Highlights
-
•
7 patients were implanted with lung-specific electromagnetic transponders (EMT).
-
•
We report no complications from implantation and no migration of the EMT.
-
•
7 non-small cell lung cancer patients underwent SBRT using EMT real-time tracking.
-
•
SBRT was delivered in free-breathing (FB) or in deep inspiration breath-hold (DIBH).
Keywords: Lung SBRT, Real-time tracking, Electromagnetic transponder, Intra-fraction motion, Deep inspiration breath-hold
Abstract
Background and purposes
Motion management is crucial for optimal stereotactic body radiotherapy (SBRT) of moving targets. We aimed to describe our clinical experience with real-time tracking of lung-specific electromagnetic transponders (EMTs) for SBRT of early stage non-small cell lung cancer in free-breathing (FB) or deep inspiration breath-hold (DIBH).
Material and methods
Seven patients were implanted with EMTs. Simulation for SBRT was performed in FB and in DIBH. We prescribed 60 Gy in 3, 5 or 8 fractions to the tumor and delivered SBRT with volumetric modulated arcs and a 6 MV flattening filter free photon beam. Patients’ setup at the linac was performed using EMT positions and cone-beam CT (CBCT) verification. Four patients were treated in DIBH because of a dosimetric benefit. We analysed patient alignment and treatment delivery parameters using DIBH or FB and EMT real-time tracking.
Results
There were no complications from the EMT implantation. Visual inspection of CBCT before and/or after SBRT revealed good alignment of structures and EMTs. The median setup time was 9.8 min (range: 4.6–34.1 min) and the median session time was 14.7 min (range: 7.3–36.5 min). EMT positions in lungs remained stable during overall treatment and allowed real-time tracking both in FB and in DIBH SBRT. The treatment beam was gated when EMT centroid position exceeded tolerance thresholds ensuring correct delivery of radiation to the tumor.
Conclusion
Using EMTs for real-time tracking of tumor motion during lung SBRT proved to be safe, accurate and easy to integrate clinically for treatments in FB or DIBH.
1. Introduction
Optimal treatment of small moving targets remains a challenge in stereotactic body radiotherapy (SBRT). Lung tumors, in particular, can move up to 30 mm [1], [2] and their motion can be unpredictable [3], [4]. In order to avoid geometrical misses, large safety margins are often added, compromising the delivery of a high dose to the target and healthy tissue sparing.
Target margin reduction could be achieved by using breath-hold techniques or other mitigation strategy which preferably requires monitoring tumor motion during treatment. A pitfall of using an external surrogate for internal motion is that the external sensor does not always correlate with tumor position and motion [5], [6]. This can be overcome by implanting radiopaque fiducials and/or performing imaging during treatment [7], [8], at the cost of additional radiation dose for the patient, expect in the case where magnetic resonance imaging is used [9], [10].
The electromagnetic localization and tracking system used in this study allows a real-time monitoring of electromagnetic transponders (EMTs) implanted in (or close to) the tumor, without adding imaging radiation dose. The EMTs are detected periodically (update rate of 24 Hz) by an electromagnetic array positioned above the patient. The EMT tracking system can be interfaced with the linac to trigger beam-holds when EMT centroid motion beyond pre-defined tolerance thresholds is detected.
In this paper, we aim to describe the clinical experience in our institution with lung SBRT using implanted EMTs for real-time tracking in free-breathing (FB) or deep inspiration breath-hold (DIBH).
2. Material and methods
2.1. Patients implantation
After providing informed consent, seven patients underwent diagnostic bronchoscopy for an inoperable suspicious pulmonary lesion (Table 1). During bronchoscopy, two to three lung-specific EMTs anchored beacons (Calypso®, Varian Medical Systems, USA) were implanted into small peripheral bronchi close to the tumor. These beacons were 16 mm long and had a 2 mm diameter. They were equipped with five anchors which deployed when inserted in small sized bronchi. The implant procedure was performed under general anesthesia by a pneumologist using the SuperDimension™ Navigation System (Medtronic, USA) enabling Electromagnetic Navigation Bronchoscopy™ procedures [11], [12], [13], [14]. The total procedure took approximately 30 min, including onsite histopathological diagnosis.
Table 1.
Geometric, setup and treatment characteristics of the seven patients treated with EMT real-time tracking.
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | |
|---|---|---|---|---|---|---|---|
| Tumor location | Apical segment of upper left lobe | Lingular segment of upper left lobe | Apical segment of lower left lobe | Overlapping anterior segment of upper left lobe and superior segment lower left lobe | Lower right lobe | Upper right lobe | Upper left lobe |
| PTV volume (cm3) | 11.5 | 9.1 | 24.7 | 77.4 | 11.5 | 7.1 | 13.2 |
| Treatment modality | FB | DIBH | FB | FB | DIBH | DIBH | DIBH |
| Days between EMT implantation and simulation CT | 15 | 9 | 32 | 8 | 7 | 7 | 41 |
| Days between simulation CT and 1st treatment | 7 | 11 | 15 | 13 | 28 | 14 | 21 |
| Prescribed dose | 8 × 7.5 Gy | 8 × 7.5 Gy | 5 × 12 Gy | 8 × 7.5 Gy | 3 × 20 Gy | 3 × 20 Gy | 5 × 12 Gy |
| Overall treatment time (days) | 17 | 19 | 10 | 21 | 7 | 8 | 14 |
| Tumor-EMT centroid distance (mm) | 4 | 13 | 18 | 10 | 11 | 15 | 17 |
| Inter-EMTs distances on planning CT (mm) | 12 | 39/45/38 | 9/60/57 | 49/54/58 | 31/26/20 | 46/46/37 | 33/32/61 |
| GTV centroid motion amplitude on 4DCT (LR/SI/AP) (mm) | <1/<1/<1 | 2/4/3 | 2/6/2 | 1/4/2 | 2/8/4 | 1/4/6 | 2/2/2 |
| Median setup time [range] (min) | 8.0 [4.6–9.9] | 11.0 [9.3–18.1] | 10.5 [5.3–14.6] | 8.7 [4.9–34.1] | 10.5 [9.4–13.0] | 19.5 [8.6–22.4] | 13.1 [11.5–13.5] |
| Median inter-EMTs distance differences [range] (mm) | 0.1 [0.0–0.2] | 1.4 [0.0–3.7] | 1.7 [0.7–4.3] | 1.8 [1.3–3.3] | 0.6 [0.3–1.4] | 2.7 [0.6–4.5] | 1.0 [0.1–1.9] |
| Median geometrical residual [range] (mm) | 0.1 [0.0–0.1] | 0.9 [0.6–1.7] | 1.3 [0.8–2.0] | 1.3 [0.8–1.7] | 0.4 [0.4–0.6] | 1.9 [1.7–2.0] | 0.8 [0.7–1.0] |
| Median session time [range] (min) | 10.3 [7.3–12.0] | 17.1 [12.4–30.0] | 16.4 [10.0–20.3] | 15.3 [7.6–36.5] | 13.0 [12.8–28.1] | 33.5 [25.5–39.3] | 22.1 [21.5–23.4] |
| Median number of beam holds per fraction [range] | 0 [0–1] | 4.5 [0–14] | 20.5 [2–36] | 2 [0–29] | 12 [9–18] | 6 [5–11] | 6 [4–8] |
Abbreviations: PTV: planning target volume, EMT: electromagnetic transponder, FB: free-breathing, DIBH: deep inspiration breath-hold, LR: left–right, SI: supero-inferior, AP: antero-posterior, P#: patient #.
2.2. Simulation for SBRT
At least one week after EMT implantation (Table 1), two simulation CTs for SBRT planning were acquired for all patients in FB: a 4DCT-scan and a 3DCT (both with 1 mm slice thickness). A DIBH CT with a 1 mm slice thickness was also performed for patients who were compliant with this technique. Patients were simulated supine on an immobilization device compatible with the Calypso tracking system (Qfix, Avondale, USA) and a custom made pillow. Delineation of target volumes and organs at risk (OAR) was performed in the contouring module of the Eclipse™ treatment planning system (v13.6, Varian Medical Systems). The quality of EMT implantation was evaluated by analyzing their coordinates with respect to the tumor and the distance separating individual EMT on the planning CT. The EMT positions were judged adequate if they were non-coplanar on axial CT sections, separated from each other by at least 10 mm, and by 70 mm at most, and if the distance between the EMT centroid position and the tumor centroid was small enough for acceptable representation of tumour motion [15], [16].
2.3. Treatment planning
An internal target volume (ITV) was generated from the 4DCT-scan by contouring the gross tumor volume (GTV) on each phase of the 4DCT. A planning target volume (PTV) was created adding an isotropic margin of 5 mm to the ITV, or to the GTV of the DIBH scan, (i.e. PTVFB = ITV + 5 mm and PTVDIBH = GTV + 5 mm). We required 98% of the PTV to be covered by 95% of the prescribed dose (60 Gy in 3, 5 or 8 fractions depending on the proximity to the chest wall and pulmonary hilium, 2–3 times a week, Table1). Treatments were planned on the FB CT and, when available, also on the DIBH CT. The treatment isocentre was set in the proximity of the EMT centroid position. The EMTs were localized on the planning CT and their individual center-of-mass coordinates relative to the treatment isocentre were transferred to the Calypso console (Calypso® 4D localization system™, version 3). For patients treated in FB, we checked that there was a correlation between EMTs and GTV centroid motion. This was performed by contouring the EMTs on each phase of the 4DCT, and then by comparing EMT centroid motion to the GTV motion on each phase. EMTs could then be used as a surrogate for GTV motion. Treatments were delivered with a 6 MV flattening filter free photon beam using two half coplanar volumetric modulated arcs, expect for patient 1 (P1) for whom two full arcs were used due to a centrally located target. For the patients who had a DIBH CT in addition to a FB CT, a dosimetric comparison was performed to evaluated difference in terms of PTV volumes and dose reduction to the OAR.
2.4. Patients setup and imaging at linac
A dry run session at the linac (TrueBeam, Varian Medical systems) was performed to check for correct Calypso tracking system and cone-beam CT (CBCT) EMT position detection for our first two patients (P1, treated in FB, and P2, treated in DIBH). Patient setup at the linac was performed by aligning the absolute position of the EMT centroid for DIBH treatment, and the mean position of the EMT centroid (determined visually) for FB treatment, to its planned position. This was followed by a CBCT imaging (in FB or in DIBH, depending on the treatment modality), which was monitored, but not gated, by the Calypso tracking system. Imaging beam was manually interrupted when EMT centroid position exceeded the tolerance threshold. When EMT centroid shifts were detected by the EMT tracking system right after CBCT-based couch corrections, CBCT was repeated. In these cases, we sought to evaluate the potential effect these shifts could have had on the dose distribution, were they not captured and adjusted, by re-calculating the plan with a corresponding displacement of the isocentre. Additional CBCT imaging between or after arcs and kV-imaging during beam-on, were acquired for selected fractions to monitor tumor position and validate the Calypso tracking system. Further imaging was performed when patient realignment was judged necessary.
2.5. Patients treatment
During treatment, real-time EMT motion was registered by the Calypso tracking system, interrupting SBRT if the tolerance thresholds for beam gating were exceeded. We set thresholds smaller than the ITV/GTV to PTV margins (5 mm). Patients treated in DIBH were coached to perform DIBH by audio communication, and the treatment beam started when EMT centroid coordinates in the three spatial directions were within a 3 mm threshold compared to the EMT centroid positions in the planning CT. For FB treatments, thresholds were defined by adding 3 mm to the EMT centroid motion amplitude on the 4DCT. Patients were asked to breathe normally during treatment, and we used EMT tracking to ensure that the tumor remained in the thresholds (range: 3.0–8.0 mm, Table 2).
Table 2.
Intra- and interfraction motion of EMT centroids positions during beam-on for the seven patients treated with EMT real-time tracking.
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | ||
|---|---|---|---|---|---|---|---|---|
| Tolerance thresholds for EMT centroid beam gating (mm) | LR | 3.0 | 3.0 | 5.0 | 5.0 | 3.0 | 3.0 | 3.0 |
| SI | 3.0 | 3.0 | 8.0 | 7.0 | 3.0 | 3.0 | 3.0 | |
| AP | 3.0 | 3.0 | 8.0 | 5.0 | 3.0 | 3.0 | 3.0 | |
| Median [min, max] EMT centroid motion excursions during all sessions (mm) | LR | 0.7 [0.6–0.7] | 2.7 [1.7–4.7] | 1.0 [0.8–1.7] | 2.7 [2.2–6.9] | 2.3 [2.1–2.9] | 2.2 [1.9–3.5] | 2.4 [1.2–2.5] |
| SI | 2.2 [1.8–2.5] | 2.0 [0.9–4.5] | 8.7 [8.1–10.7] | 9.4 [4.9–11.1] | 4.5 [3.9–4.6] | 2.0 [1.5–3.0] | 2.9 [2.2–4.2] | |
| AP | 1.5 [1.3–1.6] | 2.5 [1.7–3.3] | 5.5 [5.0–6.5] | 3.5 [3.0–5.5] | 4.6 [3.1–5.0] | 1.9 [1.7–2.7] | 1.8 [1.4–2.3] | |
| SD of median EMT positions (mm) | LR | 0.2 | 0.8 | 1.0 | 1.4 | 0.8 | 1.3 | 1.0 |
| SI | 0.6 | 1.1 | 2.8 | 0.8 | 1.0 | 0.6 | 0.7 | |
| AP | 0.6 | 0.5 | 1.1 | 0.7 | 0.5 | 0.8 | 0.6 |
Abbreviations: EMT: electromagnetic transponder, SD: standard deviation, LR: left–right, SI: supero-inferior, AP: antero-posterior, P#: patient #.
2.6. Sessions characteristics evaluation
The setup time, defined as the time between the beginning of setup with the Calypso tracking system and the start of the first treatment beam, as well as the session time, defined as the beginning of setup with the Calypso system and the end of the second treatment beam, were recorded for each SBRT fraction. Positional stability of the EMTs was determined by comparing measured (by the Calypso system at setup) vs. planned (i.e. as determined on the planning CT) inter-EMTs distances, as well as by recording geometrical residuals, which represented the discrepancy between the planned and measured EMT positions and which were computed by the Calypso tracking system at the setup.
2.7. Intra- and interfraction motion
Intra- and interfraction motion of the EMTs was analyzed using the Calypso tracking system log files, which contained EMT centroid positions and an approximate beam-on indication based on a radiation detector in the treatment room (update rate of 24 Hz). Intrafraction motion of the EMT centroid was determined by computing EMT centroid excursion motions during beam-on (defined as the difference between the 99th and the 1st percentile) in the three directions. Interfraction motions was evaluated by calculating standard deviations of median EMT positions and range of EMT excursion motions during beam-on of each fraction.
3. Results
3.1. Patients implantation
None of the patients presented complications from the EMT implantation procedure. For P1 only two EMTs were implanted due to the lack of available adjacent bronchi. For the other patients, three EMTs were successfully implanted. The EMT positions were satisfactory since they were non-coplanar on axial CT sections, distances between the EMT centroid position and the tumor centroid were between 4 and 18 mm, and median inter-EMTs distance on planning CT was 39 mm (range: 9–61 mm) (Table1).
3.2. Comparison of DIBH and FB
Among the five patients for whom both a FB and a DIBH CT were acquired (P1, P2, P5, P6 and P7), P1 was treated in FB, as tumor motion was negligible (apical tumor attached to the chest wall), while P2, P5, P6 and P7, for whom GTV motion was larger (Table 1), were treated in DIBH. For these patients, we observed a dosimetric advantage compared to FB, with a 31% median reduction in PTV volume, a better sparing of the ipsilateral lung and of adjacent OAR (main bronchus, heart or chest wall) because their distance from the PTV increased in DIBH (Table 3).
Table 3.
Dosimetric comparison of DIBH vs. FB treatment planning for the four patients treated in DIBH. Only the OAR receiving a Dmean > 2 Gy are reported.
| P2 |
P5 |
P6 |
P7 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DIBH | FB | (%) | DIBH | FB | (%) | DIBH | FB | (%) | DIBH | FB | (%) | ||
| PTV | Volume [cm3] | 9.1 | 13.2 | −31 | 11.5 | 20.9 | −45 | 7.1 | 10.1 | −30 | 13.2 | 18.7 | −29 |
| Ipsilateral lung | V5 Gy (%) | 15.6 | 27.1 | −42 | 18.7 | 23.4 | −20 | 11.8 | 17.4 | −30 | 16.2 | 19.0 | −15 |
| V20 Gy (%) | 3.6 | 7.5 | −52 | 3.8 | 5.8 | −34 | 3.1 | 5.4 | −42 | 3.2 | 4.5 | −29 | |
| V30 Gy (%) | 1.8 | 4.2 | −57 | 1.9 | 3.3 | −42 | 1.7 | 3.2 | −47 | 1.8 | 2.5 | −30 | |
| Main bronchus (P2)/heart (P5)/chest wall (P7) | Dmean (Gy) | 8.7 | 13.7 | −36 | 2.5 | 3.2 | −22 | – | – | – | 8.7 | 10.2 | −15 |
| D2% (Gy) | 22.4 | 28.2 | −21 | 7.5 | 9.2 | −18 | – | – | – | 47.5 | 66.1 | −28 | |
Abbreviations: PTV: planning target volume, FB: free-breathing, DIBH: deep inspiration breath-hold, OAR: organ at risk, Vx Gy: volume receiving x Gy, D2%: dose received by 2% of the volume, Dmean: mean dose, P#: patient #.
3.3. Treatment delivery
All but two lung SBRT fractions using EMT tracking were successfully delivered with excellent patient tolerance and no acute toxicity. EMTs were monitored real-time and automatic beam interruptions were triggered by detection of EMT centroid position out of tolerance. For P3 and P4, one fraction could not be delivered using EMT tracking because of a deficient communication cable, and a system employing an external surrogate for breathing monitoring was used instead.
3.4. Patients setup at linac
The median setup time was 9.8 min (range: 4.6–34.1 min) and the median session time was 14.7 min (range: 7.3–36.5 min) (Table 1). Differences between planned and measured inter-EMTs distances were at the most 4.5 mm, indicating stability of the EMT positions in the lung over the overall treatment time, even with the impact of breathing motion on the EMTs which could add tissue deformation. This was furthermore confirmed by geometrical residuals always smaller than 2.0 mm (Table 1).
3.5. Patients imaging at linac
During setup, CBCT-based couch shifts were applied if necessary, while monitoring that the EMT centroid position remained within the tolerance set in the EMT tracking system. Visual inspection of CBCT imaging showed good alignment of all structures and of the EMTs (Fig. 1a). For P4, in 6 setups out of 8, after applying a CBCT-based roll of about 2–3°, the EMT tracking system captured a 5 mm lateral shift of the EMT centroid position. This shift was confirmed by a subsequent CBCT imaging and corrected for. Re-calculating the treatment plan with a corresponding 5-mm lateral displacement of the isocentre showed that the PTV covered by the 95% isodose line was reduced from 98% to 88%. However the ITV was still covered by the 95% isodose line (since PTV = ITV + 5 mm). Moreover the maximum dose received by the heart and the esophagus was increased by 8%.
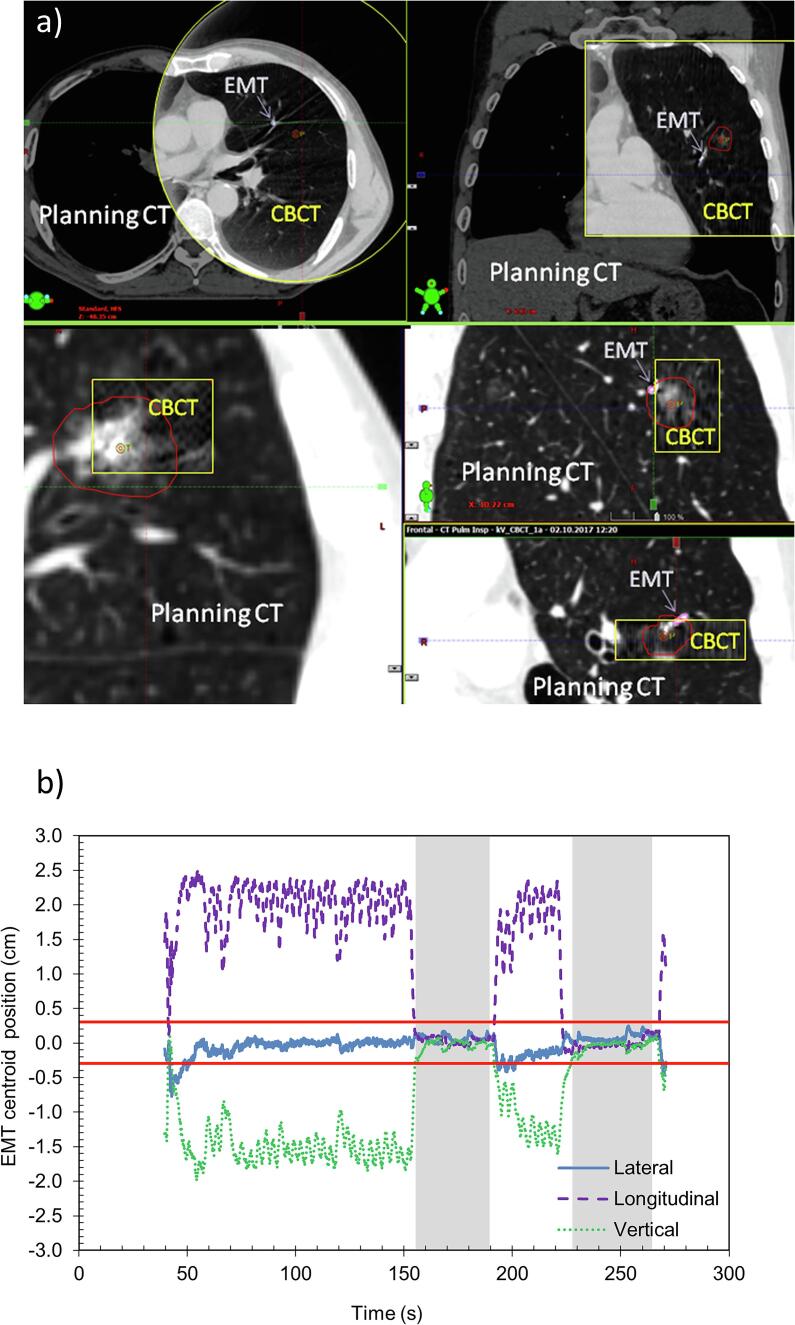
Fig. 1.
Patient 2 treated in deep inspiration breath-hold: a) example of cone-beam CT imaging at linac (framed in yellow) overlaid with planning CT, showing also the planning target volume (PTV) contour (red) and the electromagnetic transponder (EMT) (pink), b) EMT centroid positions during session 8. The shaded grey area show the moments when the treatment beam was ON. The red horizontal lines indicate the ±3 mm tolerance threshold for beam gating. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
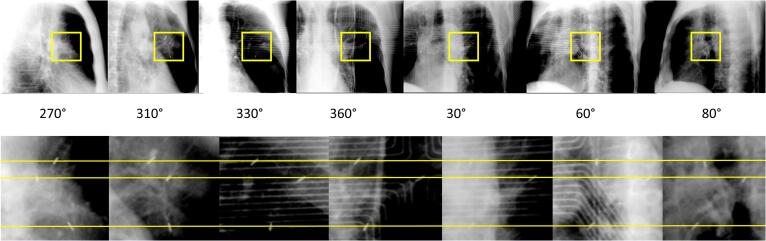
For P2 (treated in DIBH), an additional CBCT after SBRT delivery was acquired at the 1st fraction and confirmed the persistent correct positioning of the target and the EMTs. At the 3rd fraction, a kV-imaging during beam-on (18 images, every 10° of the half-beam during the first half-arc) showed that the EMTs had no supero-inferior (SI) motion (Fig. 2) as detected by the EMT tracking system.
Fig. 2.
Kilo-Voltage imaging performed during deep inspiration breath-hold (DIBH) treatment of patient 2 in a single breath-hold. A selection of images acquired every 10° from 270° to 80° imaging beam angle is shown (top), as well as a zoom on the region were the electromagnetic transponders (EMTs) are located (bottom). The horizontal yellow lines show that the EMTs had negligible supero-inferior motion during DIBH, in accordance with the EMT tracking system signal. The electromagnetic array is visible on the images acquired at angle 330°, 360°, 30° and 60°. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. Patients treatment
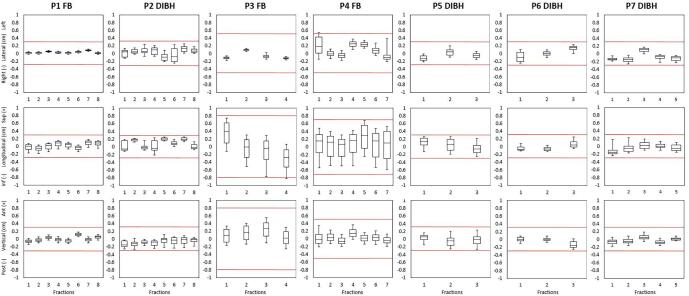
In DIBH, treatment beams started when EMT centroids were within a 3 mm threshold compared to the planned position (Fig. 1b). The EMT tracking system held the beam when patients released the DIBH before the end of the beam or when coughing (Table 1). For FB treatments, beam-hold occurred once for P1 (isolated coughing). For P3 and P4 only micro-interruptions occurred because of breathing amplitudes slightly out of tolerance (Table1). Intra-fraction motion of the EMT centroid positions during beam-on is shown in Fig. 3 and Table2.
Fig. 3.
Boxplots showing 1st percentile, 1st quartile, median, 3rd quartile and 99th percentile of the vertical, longitudinal and lateral electromagnetic transponders centroid positions registered during beam-on for each fractions of the seven patients. The red lines show the tolerance thresholds for beam gating. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The EMT tracking system used in this study has proved to be an accurate tool to monitor in real-time the prostate gland during RT [17], [18] and its accuracy has been thoroughly investigated [19], [20], [21]. Various studies have investigated its potential for motion monitoring and EMT-based gating of liver or pancreas tumors [22], [23], [24], [25], [26]. The first use of this EMT tracking system in human lungs was described by Shah et al. in an observational study evaluating the implantation, migration and feasibility of tumor tracking using smooth EMTs (i.e. EMTs without anchors) [27]. Lung-specific EMTs were first evaluated in a canine model [28], and then successfully implanted in humans [29], [30]. Schmitt et al. [31] reported on the use of anchored EMTs for real-time monitoring during long course normofractionated lung RT, analysing inter- and intrafraction motion, while the first treatment using EMT-guided real-time adaptive MLC tracking for lung SBRT was described by Booth et al.[32]. Recently, Boggs et al. presented their experience in lung SBRT with the EMT tracking system using FB or phase gating [33]. We report our clinical experience of lung SBRT, in either FB or DIBH, using EMTs for tumor real-time position tracking, which may minimize the risk of target miss compared to when using an external surrogate which does not always accurately correlate with tumor position [5], [6], [34], [35]. The use of DIBH in the treatment of four patients allowed a dosimetric benefit compared to FB and might be faster to deliver than gated treatment.
While transbronchial EMT implantation using the superDimension™ Navigation System allowed fast, safe and adequate EMTs positioning for all patients, there are limitations as to its access to the bronchi surrounding peripheral tumors. For P1 only 2 out of the 3 EMTs planned were implanted as our experienced pneumologist did not find a 3rd bronchus proximal enough to the tumor that was apical and partially in contact with the chest wall. Finally, we reported no complications from this minimally invasive procedure, being less invasive and less prone to cause pneumothorax compared to transcutaneous procedures [13], [36].
We found that patient setup with an EMT tracking system was generally fast and easily integrated in the workflow. Target motion in FB (Table 2 and Fig. 3) reproduced data reported in literature [1], namely it was greatest in the SI direction and smallest in left–right. No drift of EMT position was observed during treatment, in contrast with Schmitt et al. who reported a reduction in the distance between EMTs over the course of a long course treatment of 30 RT fractions [31]. This might be explained by our shorter overall treatment times since we used less fractions (Table 1), therefore reducing possible EMT motion caused by fixation stability, and tissue and tumor changes due to irradiation.
For two patients, one fraction could not be delivered using EMT real-time tracking because of a system breakdown. For these sessions, the real-time position management system (RPM) (Varian Medical Systems) was used to monitor and gate the breathing amplitude, following our conventional procedure for patients treated with SBRT without EMT tracking. The RPM could be activated at the linac as long as the respiratory curves were associated to the plan, and we had this backup solution ready for each patient. Even though an external sensor does not always perfectly correlate with tumor position [5], [6], the use of this backup solution was clinically judged superior than a postponement of the fraction. Besides, we did not decrease PTV margins when using the EMT tracking system compared to conventional SBRT treatments.
For P4, the EMT tracking system allowed us to capture a 5 mm lateral shift after applying a CBCT-based roll to a patient’s initial position. We can speculate that an external breathing monitoring would probably not have detected this shift since they are in general not based on the detection of an absolute position, and baseline references are acquired after patient repositioning. Our dosimetric evaluation of the potential effect of this shift indicates that table rolls should be applied carefully, in particular when using small PTV margins or if OAR are close to the PTV. As our institutional experience increases we are considering in the future to reduce the ITV to PTV margin when using EMT tracking for lung SBRT.
The Calypso EMT tracking system supports respiratory amplitude gating and this option might be used to reduce ITV margin for patients exhibiting a large amplitude breathing motion [33]. We did not use this approach for FB treatments because the CBCT at the moment is not gated by the EMT tracking system and we use CBCT images to validate both PTV and EMT positions.
Although several micro-beam interruptions occurred during treatment due to breathing amplitudes slightly larger than planned, the latency for the EMT tracking system to beam-hold is low enough (65 ms) to allow accurate treatment of the target that exits the gating limit at each respiratory cycle [23], [37].
Finally, for implanted organs with follow-up by MRI, EMTs create significant artifacts, precluding the use of MRI in the post-treatment assessment [38]. This is not a concern for patients with lung EMTs, as they are routinely followed with CT-scans.
The present study is limited by the small number of patients, but based on these promising findings, the use of EMTs for patients with early stage lung cancer treated with SBRT is now routinely assessed at our multidisciplinary tumor board, and implemented when feasible. We are among the first European hospitals to integrate EMT real-time tracking system for SBRT treatment of early lung cancer, treating successfully seven patients, and the first center to report on DIBH lung SBRT with this system.
In conclusion, this study showed that EMT implantations in lungs were performed safely and EMT positions in lungs were stable during the overall treatment time. Patient positioning resulted fast, accurate and reproducible using EMT tracking.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to thank Dr. Andreas Rimner and Dr. Michael Lovelock for useful discussions.
Funding
None.
References
- 1.Seppenwoolde Y., Shirato H., Kitamura K., Shimizu S., van Herk M., Lebesque J.V. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53:822–834. doi: 10.1016/s0360-3016(02)02803-1. [DOI] [PubMed] [Google Scholar]
- 2.Suh Y., Dieterich S., Cho B., Keall P.J. An analysis of thoracic and abdominal tumour motion for stereotactic body radiotherapy patients. Phys Med Biol. 2008;53:3623. doi: 10.1088/0031-9155/53/13/016. [DOI] [PubMed] [Google Scholar]
- 3.Shirato H., Suzuki K., Sharp G.C., Fujita K., Onimaru R., Fujino M. Speed and amplitude of lung tumor motion precisely detected in four-dimensional setup and in real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:1229–1236. doi: 10.1016/j.ijrobp.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Juhler Nøttrup T., Korreman S.S., Pedersen A.N., Aarup L.R., Nyström H., Olsen M. Intra- and interfraction breathing variations during curative radiotherapy for lung cancer. Radiother Oncol. 2007;84:40–48. doi: 10.1016/j.radonc.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Ross I.B., Seiko N., Hiroki S., George T.Y.C., Steve B.J. Residual motion of lung tumours in gated radiotherapy with external respiratory surrogates. Phys Med Biol. 2005;50:3655. doi: 10.1088/0031-9155/50/16/001. [DOI] [PubMed] [Google Scholar]
- 6.Koch N., Liu H.H., Starkschall G., Jacobson M., Forster K., Liao Z. Evaluation of internal lung motion for respiratory-gated radiotherapy using MRI: Part I—correlating internal lung motion with skin fiducial motion. Int J Radiat Oncol Biol Phys. 2004;60:1459–1472. doi: 10.1016/j.ijrobp.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Collins B.T., Erickson K., Reichner C.A., Collins S.P., Gagnon G.J., Dieterich S. Radical stereotactic radiosurgery with real-time tumor motion tracking in the treatment of small peripheral lung tumors. Radiat Oncol. 2007;2:39–40. doi: 10.1186/1748-717X-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung I.-H., Song S.Y., Jung J., Cho B., Kwak J., Je H.U. Clinical outcome of fiducial-less CyberKnife radiosurgery for stage I non-small cell lung cancer. Radiat Oncol J. 2015;33:89–97. doi: 10.3857/roj.2015.33.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crijns S.P.M., Kok J.G.M., Lagendijk J.J.W., Raaymakers B.W. Towards MRI-guided linear accelerator control: gating on an MRI accelerator. Phys Med Biol. 2011;56:4815–4825. doi: 10.1088/0031-9155/56/15/012. [DOI] [PubMed] [Google Scholar]
- 10.Menten M.J., Wetscherek A., Fast M.F. MRI-guided lung SBRT: Present and future developments. Phys Med. 2017;44:139–149. doi: 10.1016/j.ejmp.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz Y., Greif J., Becker H.D., Ernst A., Mehta A. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: the first human study. Chest. 2006;129:988–994. doi: 10.1378/chest.129.4.988. [DOI] [PubMed] [Google Scholar]
- 12.Gildea T.R., Mazzone P.J., Karnak D., Meziane M., Mehta A.C. Electromagnetic navigation diagnostic bronchoscopy. Am J Respir Crit Care Med. 2006;174:982–989. doi: 10.1164/rccm.200603-344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gex G., Pralong J.A., Combescure C., Seijo L., Rochat T., Soccal P.M. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: A systematic review and meta-analysis. Respiration. 2014;87:165–176. doi: 10.1159/000355710. [DOI] [PubMed] [Google Scholar]
- 14.Khandhar S.J., Bowling M.R., Flandes J., Gildea T.R., Hood K.L., Krimsky W.S. Electromagnetic navigation bronchoscopy to access lung lesions in 1,000 subjects: first results of the prospective, multicenter NAVIGATE study. BMC Pulm Med. 2017;17:59. doi: 10.1186/s12890-017-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seppenwoolde Y., Wunderink W., Veen S.R.W.-v., Storchi P., Romero A.M., Heijmen B.J.M. Treatment precision of image-guided liver SBRT using implanted fiducial markers depends on marker–tumour distance. Phys Med Biol. 2011;56:5445–5468. doi: 10.1088/0031-9155/56/17/001. [DOI] [PubMed] [Google Scholar]
- 16.Ueki N., Matsuo Y., Nakamura M., Mukumoto N., Iizuka Y., Miyabe Y. Intra- and interfractional variations in geometric arrangement between lung tumours and implanted markers. Radiother Oncol. 2014;110:523–528. doi: 10.1016/j.radonc.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Willoughby T.R., Kupelian P.A., Pouliot J., Shinohara K., Aubin M., Roach M., III Target localization and real-time tracking using the Calypso 4D localization system in patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;65:528–534. doi: 10.1016/j.ijrobp.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 18.Kupelian P., Willoughby T., Mahadevan A., Djemil T., Weinstein G., Jani S. Multi-institutional clinical experience with the Calypso System in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1088–1098. doi: 10.1016/j.ijrobp.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Balter J.M., Wright J.N., Newell L.J., Friemel B., Dimmer S., Cheng Y. Accuracy of a wireless localization system for radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:933–937. doi: 10.1016/j.ijrobp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Santanam L., Noel C., Willoughby T.R., Esthappan J., Mutic S., Klein E.E. Quality assurance for clinical implementation of an electromagnetic tracking system. Med Phys. 2009;36:3477–3486. doi: 10.1118/1.3158812. [DOI] [PubMed] [Google Scholar]
- 21.Franz A.M., Schmitt D., Seitel A., Chatrasingh M., Echner G., Oelfke U. Standardized accuracy assessment of the calypso wireless transponder tracking system. Phys Med Biol. 2014;59:6797–6810. doi: 10.1088/0031-9155/59/22/6797. [DOI] [PubMed] [Google Scholar]
- 22.Shinohara E.T., Kassaee A., Mitra N., Vapiwala N., Plastaras J.P., Drebin J. Feasibility of electromagnetic transponder use to monitor inter- and intrafractional motion in locally advanced pancreatic cancer patients. Int J Radiat Oncol Biol Phys. 2012;83:566–573. doi: 10.1016/j.ijrobp.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 23.James J., Cetnar A., Dunlap N.E., Huffaker C., Nguyen V.N., Potts M. Technical Note: Validation and implementation of a wireless transponder tracking system for gated stereotactic ablative radiotherapy of the liver. Med Phys. 2016;43:2794–2801. doi: 10.1118/1.4948669. [DOI] [PubMed] [Google Scholar]
- 24.Poulsen P.R., Worm E.S., Hansen R., Larsen L.P., Grau C., Høyer M. Respiratory gating based on internal electromagnetic motion monitoring during stereotactic liver radiation therapy: First results. Acta Oncol. 2015;54:1445–1452. doi: 10.3109/0284186X.2015.1062134. [DOI] [PubMed] [Google Scholar]
- 25.Betancourt R., Zou W., Plastaras J.P., Metz J.M., Teo B.K., Kassaee A. Abdominal and pancreatic motion correlation using 4D CT, 4D transponders, and a gating belt. J Appl Clin Med Phys. 2013;14:13–24. doi: 10.1120/jacmp.v14i3.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worm E.S., Høyer M., Hansen R., Larsen L.P., Weber B., Grau C. A prospective cohort study of gated stereotactic liver radiation therapy using continuous internal electromagnetic motion monitoring. Int J Radiat Oncol Biol Phys. 2018;101:366–375. doi: 10.1016/j.ijrobp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Shah A.P., Kupelian P.A., Waghorn B.J., Willoughby T.R., Rineer J.M., Mañon R.R. Real-time tumor tracking in the lung using an electromagnetic tracking system. Int J Radiat Oncol Biol Phys. 2013;86:477–483. doi: 10.1016/j.ijrobp.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Mayse M.L., Smith R.L., Park M., Monteon G.H., Silver E.H., Parikh P.J. Development of a non-migrating electromagnetic transponder system for lung tumor tracking. Int J Radiat Oncol Biol Phys. 2008;72:S430. [Google Scholar]
- 29.Bolliger C.T., Koegelenberg C.F.N., Von Groote-Bidlingmaier F., Bernasconi M., Steyn D.M., Tamm M. First report of implantation of anchored electromagnetic fiducials in human lung cancers for real-time tumor localization and tracking during radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:S578–S579. [Google Scholar]
- 30.Papachristofilou A., Petermann H., Gross M., Schratzenstaller U., Parikh P.J., Paris G. Real-time electromagnetic localization and tracking of human lung cancer using internal fiducials during radiation therapy: implications for target planning and treatment delivery. Int J Radiat Oncol Biol Phys. 2011;81:S762–S763. [Google Scholar]
- 31.Schmitt D., Nill S., Roeder F., Gompelmann D., Herth F., Oelfke U. Motion monitoring during a course of lung radiotherapy with anchored electromagnetic transponders. Strahlenther Onkol. 2017;193:840–847. doi: 10.1007/s00066-017-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Booth J.T., Caillet V., Hardcastle N., O’Brien R., Szymura K., Crasta C. The first patient treatment of electromagnetic-guided real time adaptive radiotherapy using MLC tracking for lung SABR. Radiother Oncol. 2016;121:19–25. doi: 10.1016/j.radonc.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Boggs D.H., Popple R., McDonald A., Minnich D., Willey C.D., Spencer S. Electromagnetic transponder based tracking and gating in the radiotherapeutic treatment of thoracic malignancies. Pract Radiat Oncol. 2019 doi: 10.1016/j.prro.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Ozhasoglu C., Murphy M.J. Issues in respiratory motion compensation during external-beam radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:1389–1399. doi: 10.1016/s0360-3016(01)02789-4. [DOI] [PubMed] [Google Scholar]
- 35.Mageras G.S., Yorke E. Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semin Radiat Oncol. 2004;14:65–75. doi: 10.1053/j.semradonc.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Kupelian P.A., Forbes A., Willoughby T.R., Wallace K., Mañon R.R., Meeks S.L. Implantation and stability of metallic fiducials within pulmonary lesions. Int J Radiat Oncol Biol Phys. 2007;69:777–785. doi: 10.1016/j.ijrobp.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Smith R.L., Lechleiter K., Malinowski K., Shepard D.M., Housley D.J., Afghan M. Evaluation of linear accelerator gating with real-time electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2009;74:920–927. doi: 10.1016/j.ijrobp.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 38.Zhu X., Bourland J.D., Yuan Y., Zhuang T., O'Daniel J., Thongphiew D. Tradeoffs of integrating real-time tracking into IGRT for prostate cancer treatment. Phys Med Biol. 2009;54:N393–N401. doi: 10.1088/0031-9155/54/17/N03. [DOI] [PubMed] [Google Scholar]