Abstract
Background
Breast cancer (BC) is the most prevalent cancer in adult young women in Europe. Although rare, it is one of the leading causes of death in this age group. The aim of this study is to characterize a cohort of young women regarding tumor stage, biology, treatment and survival.
Patients and methods
We present a multicenter retrospective analysis of women <35 years of age, diagnosed with BC between 2008 and 2017. A total of 207 patients from five Portuguese centers were included, from whom 172 were eligible for analysis. Data were analyzed using IBM SPPSS statistics.
Results
Median age at diagnosis was 31 years. Fifty-one percent of tumors were hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative, 20% HR-positive/HER2-positive, 8% HR-negative/HER2-positive and 20% triple-negative BC. Twenty-two percent of patients were diagnosed in stage I, 26% stage II, 45% stage III and 6% had de novo metastatic cancer. Thirty-nine percent of patients were treated with neoadjuvant chemotherapy. Mean follow-up time was 64.9 months and overall survival at 5 years, of the entire cohort and metastatic patients, was 86.5% and 26%, respectively.
Conclusions
In our study we found similar population characteristics to other cohorts <35 years of age. To our knowledge, this is one of the largest cohorts in very young women. BC in young women is an important issue and further studies are needed to provide better care and survivorship to patients.
Key words: breast cancer, young women
Highlights
-
•
We present a large cohort of women <35 years of age when compared with other series and we found similar survival rates.
-
•
Aggressive subtypes (HER2 and triple-negative) are more prevalent in this age group than in breast cancer in general.
-
•
Sixty-one percent of this cohort has unknown BRCA status. This is a concerning and important issue for young women.
-
•
Ovarian function suppression was carried out in only 68% of HR-positive patients.
-
•
We found a fourfold increase in brain metastasis in our population when compared with our series of metastatic breast cancer.
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer among women in Portugal and worldwide. The definition of ‘young women’ in the field of breast oncology is not standardized, but most of the literature refers to women aged ≤40 years.1,2 In our study we decided to include patients <35 years of age.
According to GLOBOCAN data, in 2018, approximately 2.1 million women were diagnosed with BC, which constitutes 11.6% of all malignant neoplasms.3 There were 1683 deaths due to BC in women in Portugal in 2015.4 Although rare (2.5% before 35 years of age), BC is the most commonly diagnosed cancer among women aged 20-49 years.5,6 It is also the main cause of death in the group of patients aged 30-49 years.7
Age is a major risk factor for BC, but recent studies have shown increased incidence in premenopausal women.8 The GRELL study analyzed epidemiological data from seven European countries and found the mean incidence of BC in young women to be increasing 1.2% annually between 1990 and 2008. The increase was highest in the age group between 15 and 34 years as compared with older women (34-39 years of age), especially in France and Portugal.8
BC in young women is such a relevant matter that gave rise to a periodical international consensus conference, organized by the European School of Oncology (ESO) and European Society of Medical Oncology (ESMO). The guidelines issued in this meeting should be taken into account when treating this population.9
An increase in patients diagnosed in advanced stages could be a direct consequence of lack of screening in this age group. Recent evidence found mammography screening, beginning at 40 years of age, to be associated with a relative reduction in BC mortality, which was attenuated after 10 years, although the absolute reduction remained constant.10 BC screening programs in Portugal are primarily focused on women aged 50-69 years. Delayed diagnosis may also be due to a lack of oncological awareness among practitioners who treat young women with breast alterations (during pregnancy, puerperium or lactation).11
Identification of young patients is clinically valid, as BC in that age group presents with certain biological differences and often requires special management. Typically, BC in young women has a more aggressive course, less favorable prognosis and worse survival rates compared with older subjects.12 Triple-negative BC (TNBC) and human epidermal growth factor receptor 2 (HER2)-positive disease are over-represented in young patients compared with the overall population.12 Mutations in BRCA (BC gene) 1 may explain some of the TNBC and high grade observed in young women, but the majority of these tumors are not in the context of a familial cancer syndrome.13 Also, younger women with luminal tumors seem to have less favorable outcomes than older women.2,5,14,15 This fact might be due to biological factors—prevalence of PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha) mutations—or decreased adherence to adjuvant endocrine therapy.2,16 Additionally, this high deregulation of PI3K and Myc pathways, could have a role in endocrine therapy resistance.2
The aim of this study was to characterize a cohort of young women in five oncological centers in Portugal, with homogeneous diagnostic and treatment practices, regarding tumor stage, biology, treatment and survival.
Methods
We carried out a retrospective multicenter analysis conducted in five Portuguese oncology departments in Lisbon (Prof. Doutor Fernando Fonseca Hospital, Barreiro-Montijo Hospital Center, Occidental Lisbon Hospital Center, Vila Franca de Xira Hospital and Lisbon CUF Hospitals). The study was approved by the health ethics committee (number 37/2019). Clinical data were obtained for 207 patients diagnosed with BC from January 2008 to December 2017, who were >18 and <35 years old. Thirty-five were excluded: 27 due to lack of data, 1 due to two different synchronous tumors and 7 due to histological ductal in situ carcinoma.
Epidemiological and clinical data collected from patients' medical records included: age at diagnosis, histologic and immunohistochemistry (IHC) subtypes and staging. Early BC grouped all tumor–node–metastasis stages I and II and locally advanced included stage III (according to American Joint Committee on Cancer 7th Edition). Hormone (estrogen and progesterone) receptors (HR) were considered positive if 1% or more of tumor cells demonstrated positive nuclear staining on IHC. HER2 positivity was defined as IHC 3+ and when IHC 2+ it required a silver in situ hybridization test for confirmation. If defined as IHC 1+ or 0, it was considered negative. Type of treatment was also reviewed [surgery, chemotherapy (CT), endocrine therapy, biologic agents and radiotherapy (RT)], as well as prognostic variables such as time-to-events of first relapse/progression of disease/death and metastasis sites.
Statistical analysis was carried out using IBM® SPSS® statistics software version 26.0 (IBM Corp., Armonk, NY). Categorical data were presented as counts and percentages and were analyzed with chi-square test and Fisher's exact test, as appropriate. The skewed distributions were described with medians and interquartile ranges. Normal distributions were described with means and standard deviations and were compared with the use of the Student's t-test. Survival analysis was carried out using the Kaplan–Meier method and the log-rank test was used to assess differences among survival. The outcomes of interest were overall survival (OS) and disease-free survival (DFS). OS was defined as the period of time from diagnosis to time of final analysis or death, whichever came first. DFS was defined as the period of time from diagnosis to cancer recurrence, progression of disease or death, whichever came first. All P values were two-sided and P < 0.05 was considered statistically significant.
Results
A total of 172 patients were included from an initial number of 207. Baseline characteristics of the entire cohort are presented in Table 1. Median age was 31 years (interquartile range 29.34). Ductal invasive carcinoma was found in 158 (91.9%) patients and 4 (2.3%) had lobular invasive carcinoma. Metaplastic carcinoma and invasive carcinoma (not otherwise specified) were found in two (1.2%) patients each, while one (0.6%) patient had papillary invasive carcinoma. Two patients had inflammatory carcinoma. Five (2.8%) patients were diagnosed with other histologic types of BC. Eighty-eight (51.2%) women had IHC subtype of hormone receptor (HR)-positive/HER2-negative tumors, 35 (20.3%) had HR-positive/HER2-positive, 14 (8.1%) had HR-negative/HER2-positive and 35 (20.3%) TNBC. Regarding tumor size, 74 (46%) patients had tumors of 20 mm or less, 61 (38%) were between 21 and 50 mm and 22 (14%) women had tumors larger than 50 mm. When considering tumor grade, 12 (7%) were grade 1 (well differentiated), 72 (42%) were grade 2 (moderately differentiated) and 79 (46%) were grade 3 (poorly differentiated). Proliferation index (Ki-67) was <20% in 25 (20%) tumors, between 21% and 40% in 34 (28%) tumors, 41%-60% in 23 (19%) tumors, 61%-80% in 18 (15%) tumors and >80% in 22 (18%) tumors. Distribution of patients according to staging at diagnosis was as follows: 82 (47.6%) patients had early BC (stages I and II), 77 (44.7%) had locally advanced BC (stage III), 11 (6.4%) had metastatic BC and in 2 (1.2%) patients it was unknown. Excluding metastatic patients at diagnosis, 29 (16.8%) women experienced recurrence of disease [3 (1.7%) had local recurrence and 26 (15.1%) distant metastasis]. Sixteen (43%) patients who developed metastasis had HR-positive/HER2-negative disease, 11 (30%) were HR-positive/HER2-positive, 2 (5%) patients were HR-negative/HER2-negative and 8 (22%) were TNBC (Table 2). According to IHC subtypes, HR-positive/HER2-positive more frequently developed metastasis in three or more locations (13.5% versus 8.1% in HR-positive/HER2-negative versus 2.7% in HR-negative/HER2-positive disease versus 5.4% in TNBC), while HR-positive/HER2-negative was the subtype which more frequently developed metastasis only in one site (21.6% versus 5.4% in HR-positive/HER2-positive versus 2.7% in HR-negative/HER2-positive versus 8.1% in TNBC) (Table 2). Patients with TNBC and HR-positive/HER2-positive tumors had more central nervous system (CNS) metastasis (13.5% each), while HR-negative/HER2-positive and HR-positive/HER2-negative tumors developed CNS metastasis in 5.4% each. Visceral metastases were more common in HR-positive/HER2-positive and HR-positive/HER2-negative tumors (24.3% each versus 2.7% in HR-negative/HER2-positive versus 13.5% in TNBC). Bone metastases were more frequent in HR-positive/HER2-negative tumors (35.1% versus 16.2% in HR-positive/HER2-positive versus 2.7% in HR-negative/HER2-positive versus 10.8% in TNBC) (Table 2).
Table 1.
Patients' characteristics
| Median age (years) | 31 (IQR 29.34) | |
| Histologic subtype N (%) | ||
| Ductal invasive carcinoma | 158 (92) | |
| Lobular invasive carcinoma | 4 (2) | |
| Metaplastic carcinoma | 2 (1) | |
| Papillary invasive carcinoma | 1 (0.6) | |
| Invasive carcinoma NOS | 2 (1) | |
| Others | 5 (3) | |
| Tumor subtype N (%) | ||
| HR+/HER 2− | 88 (51) | |
| HR+/HER 2+ | 35 (20) | |
| HR−/HER 2+ | 14 (8) | |
| TNBC | 35 (20) | |
| Tumor size N (%) | ||
| 0-20 mm | 74 (46) | |
| 21-50 mm | 61 (38) | |
| >50 mm | 22 (14) | |
| Proliferation index (Ki-67)aN (%) | ||
| [0%-20%] | 25 (20) | |
| [21%-40%] | 34 (28) | |
| [41%-60%] | 23 (19) | |
| [61%-80%] | 18 (15) | |
| [81%-100%] | 22 (18) | |
| Tumor gradebN (%) | ||
| G1 | 12 (7) | |
| G2 | 72 (42) | |
| G3 | 79 (46) | |
| StagingcN (%) | ||
| Early breast cancer | 82 (48) | I—38 (22) |
| II—44 (26) | ||
| Locally advanced | III—77 (45) | |
| Metastatic | IV—11 (6) | |
| BRCA-mutated N (%) | ||
| Yes | 3 (2) | |
| No | 64 (37) | |
| Unknown | 105 (61) | |
| Treatments N (%) | ||
| Mastectomy | 105 (66) | |
| Breast-conserving surgery | 54 (34) | |
| Neoadjuvant therapy | 67 (39) | |
| Taxanes | 56 (84) | |
| Platins | 2 (3) | |
| Anthracyclines | 62 (93) | |
| Alkylating agents | 6 (9) | |
| Pyrimidine analogues | 4 (6) | |
| Anti-HER 2d | 16 (24) | |
| Adjuvant therapy | 143 (83%) | |
| Taxanes | 63 (44) | |
| Platines | 7 (5) | |
| Anthracyclines | 76 (53) | |
| Alkylating agents | 21 (15) | |
| Pyrimidine analogues | 12 (8) | |
| ET | 101 (71) | |
| Anti-HER 2 | 33 (23) | |
| Metastatic therapy | 37 (22%) | |
| Taxanes | 25 (68) | |
| Platins | 10 (27) | |
| Anthracyclines | 12 (32) | |
| Alkylating agents | 10 (27) | |
| Vinca alkaloids | 14 (38) | |
| Pyrimidine analogues | 16 (43) | |
| Antimetabolites | 3 (8) | |
| ET | 12 (32) | |
| Anti-HER 2e | 12 (32) | |
| Ovarian function suppression | 84 (68) | |
| Radiotherapy | 124 (72) | |
| OS at 5 years (%) | 87 | |
| HR+/HER 2−: 92 | ||
| HR+/HER 2+: 86 | ||
| HR−/HER 2+: 83 | ||
| TNBC: 75 | ||
| DFS at 5 years (%) (non-metastatic patients) | 80 | |
| HR+/HER 2−: 83 | ||
| HR+/HER 2+: 71 | ||
| HR−/HER 2+: 91 | ||
| TNBC: 76 | ||
DFS, disease-free survival; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IQR, interquartile range; NOS, not otherwise specified; OS, overall survival; TNBC, triple-negative breast cancer.
Forty-nine missing values; Ki-67 only routinely carried out since 2010.
Nine missing values.
Two missing values.
Seven patients received pertuzumab considering neoadjuvant dual HER 2 blockade therapy protocol implementation since 2014.
Two patients received pertuzumab considering metastatic dual HER2 blockade therapy protocol implementation since 2015.
Table 2.
Metastatic disease according to IHC subtypes
| HR+/HER 2− | HR+/HER 2+ | HR−/HER 2+ | TNBC | |||||
|---|---|---|---|---|---|---|---|---|
| OS at 5 years (%) | 26 | |||||||
| 61 | 60 | 0 | 23 | |||||
| Number of patients | 16 | 43% | 11 | 30% | 2 | 5% | 8 | 22% |
| Location of metastasis | ||||||||
| CNS | 2 | 5.4% | 5 | 13.5% | 2 | 5.4% | 5 | 13.5% |
| Bone | 13 | 35.1% | 6 | 16.2% | 1 | 2.7% | 4 | 10.8% |
| Skin | 0 | 0% | 3 | 8.1% | 0 | 0% | 0 | 0% |
| Visceral | 9 | 24.3% | 9 | 24.3% | 1 | 2.7% | 5 | 13.5% |
| Number of metastatic sites | ||||||||
| 1 | 8 | 21.6% | 2 | 5.4% | 1 | 2.7% | 3 | 8.1% |
| 2 | 5 | 13.5% | 4 | 10.8% | 0 | 0% | 3 | 8.1% |
| >3 | 3 | 8.1% | 5 | 13.5% | 1 | 2.7% | 2 | 5.4% |
CNS, central nervous system; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IHC, immunohistochemistry; TNBC, triple-negative breast cancer.
Three (1.7%) patients were BRCA-mutated, in 64 (37.2%) no mutation was found and in 105 (61%) it was unknown.
Regarding surgical treatment, 105 (66%) patients underwent mastectomy and 54 (34%) breast-conserving surgery (BCS). In stage I tumors, 19 (11.9%) were treated with mastectomy and 17 (10.7%) with BCS. In stage II, 24 (15.1%) patients underwent mastectomy and 20 (13.6%) BCS. In stage III, mastectomies were carried out in 56 patients (35.1%) and BCS in 26 (10.1%). In total, 67 (39%) patients received neoadjuvant CT: patients with tumors larger than 2 cm and/or positive lymph nodes, or TNBC (if more than 0.5 cm) or HER2-positive tumors (if more than 1 cm), and inflammatory BC. Twelve percent of those patients achieved pathological complete response. One hundred and forty-three (83.1%) patients were treated with any kind of adjuvant therapy [96 (67%) had CT, 101 (71%) had endocrine therapy and 33 (23%) anti-HER2 therapy]. Thirty-one (18%) were treated with endocrine therapy exclusively. Neoadjuvant, adjuvant and metastatic adopted treatments are listed in Table 1. Among those women with HR-positive disease, 84 (68.3%) underwent ovarian function suppression, 30 (24.4%) did not and in 9 (7.3%) patients this status was unknown. One hundred and twenty-four (72.1%) patients were also treated with RT.
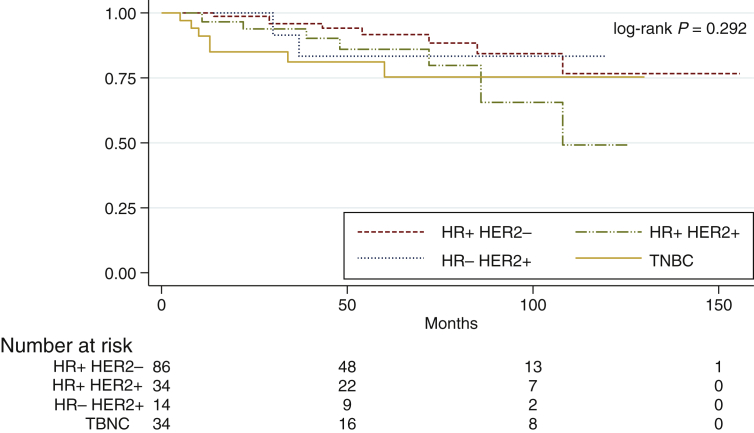
The entire cohort had an OS at 5 and 10 years of 86.5% [95% confidence interval (CI) 83.5-89.5] and 71.1% (95% CI 64.8-77.4), respectively, with a mean follow-up time of 64.9 ± 2.9 months (95% CI 59.1-70.6). Considering IHC subtypes, OS at 5 years in HR-positive/HER2 negative disease was 91.9% (95% CI 88.4-95.4), in HR-positive/HER2-positive patients it was 86.3% (95% CI 79.9-92.7) at 5 years, in HR-negative/HER2-positive patients it was 83.3% (95% CI 72.5-94.1) at 5 years and in TNBC patients OS at 5 years was 75.3% (95% CI 66.7-83.9), with no statistical significance (log-rank P = 0.292) (Figure 1).
Figure 1.
Overall survival according to IHC subtypes.
HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IHC, immunohistochemistry; TNBC, triple-negative breast cancer.
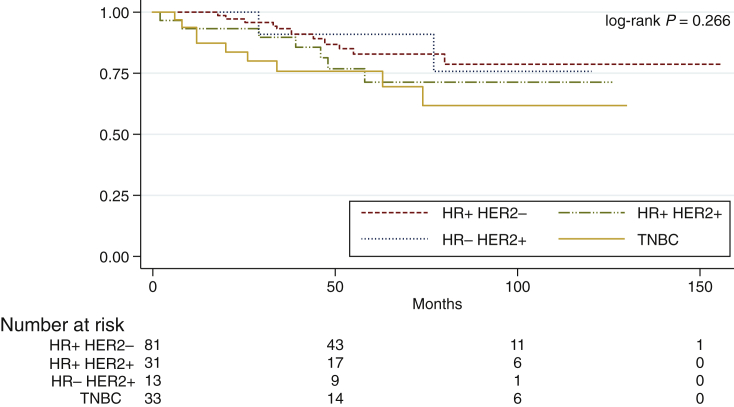
In the non-metastatic population, DFS at 5 and 10 years was 79.7% (95% CI 76-83.4) and 73.3% (95% CI 68.7-77.9), respectively, with a mean follow-up time of 64.6 months (95% CI 58.6-78.6). Patients with HR-positive/HER2-negative disease had a DFS at 5 years of 82.7% (95% CI 77.6-87.8), in HR-positive/HER2-positive patients it was 71.3% (95% CI 61.9-80.7), in HR-negative/HER2-positive it was 90.9% (95% CI 82.2-99.6) and in TNBC patients it was 75.8% (95% CI 67.6-83.9), with no statistical significance (log-rank P = 0.266) (Figure 2).
Figure 2.
Disease-free survival in non-metastatic patients according to IHC subtypes.
HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IHC, immunohistochemistry; TNBC, triple-negative breast cancer.
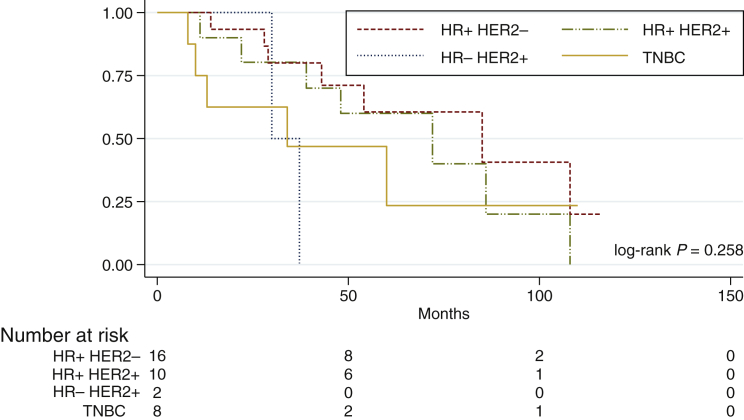
Women with metastatic disease (N = 37) had an OS (since metastatic disease diagnosis) at 5 years of 51.8% (95% CI 56.5-73.9) with a mean follow-up time of 72.7 ± 7.8 months (95% CI 57.4-88). Survival at 5 years of patients with metastatic disease was different between IHC subtypes [61% (95% CI 46.8-62.4) for HR-positive/HER2-negative versus 60% (95% CI 44.5-75.5) for HR-positive/HER2-positive versus 0% for HR-negative/HER2-positive versus 23.4% (95% CI 4.4-42-4) for TNBC], although it had no statistical significance (log-rank P = 0.258) (Figure 3). Median time from last palliative CT to death was 1.7 months.
Figure 3.
Overall survival in metastatic patients according to IHC subtypes.
HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IHC, immunohistochemistry; TNBC, triple-negative breast cancer.
Discussion
The choice of 35 years old was made considering the different cut-offs used in international literature. Indeed, there is heterogeneity of criteria for definition of younger age, with some publications referring to <30 years, <35 years, <40 years or even all premenopausal patients.1,8,17, 18, 19
Comparing with other cohorts including women <35 years old, our study included more patients in the same period of time (10 years) and had very similar population characteristics. Particularly, IHC subtype distributions were comparable, with approximately 70% of HR-positive disease, 30% of HER2-positive disease and 20% of TNBC.20, 21, 22 One study also had around 45% of poorly differentiated tumors, while the other two studies had higher percentages (60%-80%).20, 21, 22 Concerning metastatic patients at diagnosis, we found 11 (6%) in our study, which is exactly the same proportion found by Liukkonen et al.21 and slightly higher than that found in an Israeli cohort (4.7%).20,21 We had more patients receiving neoadjuvant CT (N = 67; 39%) than the other two studies (3%-22%), which might be due the period of analysis and clinical practices contemporary to the period of analysis.20,21 These two studies were carried out between 1997 and 2007 and 2000 and 2010. Regarding surgical treatment, our population had the same proportion of BCS (N = 54; 34%) and mastectomies (N = 105; 66%) than in the study by Liukkonen et al.21 (32% BCS and 66% mastectomies). The Israeli study had fewer mastectomies (40%) and more BCS (55%).20 OS at 5 years was approximately 85% in all of these studies.20,21 According to CONCORD-3, Portugal has the fifth best BC survival in Europe.23
TNBC and HER2-positive disease are more prevalent in younger patients than in older women.6 As expected, the main histological subtype was ductal invasive carcinoma and this pattern does not differ from general BC patients.24
BC in younger women is often diagnosed in more advanced stages of disease. The main reason for that is the lack of screening, which is not recommended in this age group, but also a longer delay in diagnosis is reported when compared with patients >40 years of age.25 Another possible reason might be the fact that these patients are only diagnosed when they have symptoms. Mammography is widely used to diagnose BC, but young women have dense breast parenchyma, which could reduce the sensitivity and accuracy of digital mammography.26 Also, there is no evidence of a mortality benefit from mammographic screening of women under the age of 35 years.6
Due to over-representation of more aggressive molecular subtypes and advanced stages of disease at diagnosis, an important number of women in our study developed metastasis (N = 26; 15%). HER2-positive disease was the subtype which developed more CNS metastasis, while luminal-type tumors were those which spread more frequently to bone, as expected. In proportion, the prevalence of IHC subtypes at metastasis is higher in HER2-positive disease (35%) and TNBC (22%). We also want to highlight the great amount of CNS metastatic involvement, which is higher than that described for all ages of BC patients, as we also saw in our previous work.27,28 In a previous retrospective analysis carried out in one of the hospitals included in this study, we found, from an overall population of 217 metastatic BC patients of all ages, 21 (9.7%) with brain metastasis, with a median age of 45 years old (minimum 28 and maximum 66), which is much less than what we found in this young population (37.8%). It should be highlighted that our previous work included a large number of metastatic elderly patients. Other studies found that the most consistent features for predicting CNS metastasis include estrogen receptor negativity and young age.29 In this analysis, the majority of patients who developed CNS metastasis had HER2-positive disease or TNBC.
Genetic factors are even more meaningful in younger patients, because lifestyle and environmental issues (lifetime exposure to endogenous and exogenous estrogen, tobacco use, dietary and lack of physical activity) usually only have impact in the development of malignancy after decades of exposure.30 Since this is a retrospective study, we only had access to BRCA status of 39% of our population and found a lower than expected percentage of BRCA mutation.31,32 This in an important limitation of our study, since it does not allow us to characterize possible genetic causes in our population. For further improvement, these patients should have BRCA mutation testing. This is relevant since this allows specific management and treatment decision-making that may improve survival, like pharmacologic interventions and risk-reducing surgeries (bilateral oophorectomy and mastectomy). Additionally, family members should also have genetic counseling.
It is known that age should not be the only reason for overtreatment and age alone should not determine intensity of treatment.33 Neoadjuvant CT was offered to 39% of patients. Twelve percent achieved complete pathological response and this number is similar to that found in literature (15%).34 Eighty-three percent of patients received adjuvant treatment and decision about adjuvant CT regimens followed local oncological centers practices. We want to highlight that the low percentage of ovarian function suppression might be due to the fact that positive results of ovarian suppression function in DFS for premenopausal women in SOFT and TEXT trials were only known since 2014.35,36 We also found that the median time from last palliative CT treatment to death was 1.7 months. This value could indicate that some patients might have received futile CT.37 When prescribing palliative antineoplastic treatment, physicians should always balance expected benefit with possible secondary effects and its impact in quality of life. Early identification of no benefit is crucial, as it translates into better care for these patients. Also, it is important to integrate supportive care, ideally in articulation with a dedicated supportive care team.
BC 5-year survival in women <35 years of age is between 75% and 80% and >35 years of age ranges from 80% to 86%.5 Including all patients, OS at 5 years was 87% and in HR-positive/HER2-negative disease patients (92%) it was better when compared with HER2-positive, either HR-positive (86%) or -negative (83%), and TNBC (75%), although with no statistical significance.
Besides the previously mentioned limitation of low prevalence of testing for germline genetic alterations, and since this is a retrospective study, we could not access sociodemographic characteristics of these patients, like educational degree, marital status, reproductive history, estrogen exposure, tobacco use and alcohol consumption. These are very important issues to be discussed with these patients, not only for treatment decision-making, but also during survivorship. BC in young women is becoming more prevalent and a major clinical issue. Cancer diagnosis and oncological treatment strongly impacts quality of life. Management of these patients needs a dedicated approach involving a multidisciplinary team. Besides antineoplastic treatment, there are survivorship issues that need to be taken into consideration. Health care professionals should address physical and psychological morbidity during and after antineoplastic treatments.38 These patients might experience modification of body image and sexual functioning, prematurely induced menopause and infertility. Other issues that also need to be taken into account are sustaining careers and parenting of young children.13,39,40 Furthermore, the risk of secondary malignancies needs to be addressed in these young patients. Further studies characterizing this population are needed to provide better care to these patients. Guidelines like ESO-ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women are measures carried out by experts that bring a great benefit by standardizing and spreading the best treatment than can be provided to these patients.9
It would be interesting to follow a group of such patients prospectively focusing mainly on lifestyle, therapeutics and survivorship issues.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Rossi L., Mazzara C., Pagani O. Diagnosis and treatment of breast cancer in young women. Curr Treat Options Oncol. 2019;20(12):86. doi: 10.1007/s11864-019-0685-7. [DOI] [PubMed] [Google Scholar]
- 2.Azim H.A., Jr., Partridge A.H. Biology of breast cancer in young women. Breast Cancer Res. 2014;16(4):427. doi: 10.1186/s13058-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.DGS . DGS; Lisboa, Portugal: 2017. Programa Nacional Para as Doenças Oncológicas; p. 11. [Google Scholar]
- 5.Anders C.K., Johnson R., Litton J. Breast cancer before age 40 years. Semin Oncol. 2009;36(3):237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gentilini O., Partridge A.H., Pagani O. Springer International Publishing; Switzerland: 2020. Breast Cancer in Young Women; pp. 1–33. [Google Scholar]
- 7.Konat-Baska K., Matkowski R., Blaszczyk J. Does breast cancer increasingly affect younger women? Int J Environ Res Public Health. 2020;17:4884–4894. doi: 10.3390/ijerph17134884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclere B., Molinie F., Tretarre B. Trends in incidence of breast cancer among women under 40 in seven European countries: a GRELL cooperative study. Cancer Epidemiol. 2013;37:544–549. doi: 10.1016/j.canep.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Paluch-Shimon S., Cardoso F., Partridge A. ESO-ESMO 4th international consensus guidelines for breast cancer in young women (BCY4) Ann Oncol. 2020;31(6):674–696. doi: 10.1016/j.annonc.2020.03.284. [DOI] [PubMed] [Google Scholar]
- 10.Duffy S., Vulkan D., Cuckle H. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21(9):1165–1172. doi: 10.1016/S1470-2045(20)30398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radecka B., Litwiniuk M. Breast cancer in young women. Ginekol Pol. 2016;87(9):659–663. doi: 10.5603/GP.2016.0062. [DOI] [PubMed] [Google Scholar]
- 12.Brenner D.R., Brockton N.T., Kotsopoulos J. Breast cancer survival among young women: a review of the role of modifiable lifestyle factors. Cancer Causes Control. 2016;27:459–472. doi: 10.1007/s10552-016-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman R.A., Partridge A.H. Management of breast cancer in very young women. Breast. 2013;22(2):S176–S179. doi: 10.1016/j.breast.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Zhong W., Tan L., Jiang W.G. Effect of younger age on survival outcomes in T1N0M0 breast cancer: a propensity score matching analysis. J Surg Oncol. 2019;119(8):1039e1046. doi: 10.1002/jso.25457. [DOI] [PubMed] [Google Scholar]
- 15.Partridge A., Hughes M., Warner E. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34:3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 16.Fu J., Zhong C., Wu L. Young patients with hormone receptor-positive breast cancer have a higher long-term risk of breast cancer specific death. J Breast Cancer. 2019;22:96e108. doi: 10.4048/jbc.2019.22.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkum N., Dermime S., Ajarim D. Being 40 or younger is an independent risk factor for relapse in operable breast cancer patients: the Saudi Arabia experience. BMC Cancer. 2007;7:222. doi: 10.1186/1471-2407-7-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makanjuola D., Alkushi A., Alzaid M. Breast cancer in women younger than 30 years: prevalence rate and imaging findings in a symptomatic population. Pan Afr Med J. 2014;19:35–44. doi: 10.11604/pamj.2014.19.35.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols H.B., Schoemaker M.J., Wright L.B. The premenopausal breast cancer collaboration: a pooling project of studies participating in the National Cancer Institute Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1360–1369. doi: 10.1158/1055-9965.EPI-17-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passhak M., Shachar S.S., Bar-Sela G. Breast cancer in young women age 35 and under: patterns of care and outcome. J Clin Oncol. 2014;32(15):e11534. doi: 10.1111/tbj.12966. [DOI] [PubMed] [Google Scholar]
- 21.Liukkonen S., Leidenius M., Saarto T. Breast cancer in very young women. Eur J Surg Oncol. 2011;37(12):1030–1037. doi: 10.1016/j.ejso.2011.08.133. [DOI] [PubMed] [Google Scholar]
- 22.Das U., Lakshmaiah K., Lokanatha D. Breast cancer in women of younger than 35 years: a single center study. J Mol Biomark Diag. 2015;6(6):1000261–1000265. [Google Scholar]
- 23.Allemani C., Matsuda T., Di Carlo V. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C.I., Uribe D.J., Daling J.R. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93(9):1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Partridge A.H., Hughes M.E., Ottensen R.A. The effect of age on delay in diagnosis and stage of breast cancer. Oncologist. 2012;17(6):775–782. doi: 10.1634/theoncologist.2011-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J., Lin Q., Cui C. Correlation between imaging features and molecular subtypes of breast cancer in young women (≤30 years old) Jpn J Radiol. 2020;38:1062–1074. doi: 10.1007/s11604-020-01001-8. [DOI] [PubMed] [Google Scholar]
- 27.Barnholtz-Sloan J.S., Sloan A.E., Davis F.G. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 28.Batista M.V., Tomás T.C., Eiriz I.F. Brain metastasis of breast cancer: a 10-year single institution retrospective analysis. Ann Oncol. 2019;30(suppl 3):P56. [abstract] [Google Scholar]
- 29.Palmieri D., Smith Q.R., Lockman P.R. Brain metastases of breast cancer. Breast Dis. 2006-2007;26:139–147. doi: 10.3233/bd-2007-26112. [DOI] [PubMed] [Google Scholar]
- 30.Pizot C., Boniol M., Mullie P. Physical activity, hormone replacement therapy and breast cancer risk: a meta-analysis of prospective studies. Eur J Cancer. 2016;52:138–154. doi: 10.1016/j.ejca.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 31.Peto J., Collins N., Barefoot R. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst. 1999;91(11):943–949. doi: 10.1093/jnci/91.11.943. [DOI] [PubMed] [Google Scholar]
- 32.Malone K.E., Daling J.R., Neal C. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer. 2000;88(6):1393–1402. doi: 10.1002/(sici)1097-0142(20000315)88:6<1393::aid-cncr17>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso F., Senkus E., Costa A. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chollet P., Amat S., Cure H. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer. 2002;86:1041–1046. doi: 10.1038/sj.bjc.6600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francis P.A., Regan M.M., Fleming G.F. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMc1502618. [DOI] [PubMed] [Google Scholar]
- 36.Pagani O., Regan M.M., Walley B.A. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braga S., Miranda A., Fonseca R. The aggressiveness of cancer care in the last three months of life: a retrospective single center analysis. Psychooncology. 2007;16:863–868. doi: 10.1002/pon.1140. [DOI] [PubMed] [Google Scholar]
- 38.Poggio F., Lambertini M., Bighin C. Management of young women with early breast cancer. ESMO Open. 2018;3:e000458. doi: 10.1136/esmoopen-2018-000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Partridge A.H., Ruddy K.J., Kennedy J. Model program to improve care for a unique cancer population: young women with breast cancer. J Oncol Pract. 2012;8(5):e105–e110. doi: 10.1200/JOP.2011.000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali A., Warner E. PYNK: breast cancer program for young women. Curr Oncol. 2013;20(1):e34–e39. doi: 10.3747/co.20.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]