Abstract
Background
There is no clinically applicable prognostic model designed for patients with de novo metastatic nasopharyngeal carcinoma (mNPC) treated with chemotherapy followed by locoregional radiotherapy (LRRT). We sought to develop a predictive tool of overall survival for individualized prediction and risk stratification in this heterogeneous patient population.
Patients and methods
A total of 244 eligible patients with de novo mNPC, who were treated with platinum-based first-line chemotherapy followed by LRRT, were included in this retrospective study. We divided patients into the training and validation sets based on the date of initial treatment, with 152 patients treated between 2008 and 2013 comprising the training set for model development and 92 patients treated at a later time (2014 to 2015) forming the validation set. We applied Cox proportional hazards model to examine factors associated with overall survival (OS). We developed and subsequently validated a prognostic model to predict OS. We assessed the performance of this prognostic model and stratified patients based on prognostic scores obtained from this proposed model.
Results
The median OS of the entire cohort was 60.9 months. C-creative protein, number of metastatic sites, liver metastasis, post-treatment Epstein–Barr virus DNA, and response of metastasis were significantly associated with OS. A prognostic model for individual survival prediction was developed and graphically represented as a nomogram. The model showed favorable discrimination (C-index: 0.759), predictive accuracy [time dependent area under the curve (tAUC) at 5 years: 0.800], and calibration, and was further validated in an independent dataset. A risk stratification derived from the model can stratify these patients into three prognostic subgroups with significantly different survival.
Conclusion
We developed and validated a prognostic model that exhibited adequate performance in individualized prediction and risk stratification for patients with de novo mNPC treated with chemotherapy followed by LRRT.
Key words: prognostic model, risk stratification, de novo metastatic nasopharyngeal carcinoma, locoregional radiotherapy
Highlights
-
•
We developed and validated a prognostic model for patients with de novo mNPC treated with chemotherapy followed by LRRT.
-
•
The prognostic model exhibited adequate performance in individualized prediction and risk stratification.
-
•
The prognostic model can stratify patients into three prognostic subgroups with distinctly different survival.
-
•
The prognostic model could be helpful in making individualized treatment recommendations in clinical practice.
Introduction
Nasopharyngeal carcinoma (NPC) is endemic in southern China, Southeast Asia, and North Africa, whereas its incidence is drastically lower in other regions. According to the Global Cancer Statistics 2018 released by the International Agency for Research on Cancer, approximately 130 000 new cases of NPC are diagnosed worldwide annually.1 The incidence of patients with de novo metastatic NPC (mNPC) at presentation accounts for roughly 4%-10% of diagnosed NPC cases.2, 3, 4 NPC is highly sensitive to chemoradiotherapy. Platinum-based chemotherapy is generally regarded as the primary treatment of patients with de novo metastatic NPC, with a response rate of 70%-80%.4, 5, 6 For selected patients with distant metastases at limited sites or with small tumor burden, locoregional radiotherapy (LRRT) to the primary tumor and nodal regions is recommended by the contemporary National Comprehensive Cancer Network (NCCN) guidelines.7
Recently, a randomized phase III clinical trial, for the first time, demonstrated that LRRT added to chemotherapy significantly improved overall survival (OS) in patients with de novo mNPC who showed a satisfactory response to chemotherapy.8 The study suggested that incorporating LRRT with chemotherapy could be an effective treatment modality for de novo mNPC. However, the results also indicated that the role of LRRT among the nonresponders to chemotherapy remains unclear. Previous studies have shown that benefit from treatment is heterogeneous across the entire spectrum of LRRT-treated patients.9, 10, 11, 12, 13, 14, 15, 16 Given that the optimal candidates for this treatment strategy remain undefined, patients with de novo mNPC must be appropriately stratified beforehand to avoid overtreatment.
Although a substantial portion of patients with de novo mNPC would receive LRRT after first-line chemotherapy in clinical practice, currently, there is no validated prognostic model designed explicitly for predicting the prognosis of these patients. There is an urgent unmet need to develop a clinically useful tool to predict the OS of this heterogeneous patient population. A helpful model would enhance face-to-face discussions with patients and assist in making treatment decisions. Therefore, the current study sought to develop and validate a predictive tool to make individualized predictions and facilitate personalized recommendations for LRRT in patients with de novo mNPC.
Methods
Study population
This retrospective study included consecutive patients treated with platinum-containing first-line chemotherapy followed by LRRT for de novo metastatic NPC between January 2008 and December 2015 at the Sun Yat-sen University Cancer Center. Inclusion criteria for the current study consisted of treatment-naïve histologically confirmed NPC with metastatic disease at the time of presentation, available TNM classifications, without previous or synchronous malignancies at other sites, platinum-based chemotherapy as first-line treatment at least for two cycles followed by LRRT, and measurable primary and metastatic lesions assessed and monitored at least at baseline and after first-line chemotherapy. The study population was divided into the training and validation sets aiming at a ratio of 2 : 1. Finally, 152 (62.3%) patients treated between 2008 and 2013 comprised the training set for model development, whereas 92 (37.7%) patients treated at a later time (2014 to 2015) were applied to validation. This study obtained approval from the Research Ethics Committee of Sun Yat-sen University Cancer Center (registration number 2020-FXY-277). Informed consent was waived due to the retrospective nature of this study and the anonymous processing of patient data. All personal data were anonymized before analysis.
Baseline evaluation and treatment
The routine baseline evaluation consisted of a detailed medical history, comprehensive physical examinations, hematological testing, biochemical profiling, plasma Epstein–Barr Virus (EBV) DNA titer, nasopharynx biopsy, abdominal ultrasonography, chest X-ray, emission computerized tomography (ECT) bone imaging, magnetic resonance imaging (MRI) of the nasopharynx and neck, MRI or computed tomography (CT) of metastatic sites, and/or positron emission tomography-CT (PET-CT) if applicable. All the patients received platinum-containing first-line chemotherapy. The chemotherapy regimens administrated in the current study included 5-fluorouracil and platinum (FP); gemcitabine and platinum (GP); taxane and platinum (TP); taxane, platinum, and 5-fluorouracil (TPF). All the patients received LRRT after first-line chemotherapy. Techniques of radiotherapy and protocol of target volume delineation utilized in this study have been described in previous studies.17 The details of treatment-related information are summarized in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2020.100004.
Objectives and variables of interest
The primary endpoint of this study was OS, which was measured from the date of initiation of first-line chemotherapy to the date of death as a result of any cause or last date of follow-up. The objective of this study was to develop and validate a prognostic model allowing patients with de novo mNPC to be stratified based on their survival expectations. In the current study, variables of interest were selected based on a review of the literature. All variables included in this study were supposed to be underlying prognostic factors or confounding factors involving demographic information, clinical characteristics, tumor features, and treatment-related factors.
Efficacy evaluation and follow-up
Therapeutic responses were assessed after completion of first-line chemotherapy, after completion of combined treatment, and every 3 months after that using EBV DNA titer and radiological examination. The plasma EBV DNA titer was measured using a quantitative polymerase chain reaction assay. Tumor response was measured according to the Response Evaluation Criteria in Solid Tumors guidelines (version 1.1) and evaluated by MRI for the primary tumor, and MRI, CT, ECT, or PET-CT for the metastatic lesions accordingly.
Statistical analysis
No prior sample size calculation was conducted due to a lack of evidence in building a prognostic model for stratifying patients. However, the number of events in this study reached 123, with an exceeding ratio of 10 events per variable in multiple modeling, suggesting sufficient power of estimation.18 Variables with missing values were C-reactive protein (CRP), baseline and post-treatment EBV DNA. The pattern of missing values in the dataset and the combinations of missing values were presented in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2020.100004. Continuous data were given as medians with interquartile ranges (IQR) and compared with the Wilcoxon rank-sum test. Categorical data were reported as frequencies with percentages and compared with the chi-square test, continuity corrected chi-square test, or Fisher's exact test, where appropriate. Survival curves were estimated using the Kaplan–Meier method and compared with the log-rank test. The Cox proportional hazards model was conducted to calculate the corresponding hazard ratios (HRs) and 95% confidence interval (CIs). The patients with missing values in the training dataset were excluded in the univariable analyses. If a variable met the predetermined significance threshold (P = 0.1) in univariable analysis, it would enter further multivariable Cox regression analysis. Variables with the missing data were imputed using multivariate imputation by chained equations algorithm before multiple modeling.19 Model selection in multiple modeling was based on the Akaike information criterion (AIC). A prognostic model was developed using independent risk factors identified in the training set and graphically presented as a nomogram. The discrimination, predictive accuracy, calibration, and clinical usefulness of the prognostic model were assessed using the concordance index (C-index), time-dependent area under curve (tAUC) at different time points, calibration plot, and decision curves analysis, respectively. The robustness of the model was confirmed via bootstrapping with 1000 resamples and validated in an independent validation set.
All statistical analyses were conducted using the R package (version 4.0.2). A P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
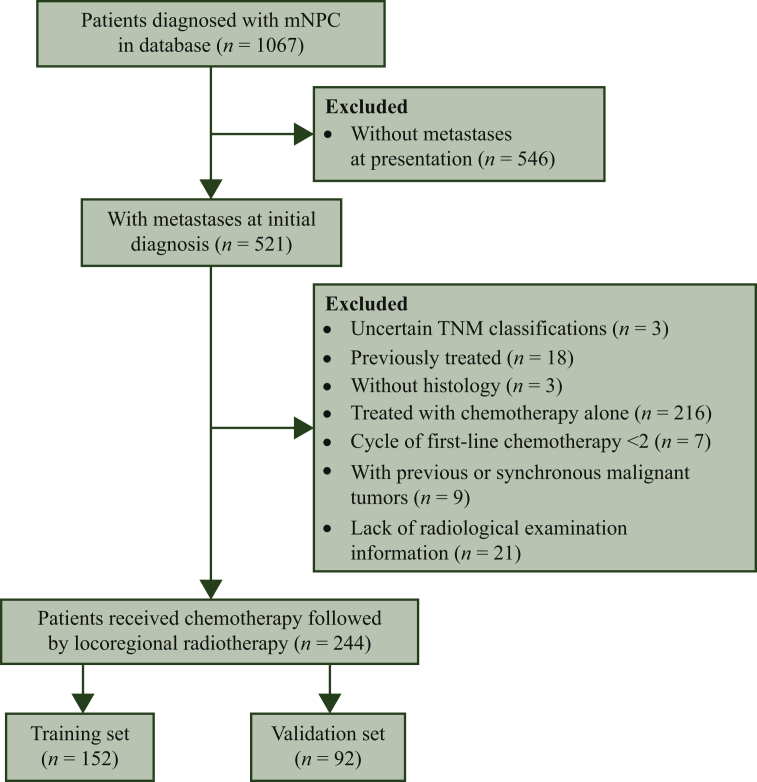
A total of 244 patients were included in final analyses and divided into the training (n = 152) and validation (n = 92) sets (Figure 1). A comparison of the patient characteristics in the training and validation sets is shown in Table 1. Although some significant differences existed between the groups, they were generally comparable. Among patients with available EBV DNA data, 205 (89.1%) patients had detectable EBV DNA at baseline evaluation, and 132 (60.8%) patients achieved EBV DNA clearance after the first-line chemotherapy. Among all the patients, 174 (71.3%) patients experienced radiological objective response after first-line chemotherapy, including 36 (16.1%) complete responses (CRs) and 138 (56.6%) partial response (PR). As for treatment details, 147 (60.2%) patients received greater than or equal to six cycles of first-line chemotherapy, 234 (95.9%) patients underwent radical radiotherapy dose (≥66 Gy), 131 (53.7%) patients received concurrent treatment, and 71 (29.1%) patients received local therapy to the metastatic lesion.
Figure 1.
Flow chart of study design.
mNPC, metastatic nasopharyngeal carcinoma; TNM, tumor, node, metastasis.
Table 1.
Comparison of patient characteristics in the training and validation sets
| Characteristics | Training set |
Validation set |
P value |
|---|---|---|---|
| n = 152 (% or interquartile) | n = 92 (% or interquartile) | ||
| Age (year) | 45.0 (39.0-53.0) | 46.0 (40.0-52.2) | 0.426 |
| Sex | 0.982 | ||
| Female | 20 (13.2) | 13 (14.1) | |
| Male | 132 (86.8) | 79 (85.9) | |
| Comorbidity | 0.410 | ||
| Absent | 100 (65.8) | 66 (71.7) | |
| Present | 52 (34.2) | 26 (28.3) | |
| KPS | <0.001 | ||
| <80 | 50 (32.9) | 9 (9.78) | |
| ≥80 | 102 (67.1) | 83 (90.2) | |
| Smoking | 0.050 | ||
| No | 87 (57.2) | 65 (70.7) | |
| Yes | 65 (42.8) | 27 (29.3) | |
| Drinking: | 0.197 | ||
| No | 135 (88.8) | 87 (94.6) | |
| Yes | 17 (11.2) | 5 (5.43) | |
| Body mass index (kg/m2) | 21.4 (19.0-23.9) | 21.4 (19.1-23.6) | 0.924 |
| Histology | 0.543 | ||
| Type II | 9 (5.92) | 3 (3.26) | |
| Type III | 143 (94.1) | 89 (96.7) | |
| T category | 0.139 | ||
| T1 | 6 (3.95) | 8 (8.70) | |
| T2 | 20 (13.2) | 7 (7.61) | |
| T3 | 77 (50.7) | 40 (43.5) | |
| T4 | 49 (32.2) | 37 (40.2) | |
| N category | 0.014 | ||
| N0 | 3 (1.97) | 3 (3.26) | |
| N1 | 28 (18.4) | 13 (14.1) | |
| N2 | 78 (51.3) | 32 (34.8) | |
| N4 | 43 (28.3) | 44 (47.8) | |
| No. of metastatic sites | 0.014 | ||
| Multiple | 26 (17.1) | 29 (31.5) | |
| Single | 126 (82.9) | 63 (68.5) | |
| Number of metastatic lesions | 1.000 | ||
| Multiple | 108 (71.1) | 65 (70.7) | |
| Single | 44 (28.9) | 27 (29.3) | |
| Liver metastasis | 0.794 | ||
| Absent | 124 (81.6) | 73 (79.3) | |
| Present | 28 (18.4) | 19 (20.7) | |
| Bone metastasis: | 0.727 | ||
| Absent | 45 (29.6) | 30 (32.6) | |
| Present | 107 (70.4) | 62 (67.4) | |
| Lung metastasis | 0.879 | ||
| Absent | 115 (75.7) | 68 (73.9) | |
| Present | 37 (24.3) | 24 (26.1) | |
| Pretreatment EBV DNA | 0.008 | ||
| Undetectable | 17 (11.2) | 8 (8.70) | |
| Detectable | 121 (79.6) | 84 (91.3) | |
| Missing | 14 (9.21) | 0 (0.00) | |
| Lactate dehydrogenase (U/l) | 194 (167-246) | 205 (171-248) | 0.214 |
| Alkaline phosphatase (U/l) | 75.0 (64.8-88.0) | 85.7 (69.8-101) | <0.001 |
| C-reactive protein (g/ml) | 3.71 (1.12-12.2) | 3.00 (1.38-8.12) | 0.724 |
| Albumin (g/l) | 44.0 (41.5-45.6) | 43.0 (40.9-45.1) | 0.145 |
| Neutrophil (109/l) | 4.50 (3.40-5.49) | 4.55 (3.60-5.80) | 0.395 |
| Hemoglobin (g/l) | 143 (133-151) | 142 (131-151) | 0.574 |
| Thrombocyte (109/l) | 245 (198-287) | 252 (219-300) | 0.096 |
| Chemotherapy regimen | 0.295 | ||
| GP | 6 (3.95) | 7 (7.61) | |
| FP | 45 (29.6) | 29 (31.5) | |
| TP | 44 (28.9) | 18 (19.6) | |
| TPF | 57 (37.5) | 38 (41.3) | |
| Cycle of first-line chemotherapy | 0.576 | ||
| <6 | 63 (41.4) | 34 (37.0) | |
| ≥6 | 89 (58.6) | 58 (63.0) | |
| Radiotherapy dose (Gy) | 1.000 | ||
| <66 | 6 (3.95) | 4 (4.35) | |
| ≧66 | 146 (96.1) | 88 (95.7) | |
| Concurrent treatment | 0.176 | ||
| No | 76 (50.0) | 37 (40.2) | |
| Yes | 76 (50.0) | 55 (59.8) | |
| Local treatment to metastasis | 0.615 | ||
| No | 110 (72.4) | 63 (68.5) | |
| Yes | 42 (27.6) | 29 (31.5) | |
| Post-treatment EBV DNA | <0.001 | ||
| Undetectable | 89 (58.6) | 43 (46.7) | |
| Detectable | 37 (24.3) | 48 (52.2) | |
| Missing | 26 (17.1) | 1 (1.09) | |
| Response of primary tumor | 0.112 | ||
| Complete response | 15 (9.87) | 18 (19.6) | |
| Partial response | 130 (85.5) | 68 (73.9) | |
| Stable disease | 5 (3.29) | 3 (3.26) | |
| Progression disease | 2 (1.32) | 3 (3.26) | |
| Response of metastasis | 0.479 | ||
| Complete response | 22 (14.5) | 14 (15.2) | |
| Partial response | 89 (58.6) | 49 (53.3) | |
| Stable disease | 36 (23.7) | 22 (23.9) | |
| Progression disease | 5 (3.29) | 7 (7.61) |
EBV DNA, Epstein–Barr virus DNA; FP, 5-fluorouracil and platinum; GP, gemcitabine and platinum; KPS, Karnofsky Performance Scale; TP, taxane and platinum; TPF, taxane, platinum and 5-fluorouracil.
Survival outcomes
The median follow-up was 62.7 (95% CI 58.9-72.2) months in the entire cohort. A total of 123 (50.4%) death events were observed (75 in the training set; 48 in the validation set). The median OS of the entire cohort was 60.9 (95% CI 44.5-83.8) months, with 1-year, 3-year, and 5-year OS probability being 91.7%, 63.5%, and 49.4%, respectively. No significant survival difference was detected between the training and validation sets [median OS 68.7 (95% CI 48.5-NA) months versus 43.1 (95% CI 36.8-NA) months, P = 0.072; Supplementary Figures S2A and B, available at https://doi.org/10.1016/j.esmoop.2020.100004].
Development of the prognostic model
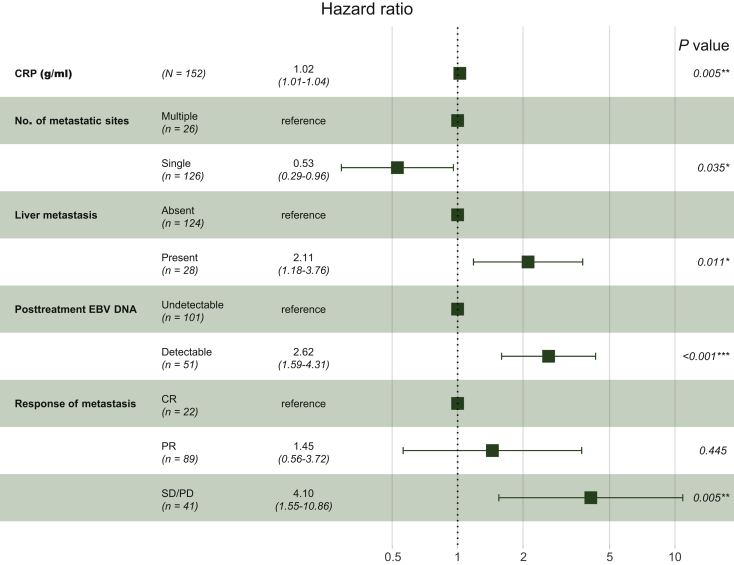
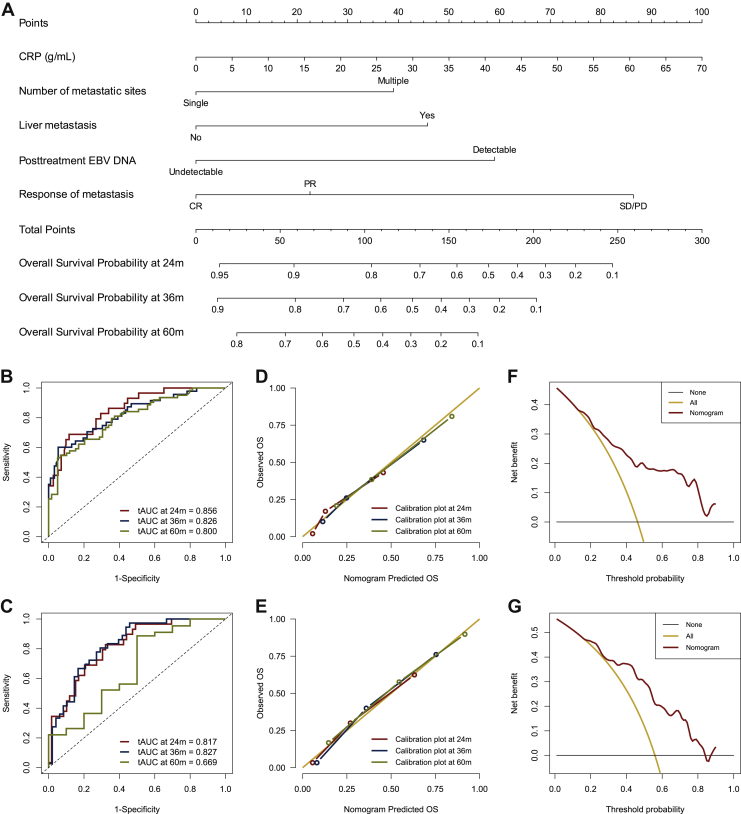
The results of univariate Cox regression models in the training set are summarized in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2020.100004. Variables that met the predetermined significance threshold (P = 0.1) in univariable analyses were entered into multivariable Cox regression analyses. Multicollinearity diagnostic tests, including pair-wise correlations, variance inflation factors plot, and Eigenvalues plot, suggested that severe multicollinearity issues would not exist (Supplementary Figures S3 and S4, available at https://doi.org/10.1016/j.esmoop.2020.100004). Proportional hazards diagnostic tests indicated that the proportional hazards assumption was fulfilled in the multivariable modeling (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2020.100004). In the multivariate stepwise Cox regression model, the model that had the lowest AIC of 631.4 was selected for final modeling. Results of the final multivariate Cox regression model revealed that CRP, number of metastatic sites, liver metastasis, post-treatment EBV DNA, and response of metastasis were significantly associated with OS (Figure 2). Based on the above five independent prognostic factors, a prognostic model for individual survival prediction was developed and graphically represented as an easy-to-use nomogram (Figure 3A). By using the nomogram, one can predict the OS probability of individual patients before the implementation of LRRT. The example of its use is specified in Supplementary Materials (Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2020.100004).
Figure 2.
Results of the final multivariate Cox regression model were presented in a forest plot.
AIC, Akaike information criteria; CR, complete response; CRP, C-reactive protein; EBV DNA, Epstein–Barr virus DNA; PR, partial response; SD/PD, stable disease/progression disease.
Figure 3.
Nomogram of the current model for individual survival prediction (A). Time independent AUC of the current model at 2-, 3-, and 5-year in the training set (B) and validation set (C). Calibration plot of the current model at 2-, 3-, and 5-year in the training set (D) and validation set (E). Decision curves analysis of the current model at 5 years in the training set (F) and validation set (G).
AUC, area under curve; CR, complete response; CRP, C-reactive protein; EBV DNA, Epstein–Barr virus DNA; OS, overall survival; PR, partial response; SD/PD, stable disease/progression disease; tAUC, time dependent AUC.
Assessment of the performance of prognostic model
The prognostic model yielded adequate discriminative ability and predictive accuracy. The C-index was 0.759 (95% CI 0.705-0.814) and 0.748 (95% CI 0.680-0.817) in the training and validation sets, respectively. Bootstrapping with 1000 resamples in the training set yielded similar discrimination, with a bias-corrected C-index of 0.761 (95% CI 0.706-0.816). The 2-year, 3-year, and 5-year tAUCs of the prognostic model in the training set were 0.856, 0.826, and 0.800, respectively (Figure 3B); and respective tAUCs in the validation set were 0.817, 0.827, and 0.699, respectively (Figure 3C). Calibration curves in the training and validation sets showed good agreement between predicted survival and actual survival (Figures 3D and E). Decision curves analysis demonstrated that the prognostic model had favorable clinical usefulness. The proposed model conferred more net benefits compared with both the treat-all-patients scheme and the treat-none scheme in the training and validation sets (Figures 3F and G).
Risk stratification
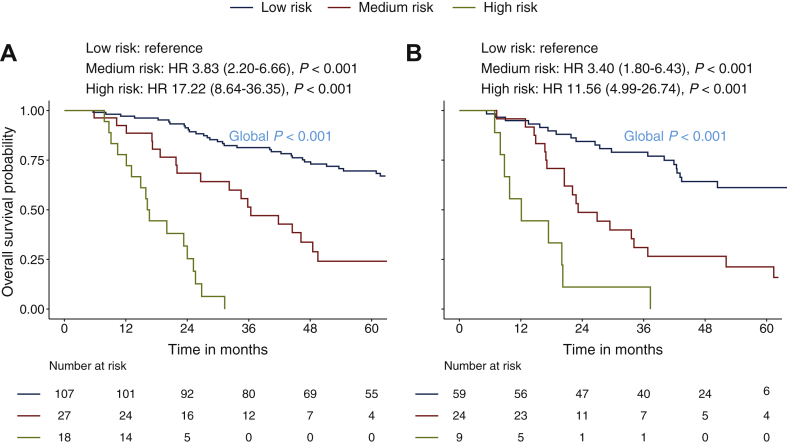
In the current study, the cut-off values used are defined based on probability points that divide the predicted 3-year OS rate into three segments of probability using the entire dataset: <25.0%, 25.0%-49.9%, ≥50.0%. These cut-off values approximately fit the nomogram scores of 180 and 127 that have been applied accordingly as the reference to define the risk stratifications: low risk (<127 points), medium risk (127-180 points), high risk (>180 points). The comparison of patient characteristics stratified by three risk subgroups is summarized in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2020.100004. The patients in higher levels of risk subgroups were significantly associated with unfavorable prognostic factors. The 3-year OS rate of low-, medium-, and high-risk patients in the training set was 81.3%, 51.3%, and 0% respectively. With low-risk patients being the reference, the HRs for medium- and high-risk patients were 3.83 (95% CI 2.20-6.66; P < 0.001) and 17.22 (95% CI 8.64-36.35; P < 0.001), respectively. In the validation set, the 3-year OS rate of these three strata was 79.1%, 31.0%, and 11.1%, respectively. With stratum one as reference, the HRs for strata two and three were 3.40 (95% CI 1.80-6.43; P < 0.001) and 11.56 (95% CI 4.99-26.74; P < 0.001), respectively. Survival curves stratified based on the three strata showed significant survival differences both in the training and validation sets (both with global log-rank P < 0.001, Figures 4A and B).
Figure 4.
Kaplan–Meier curves stratified based on the risk stratification in the training set (A) and validation set (B): low risk (<127 points), medium risk (127-180 points), high risk (>180 points).
HR, hazard ratio.
Discussion
To our knowledge, this is the first study to investigate a robust prognostic tool to generate individualized predictions and risk stratifications regarding the prognosis of patients with de novo mNPC who were treated with chemotherapy followed by LRRT. We developed and validated a prognostic model based on conventional clinical and post-chemotherapy response indices. The prognostic model was represented as an easy-to-use nomogram and can predict individual survival with good performance. Based on the scores derived from the nomogram, we defined a risk stratification that stratified patients into three prognostic subgroups with significantly different survival. Given that there is no specific model designed for stratifying ideal LRRT candidates in patients with de novo mNPC, the current prognostic model could serve as a clinically useful tool to make individualized treatment recommendations in this heterogeneous patient population.
NPC is sensitive to chemotherapy. Platinum-based systemic chemotherapy is considered the mainstay treatment of patients with de novo mNPC. The large phase III trial NCT01528618 conducted by Zhang et al. revealed that the GP regimen was superior to the FP regimen, which led to the recommendation for it to be used as the gold standard for patients with recurrent or metastatic NPC. In our study, 13 (5.3%) patients received a GP regimen, whereas 74 (30.3%) patients received an FP regimen. The relatively low rate of GP regimen used in this study could be that the study population included were treated between 2008 and 2015 when the GP regimen was not a mainstream proposal in our institution. We did not observe a statistically significant difference concerning OS between patients who received different chemotherapy regimens in univariable analysis. However, we found that patients who received the GP regimen had the highest objective response rate (76.9%), whereas patients who received the FP regimen had the lowest objective response rate (67.6%). The result was in line with trial NCT01528618 in one way or another. But we should note that the study population in our study was quite different from this trial. The primary eligibility criteria of trial NCT01528618 were that the patient had metastatic disease after curative radiotherapy or local recurrence after curative radiotherapy, which was unsuitable for local treatment or was primarily metastatic. Also, the trial excluded patients if they were suitable for local treatment. On the contrary, patients included in our study were all diagnosed with primarily metastatic NPC and treated with LRRT, which means that most patients in our study would not meet the eligibility criteria of trial NCT01528618. Nevertheless, the GP regimen with a relatively higher objective response rate did not result in a superior OS compared with the FP regimen in our study. A potential explanation was that the subsequent LRRT might dilute the superiority of the GP regimen. Other reasons included observed bias and insufficient sample size.
NPC is also sensitive to radiotherapy. Palliative chemotherapy can lead to a median OS of approximately 10-15 months in patients with de novo mNPC, which can be further improved if the patient is suitable for combined therapy such as LRRT to the primary tumor and nodal regions and/or local treatment to the metastatic lesions.5,9, 10, 11, 12, 13, 14, 15, 16 A recent phase III randomized clinical trial reported the efficacy of definitive LRRT in addition to palliative chemotherapy in patients with de novo mNPC.8 The study demonstrated that high-dose LRRT to the primary and nodal regions resulted in a significant survival benefit in a highly selected subgroup of patients with de novo mNPC, who had shown satisfactory response to first-line chemotherapy. The results indicate chemotherapy followed by high-dose LRRT could be a promising treatment strategy and should be considered for chemotherapy-sensitive patients with de novo mNPC. Our results here are consistent with the ones included in the recent report. The response of metastatic lesions to chemotherapy was also identified as a significant independent prognostic factor: responders to first-line chemotherapy had a more favorable prognosis.
Although published series conducted in locoregionally advanced NPC have indicated that concurrent chemoradiotherapy (CCRT) is associated with a survival benefit, the optimal cumulative cisplatin dose (CCD) during CCRT remains controversial, especially for patients who receive induction chemotherapy (IC) plus CCRT. Previous studies showed that the CCD of CCRT after IC was not an independent prognostic factor for OS.20, 21, 22, 23, 24 However, a dose of CCD ≥200 mg/m2 had the trend to prolong OS benefit in those with an unfavorable response to IC. In our study, we did not observe a statistically significant difference in OS between de novo mNPC patients treated with radiotherapy alone versus concomitant chemoradiotherapy after first-line chemotherapy. However, the objective response rate (ORR) of the primary tumor for these patients at the end of first-line chemotherapy was as high as 94.7% (231 out of 244 patients). The nonresponders were uncommon, including 8 (3.3%) patients with stable disease and 5 (2.1%) patients with progressive disease. Besides, 229 (93.9%) patients had received more than four cycles of first-line chemotherapy before LRRT, which meant that the CCD in these patients was considerable. Therefore, we suppose the high ORR and CCD might explain the non-significant difference between patients treated with radiotherapy alone versus concomitant chemoradiotherapy.
Other known risk factors reported in previous studies,9, 10, 11,16,25, 26, 27 including liver metastasis and the number of metastatic sites, were also found to be significant prognostic factors in this study. As anatomy-based factors are insufficient for assessing prognosis or treatment benefits in patients with mNPC, many studies have investigated whether prognostic biomarkers would better predict survival. Our previous work has demonstrated that elevated baseline CRP level was associated with unfavorable clinicopathologic characteristics and helped to predict prognosis in patients with mNPC.28 Beyond pretreatment CRP, unfavorable EBV DNA response after palliative chemotherapy is also an adverse prognosticator for survival outcomes.9,29, 30, 31 Our results provided additional support for the previous finding. In the current study, several adverse prognostic biomarkers, including baseline CRP and post-treatment EBV DNA titer, were closely related to the prognosis of patients with de novo mNPC being treated with chemotherapy followed by LLRT.
In the past two decades, the treatment of patients with de novo mNPC has witnessed great progress, with a shift toward personalized treatment. However, the optimal treatment strategy is not yet well established for de novo mNPC.4,12,32,33 The concept of aggressive treatment to the primary tumor and nodal regions is also evolving. LRRT has now been listed as a treatment option for a subgroup of patients with de novo mNPC in the contemporary NCCN guidelines. In the absence of prospective data to clarify the optimal LRRT candidates, using prognostic models rather than depending on the physician's experience may help in refining case selection and avoiding futile LRRT. The prognostic models based on clinical characteristics, biomarkers, or treatment response can be applied to better stratify patients into different prognostic subgroups.4 Therefore, we created a validated prognostic model for individual survival prediction in patients with de novo mNPC. This model is based on an optimized selection of conventional clinical (CRP, liver metastasis, and the number of metastatic sites) and post-chemotherapy response (response of metastasis and post-treatment EBV DNA) indices. These indices can be easily collected before LRRT, thus affecting clinical risk stratification and subsequent treatment decision. The risk stratification derived from the prognostic model allows patients to be easily classified into three prognostic strata with heterogeneous survival. Beyond prognostication, the risk stratification also has the potential to identify which individuals are likely to benefit from LRRT, which makes up another dimension of its clinical utility. In the current study, low-risk patients had significantly longer survival than medium- or high-risk patients treated with chemotherapy followed with LRRT, which might represent the optimal LRRT candidates. On the contrary, patients stratified into the high-risk subgroup showed unfavorable survival after LRRT. These individuals are unlikely to benefit from LRRT because of quick tumor progression and should be referred for sequential consolidation chemotherapy or targeted therapies. As for the patients with medium risk, whether the benefit of LRRT outweighs the potential radiotherapy-related toxicities should be considered in order to achieve better cost-effectiveness.
The study has some inevitable limitations that should be noted. The first limitation is that potential selection bias from heterogeneous study population and incomplete data collection were unavoidable because of the nature of the retrospective study. However, the risk of selection bias has been minimized by recruiting all eligible consecutive patients and a large cohort of de novo mNPC candidates of LRRT to date. The second limitation is that no external validation of the prognostic model was conducted due to our inability to obtain high-quality data from other centers. Further multicentric external validation would be necessary and strengthen our findings. The third limitation is that NPC in endemic regions is mainly EBV-related and nonkeratinizing whereas keratinizing subtype is more frequently reported in nonendemic areas. Given this limitation, the generalization of the proposed model as a robust prognostic tool in other settings warrants further validation.
In conclusion, we developed and validated a prognostic model that exhibited adequate performance in individualized prediction and risk stratification for patients with de novo mNPC treated with chemotherapy followed by LRRT. The proposed model is capable of stratifying patients into three prognostic subgroups with distinctly different survival. This prognostic tool has the potential to provide patients with more realistic expectations of survival when considering the initiation of LRRT and could be helpful in making individualized treatment recommendations in clinical practice. Further validations in patients from other centers and nonendemic regions is warranted.
Acknowledgments
Funding
This study was supported by the National Natural Science Foundation of China [grant number 81672680 and 81802712], the Guangdong Medical Research Foundation [grant number A2017492], and the Young Teacher Training Project of Sun Yat-Sen University [grant number 19ykpy188].
Disclosure
The authors declare no conflicts of interest.
Ethics approval
The study protocol was approved by the institutional review board of Sun Yat-Sen University Cancer Center.
Data sharing
The authenticity of this article has been validated by uploading the key raw data on to the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number, RDDA2020001681.
Contributor Information
W.-X. Xia, Email: xiawx@sysucc.org.cn.
Y.-Q. Xiang, Email: xiangyq@sysucc.org.cn.
Supplementary data
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Lee A.W.M., Ng W.T., Chan L.K. The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol. 2012;48:1007–1013. doi: 10.1016/j.oraloncology.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Pan J.J., Ng W.T., Zong J.F. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122:546–558. doi: 10.1002/cncr.29795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y.-P., Chan A.T.C., Le Q.-T., Blanchard P., Sun Y., Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 5.Bensouda Y., Kaikani W., Ahbeddou N. Treatment for metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:79–85. doi: 10.1016/j.anorl.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L., Huang Y., Hong S. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388:1883–1892. doi: 10.1016/S0140-6736(16)31388-5. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network National Comprehensive Cancer Network Head and Neck Cancers, version 2.2020 — 9 June 2020. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf Available at: Accessed October 26, 2020. [DOI] [PubMed]
- 8.You R., Liu Y.P., Huang P.Y. Efficacy and safety of locoregional radiotherapy with chemotherapy vs chemotherapy alone in de novo metastatic nasopharyngeal carcinoma: a multicenter phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1345–1352. doi: 10.1001/jamaoncol.2020.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X.S., Liu L.T., Liu S.L. Identifying optimal candidates for local treatment of the primary tumor among patients with de novo metastatic nasopharyngeal carcinoma: a retrospective cohort study based on Epstein-Barr virus DNA level and tumor response to palliative chemotherapy. BMC Cancer. 2019;19:92. doi: 10.1186/s12885-019-5281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu S-x, He X-h, Dong M. Systemic chemotherapy followed by locoregional definitive intensity-modulated radiation therapy yields prolonged survival in nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis. Med Oncol. 2015;32:224. doi: 10.1007/s12032-015-0663-2. [DOI] [PubMed] [Google Scholar]
- 11.Zeng L., Tian Y.M., Huang Y. Retrospective analysis of 234 nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis: therapeutic approaches and prognostic factors. PLoS One. 2014;9:e108070. doi: 10.1371/journal.pone.0108070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rusthoven C.G., Lanning R.M., Jones B.L. Metastatic nasopharyngeal carcinoma: patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother Oncol. 2017;124:139–146. doi: 10.1016/j.radonc.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Fandi A., Bachouchi M., Azli N. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol. 2000;8:1324–1330. doi: 10.1200/JCO.2000.18.6.1324. [DOI] [PubMed] [Google Scholar]
- 14.Hui E.P., Leung S.F., Au J.S. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer. 2004;101:300–306. doi: 10.1002/cncr.20358. [DOI] [PubMed] [Google Scholar]
- 15.Chen M.Y., Jiang R., Guo L. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32:604–613. doi: 10.5732/cjc.013.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S., Tham I.W., Pan J., Han L., Chen Q., Lu J.J. Combined high-dose radiation therapy and systemic chemotherapy improves survival in patients with newly diagnosed metastatic nasopharyngeal cancer. Am J Clin Oncol. 2012;35:474–479. doi: 10.1097/COC.0b013e31821a9452. [DOI] [PubMed] [Google Scholar]
- 17.Lai S., Li W., Chen L. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Peduzzi P., Concato J., Feinstein A., Holford T. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 19.van Buuren S., Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(2011):67. [Google Scholar]
- 20.Lv J., Qi Z., Zhou G. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients receiving additional induction chemotherapy. Cancer Sci. 2018;109:751–763. doi: 10.1111/cas.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S., Sun X., Yan J. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients based on induction chemotherapy response. Radiother Oncol. 2019;137:83–94. doi: 10.1016/j.radonc.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Liu S., Sun X., Liu L. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients based on plasma Epstein-Barr virus DNA level after induction chemotherapy. Aging. 2020;12:4931–4944. doi: 10.18632/aging.102920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng L., Chen J., Zhu G. Treatment effects of cumulative cisplatin dose during radiotherapy following induction chemotherapy in nasopharyngeal carcinoma: propensity score analyses. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920937424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen D., Li Z., Chen F. Individualized cumulative cisplatin dose for locoregionally-advanced nasopharyngeal carcinoma patients receiving induction chemotherapy and concurrent chemoradiotherapy. Oral Oncol. 2020;107 doi: 10.1016/j.oraloncology.2020.104675. [DOI] [PubMed] [Google Scholar]
- 25.Ong Y.K., Heng D.M., Chung B. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer. 2003;39:1535–1541. doi: 10.1016/s0959-8049(03)00310-1. [DOI] [PubMed] [Google Scholar]
- 26.Cao X., Han Y., He L., Xiang J., Wen Z. Risk subset of the survival for nasopharyngeal carcinoma patients with bone metastases: who will benefit from combined treatment? Oral Oncol. 2011;47:747–752. doi: 10.1016/j.oraloncology.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Pan C.-C., Lu J.I.N., Yu J.-R. Challenges in the modification of the M1 stage of the TNM staging system for nasopharyngeal carcinoma: a study of 1027 cases and review of the literature. Exp Ther Med. 2012;4:334–338. doi: 10.3892/etm.2012.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia W.X., Ye Y.F., Lu X. The impact of baseline serum C-reactive protein and C-reactive protein kinetics on the prognosis of metastatic nasopharyngeal carcinoma patients treated with palliative chemotherapy. PLoS One. 2013;8:e76958. doi: 10.1371/journal.pone.0076958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W.Y., Twu C.W., Chen H.H. Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res. 2010;16:1016–1024. doi: 10.1158/1078-0432.CCR-09-2796. [DOI] [PubMed] [Google Scholar]
- 30.Hsu C.L., Chang K.P., Lin C.Y. Plasma Epstein-Barr virus DNA concentration and clearance rate as novel prognostic factors for metastatic nasopharyngeal carcinoma. Head Neck. 2012;34:1064–1070. doi: 10.1002/hed.21890. [DOI] [PubMed] [Google Scholar]
- 31.An X., Wang F.-H., Ding P.-R. Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer. 2011;117:3750–3757. doi: 10.1002/cncr.25932. [DOI] [PubMed] [Google Scholar]
- 32.Lee A.W., Ng W.T., Chan Y.H., Sze H., Chan C., Lam T.H. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104:272–278. doi: 10.1016/j.radonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Chan O.S., Ngan R.K. Individualized treatment in stage IVC nasopharyngeal carcinoma. Oral Oncol. 2014;50:791–797. doi: 10.1016/j.oraloncology.2014.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.