2147/LRA.S112756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaric D., Pace N.L. Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics. Cochrane Database Syst Rev. 2009;2:CD003006. doi: 10.1002/14651858.CD003006.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Gebhardt V., Zawierucha V., Schöffski O., Schwarz A., Weiss C., Schmittner M.D. Spinal anaesthesia with chloroprocaine 1% versus total intravenous anaesthesia for outpatient knee arthroscopy: a randomised controlled trial. Eur J Anaesthesiol. 2018;35:774–781. doi: 10.1097/EJA.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 4.Liu S.S., Strodtbeck W.M., Richman J.M., Wu C.L. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634–1642. doi: 10.1213/01.ANE.0000180829.70036.4F. [DOI] [PubMed] [Google Scholar]
- 5.Borgeat A., Ekatodramis G., Shenker C. Postoperative nausea and vomiting in regional anaesthesia. Anesthesiology. 2003;98:530–547. doi: 10.1097/00000542-200302000-00036. [DOI] [PubMed] [Google Scholar]
- 6.Saporito A., Marcello C., Perren A. Does spinal chloroprocaine pharmacokinetic profile actually translate into a clinical advantage in terms of clinical outcomes when compared to low-dose spinal bupivacaine? A systematic review and meta-analysis. J Clin Anesth. 2019;52:99–104. doi: 10.1016/j.jclinane.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Nair G.S., Abrishami A., Lermitte J., Chung F. Systematic review of spinal anaesthesia using bupivacaine for ambulatory knee arthroscopy. Br J Anaesth. 2009;102:307–315. doi: 10.1093/bja/aen389. [DOI] [PubMed] [Google Scholar]
- 8.Teunkens A., Vermeulen K., Van Gerven E., Fieuws S., Van de Velde M., Rex S. Comparison of 2-chloroprocaine, bupivacaine, and lidocaine for spinal anaesthesia in patients undergoing knee arthroscopy in an outpatient setting: a double-blind controlled trial. Reg Anesth Pain Med. 2016;41:576–583. doi: 10.1097/AAP.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 9.Sahin A., Turker G., Bekar A., Bilgin H., Korfali G. A comparison of spinal anesthesia characteristics following intrathecal bupivacaine or levobupivacaine in lumbar disc surgery. Eur Spine J. 2014;23:695–700. doi: 10.1007/s00586-013-3082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohta M. Ropivacaine: is it a good choice for spinal anesthesia? J Anaesthesiol Clin Pharmacol. 2015;31:457–458. doi: 10.4103/0970-9185.169050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiteside J.B., Burke D., Wildsmith J.A. Comparison of ropivacaine 0.5% (in glucose 5%) with bupivacaine 0.5% (in glucose 8%) for spinal anaesthesia for elective surgery. Br J Anaesth. 2003;90:304–308. doi: 10.1093/bja/aeg077. [DOI] [PubMed] [Google Scholar]
- 12.Zayas V.M., Liguori G.A., Chisholm M.F., Susman M.H., Gordon M.A. Dose response relationships for isobaric spinal mepivacaine using the combined spinal epidural technique. Anesth Analg. 1999;89:1167–1171. [PubMed] [Google Scholar]
- 13.Snoeck M. Articaine: a review of its use for local and regional anesthesia. Local Reg Anesth. 2012;5:23–33. doi: 10.2147/LRA.S16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodgson P.S., Liu S.S., Batra M.S., Gras T.W., Pollock J.E., Neal J.M. Procaine compared with lidocaine for incidence of transient neurologic symptoms. Reg Anesth Pain Med. 2000;25:218–222. doi: 10.1016/s1098-7339(00)90001-4. [DOI] [PubMed] [Google Scholar]
- 15.Johnson M., Swanson J. Procaine spinal neurotoxcity. Anesthesiology. 2008;109:349–351. doi: 10.1097/ALN.0b013e31817fdeb8. [DOI] [PubMed] [Google Scholar]
- 16.Camponovo C., Fanelli A., Ghisi D., Cristina D., Fanelli G. A prospective, double-blinded, randomized, clinical trial comparing the efficacy of 40 mg and 60 mg hyperbaric 2% prilocaine versus 60 mg plain 2% prilocaine for intrathecal anesthesia in ambulatory surgery. Anesth Analg. 2010;111:568–572. doi: 10.1213/ANE.0b013e3181e30bb8. [DOI] [PubMed] [Google Scholar]
- 17.Goldblum E., Atchabahian A. The use of 2-chloroprocaine for spinal anaesthesia. Acta Anaesthesiol Scand. 2013;57:545–552. doi: 10.1111/aas.12071. [DOI] [PubMed] [Google Scholar]
- 18.Orr T., Baruah R. Consent in anaesthesia, critical care and pain medicine. BJA Educ. 2018;18:135–139. doi: 10.1016/j.bjae.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camponovo C., Wulf H., Ghisi D. Intrathecal 1% 2-chloroprocaine vs. 0.5% bupivacaine in ambulatory surgery: a prospective, observer-blinded, randomised, controlled trial. Acta Anaesthesiol Scand. 2014;58:560–566. doi: 10.1111/aas.12291. [DOI] [PubMed] [Google Scholar]
- 20.Hausman M.S., Jr., Jewell E.S., Engoren M. Regional versus general anaesthesia in surgical patients with chronic obstructive pulmonary disease: does avoiding general anaesthesia reduce the risk of postoperative complications? Anesth Analg. 2015;120:1405–1412. doi: 10.1213/ANE.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 21.Members of the Working Party. Nightingale C.E., Margarson M.P., Shearer E. Peri-operative management of the obese surgical patient 2015: association of anaesthetists of great britain and Ireland society for obesity and bariatric anaesthesia. Anaesthesia. 2015;70:859–876. doi: 10.1111/anae.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez G., Faber P. Obstructive sleep apnoea. BJA Educ. 2011;11:5–8. [Google Scholar]
- 23.Turnbull D., Shepherd D. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–729. doi: 10.1093/bja/aeg231. [DOI] [PubMed] [Google Scholar]
- 24.Cook T.M., Counsell D., Wildsmith J.A.W. Major complications of central neuraxial block: report on the third national audit Project of the royal college of anaesthetists. Br J Anaesth. 2009;102:179–190. doi: 10.1093/bja/aen360. [DOI] [PubMed] [Google Scholar]
- 25.Gebhardt V., Herold A., Weiss C., Samakas A., Schmittner M.D. Dosage finding for low-dose spinal anaesthesia using hyperbaric prilocaine in patients undergoing perianal outpatient surgery. Acta Anaesthesiol Scand. 2013;57:249–256. doi: 10.1111/aas.12031. [DOI] [PubMed] [Google Scholar]