Introduction
Maternal cardiac arrest is a rare event, with an incidence of one in 12,000–36,000 women per year in the developed world.1 A prompt, coordinated response by a multidisciplinary team of anaesthetists, obstetricians, neonatologists and nurses is essential.2,3 This article reviews the common aetiologies and the organised response that affords the best chance of maternal and neonatal survival – advanced cardiac life support, resuscitative hysterotomy, and treatment of the underlying aetiology.
Aetiology of cardiac arrest and maternal mortality
The rates and common aetiologies of maternal mortality significantly differ between different parts of the world.4 Although pre-existing disease, cardiovascular disease, cardiomyopathies, infection, and haemorrhage predominate as causes of maternal mortality in settings with well-resourced healthcare, the majority of maternal mortality in resource-poor areas results from haemorrhage.4,5 Failure to rescue patients in cardiac arrest caused by haemorrhage and hypovolaemia in resource-poor areas is an urgent problem, which is beyond the scope of this review.
In 2015, the overall maternal mortality was nine per 100,000 in the UK and 14 per 100,000 in the USA.4 According to the World Health Organization, in 2017 the maternal mortality rate was 462 per 100,000 live births in low-income countries. Of all pregnant women in the UK who received basic life support between July 2011 and June 2014, the survival rate was 58%.3 Coordinated resuscitative efforts, including resuscitative hysterotomy, yield a good chance of survival when the cause of cardiac arrest is a complication related to anaesthesia (e.g. hypoxia, cardiac causes, anaphylaxis, and drug toxicity).3 Survival is lower when cardiac arrest results from hypovolaemia, venous thromboembolism, sepsis, amniotic fluid embolism, intracerebral bleed, aortic dissection, asthma, and pulmonary artery rupture.3
Twenty-five percent of the cardiac arrests in the UK were related to anaesthesia (problem with tracheal intubation, cardiovascular collapse after epidural top-up, total spinal anaesthetic) with obesity complicating the majority of these cases. The predominant causes of maternal arrest in the USA are haemorrhage, heart failure, amniotic fluid embolism and sepsis.2 Stillbirth, Caesarean delivery, severe pre-eclampsia and placenta praevia are the obstetric issues associated with maternal arrest.2 Racial disparities in the USA and UK may be a contributing factor, indicated by an approximately three times higher mortality among black than white women in both nations.2,5 Aetiologies of maternal cardiac arrest with specific treatments are presented in Table 1.
Table 1.
Differential diagnosis of maternal cardiac arrest and suggested management. ECMO, extracorporeal membrane oxygenation; HELLP, haemolysis, elevated liver enzymes, low platelet count
| Diagnosis | Action item | ||
|---|---|---|---|
| Complications of anaesthesia | High neuraxial block | Treat hypotension aggressively (e.g. low dose adrenaline [epinephrine]) Support airway and breathing |
|
| Loss of airway, aspiration, respiratory depression | Support airway and breathing Difficult airway algorithm |
||
| Hypotension | Treat with vasopressors Lower head of bed to improve cerebral perfusion Volume replacement Obtain more intravenous access |
||
| Systemic toxicity from local anaesthetics | Give intralipid Consider cardiopulmonary bypass or ECMO |
||
| Bleeding | Coagulopathy | Fibrinogen replacement Fresh frozen plasma Cryoprecipitate Platelets Consider tranexamic acid 1 g i.v. |
|
| Uterine atony | Give uterotonics Bakri balloon Compression suture Uterine artery embolisation Hysterectomy |
||
| Placenta accreta | Consider uterine artery embolisation Consider hysterectomy |
||
| Placental abruption | Delivery if indicated Monitor for coagulopathy |
||
| Placenta praevia | Delivery if indicated Prepare for lower uterine segment atony |
||
| Uterine rupture | Uterine repair or hysterectomy | ||
| Trauma | Call general surgeon Activate massive transfusion |
||
| Transfusion reaction | Stop transfusion Notify blood bank Adrenaline Steroids Send tryptase |
||
| Cardiovascular | Cardiomyopathy | Inotrope infusion Call for ECMO |
|
| Myocardial infarction | Inotrope infusion Call for ECMO Call for cardiac surgeon Call cardiac catheterisation laboratory Send cardiac enzymes |
||
| Aortic dissection | Call cardiac surgeon Activate massive transfusion |
||
| Arrhythmias | Ventricular fibrillation Unstable ventricular tachycardia (VT) |
Defibrillate Amiodarone Lidocaine |
|
| Torsade de pointes | Defibrillate Magnesium |
||
| Stable VT | Amiodarone Lidocaine |
||
| Supraventricular tachycardia | Adenosine | ||
| Atrial fibrillation | Amiodarone Cardioversion |
||
| Drugs | Anaphylaxis | Adrenaline Steroids Diphenhydramine Ranitidine |
|
| Illicit | Opioid overdose | Naloxone | |
| Benzodiazepine overdose | Flumazenil | ||
| Cocaine coronary vasospasm | Oxygen, aspirin, nitrates, thrombolytic therapy, or acute percutaneous coronary intervention | ||
| Drug error | Identify, discontinue agent and treat | ||
| Magnesium toxicity | Stop magnesium Give calcium chloride 10 ml in 10% solution or calcium gluconate 30 ml in 10% solution |
||
| Insulin overdose | Give glucose/dextrose Glucagon |
||
| Oxytocin overdose | Treat hypotension | ||
| Embolic | Pulmonary embolus | Call Interventional radiology Call cardiac surgeon Prepare catheterisation laboratory Echocardiography Start heparin i.v. Consider thrombolytics in cardiac arrest Nitric oxide |
|
| Coronary thrombus | Call cardiac surgeon Catheterisation laboratory Nitroglycerine Nitric oxide if right heart failure |
||
| Amniotic fluid embolism/Anaphylactoid syndrome of pregnancy | Adrenaline Initiate cardiopulmonary resuscitation Call for extracorporeal membrane oxygenator Call for transoesophageal echocardiography Prepare for coagulopathy and need for massive transfusion protocol Consider unproven ‘A-OK’ therapy: atropine, ondansetron, ketorolac Consider steroids Consider nitric oxide |
||
| Air | Flood field if uterine venous sinuses open Internalise uterus |
||
| Fever | Infection, sepsis | Give broad spectrum antibiotics Fluids, volume replacement Vasopressors Place arterial line Perform echocardiogram Inotrope if low cardiac output |
|
| General non-obstetric causes of cardiac arrest | Hypotension | Treat with vasopressors Lower head of bed to improve cerebral perfusion Fluids, volume replacement Obtain more intravenous access Call for transthoracic echocardiography |
|
| Hypoxia | Airway control 100% oxygen |
||
| Hypothermia | Warm patient Warm fluids Blankets Increase room temperature |
||
| Hyperkalaemia | Calcium Insulin and glucose Furosemide Albuterol Sodium bicarbonate to correct acidosis Intubate and hyperventilate Polystyrene sulphonate (potassium binder) Consider haemodialysis |
||
| Hypoglycaemia | Give glucose/dextrose Glucagon |
||
| Hypercarbia/acidosis | Intubate trachea and optimise ventilation Determine cause of acidosis Sodium bicarbonate |
||
| Thrombus | See pulmonary embolus above | ||
| Trauma | Call general or trauma surgeon | ||
| Toxin | Give antidote if agent known | ||
| Tension pneumothorax | Needle decompression Insert chest tube |
||
| Tamponade | Call for ECMO Call cardiac surgeon |
||
| Hypertension | Pre-eclampsia/eclampsia/HELLP | Antihypertensive agents: labetalol (avoid in asthmatics), hydralazine, nicardipine Magnesium |
|
| Intracranial haemorrhage with increased intracranial pressure | Call neurosurgeon BP goal: systolic <140 mmHg Elevate head of bed 30° Reverse coagulopathy if present Hypertonic saline/mannitol |
||
Implications of pregnancy for cardiopulmonary resuscitation
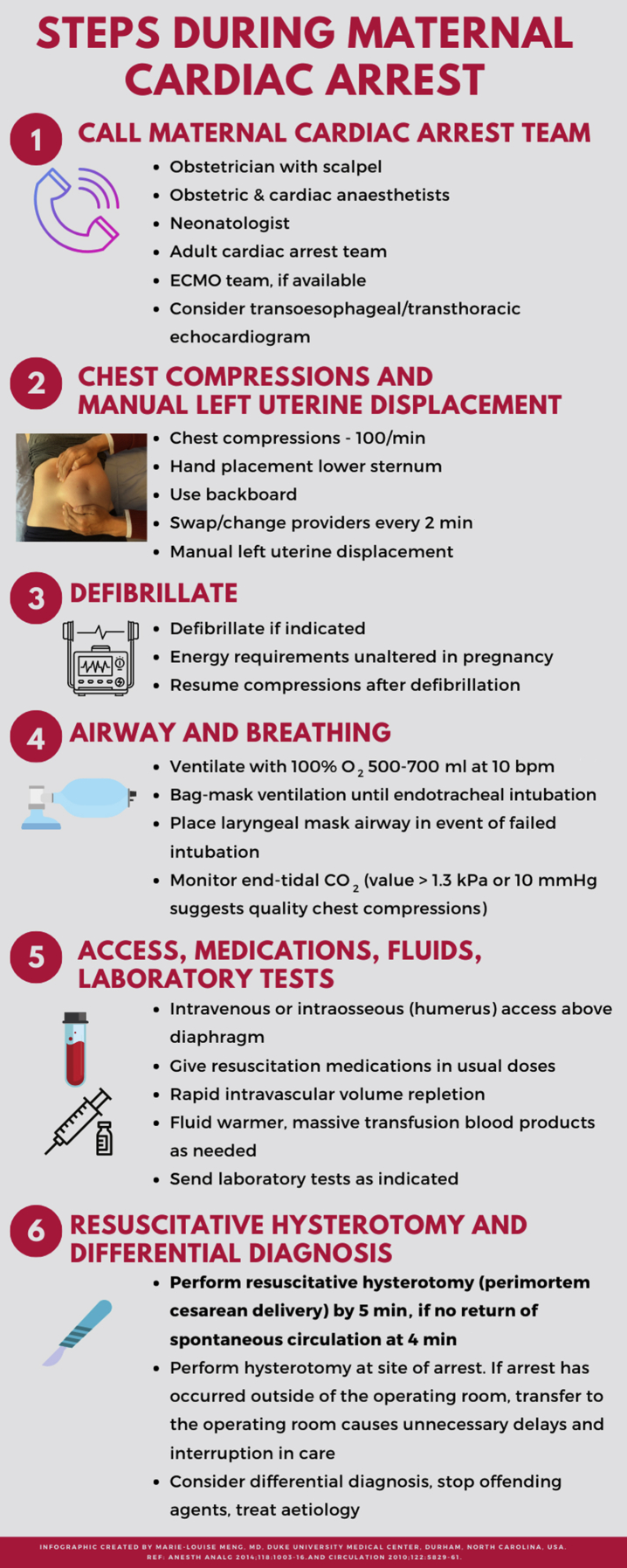
Figure 1 summarises the actions that should take place when maternal cardiac arrest occurs.
Fig 1.
Steps during maternal cardiac arrest. ECMO, extracorporeal membrane oxygenation.
Chest compressions
Chest compressions should be hard and fast, performed on a firm flat surface – achieving at least 5 cm depth and at a rate of 100 compressions min−1. A backboard may be required if resuscitation is taking place on a typical hospital mattress. The optimal hand position during chest compression is the lower sternum.6,7 There is no scientific evidence to support a higher hand position on the sternum, despite the fact that the gravid uterus may cause superior and anterolateral displacement of the myocardium. When possible, transoesophageal guidance can be used to confirm adequate compression location.
Left uterine displacement
The enlarging gravid uterus impairs resuscitative efforts because of reduced venous return secondary to obstruction of the vena cava in the supine position, beginning at 20 weeks' gestation. Gestational age 20 weeks can be assumed if the fundus reaches or exceeds the level of the umbilicus. To prevent decreases in preload, left uterine displacement must be performed during resuscitation. Manual left uterine displacement is preferred over left lateral tilt as it is more difficult to create the appropriate compression force vector when left lateral tilt is used. Manual left uterine displacement can be performed from the left side of the patient, using two hands to pull the uterus upward and to the left or from the right side of the patient by pushing the uterus up and to the left.6
Airway and ventilation
Decreases in functional residual capacity and increased oxygen consumption by the fetus lead to rapid development of hypoxaemia. Vascular engorgement results in oedema of the oro- and nasopharynx, larynx and trachea, and may result in difficult intubation and necessitate smaller tracheal tubes. Videolaryngoscopy has been recommended for the first intubation attempt, if possible, to increase the chances of success. Confirmation of tracheal intubation with a capnometer is advised. Nasopharyngeal airways should be used with caution because of the increased risk of bleeding. Ventilation with 100% oxygen with tidal volumes of 500–700 ml at 10 bpm is recommended.
Intravenous access
Large-bore intravenous access should be obtained above the diaphragm as uterine compression and bleeding may prevent adequate resuscitation from below the diaphragm. Ultrasound-guided venous access or intraosseous access in the proximal humerus may be necessary in cases where access is difficult.
Drugs
During pregnancy, circulating blood volume increases out of proportion to the increase in haemoglobin, creating a physiologic anaemia. Increased blood volume and decreased protein binding may theoretically mildly alter the volume of distribution of administered drugs, but these changes are negligible in the low flow state of arrest. Drugs of resuscitation should be administered according to standard guidelines, with none considered to be contraindicated.6 Oxytocin should be discontinued because of its vasodilatory and negative inotropic effects. Neuraxial local anaesthetic infusions should also be discontinued. Magnesium should be discontinued if it is contributing to significant hypotension.
Defibrillation
Defibrillation should be performed for a shockable rhythm and is considered to be safe for the fetus during maternal cardiac arrest. No changes to the energy requirements are necessary with unchanged thoracic impedance in pregnancy. The theoretical risk of burns from fetal external and internal monitors should not delay the initiation of defibrillation because of waiting to disconnect or remove from the mother.
Perimortem Caesarean delivery/resuscitative hysterotomy
Maternal and neonatal survival are improved when the time from arrest to resuscitative hysterotomy is less than 5 min vs greater than 5 min–61% vs 35% for the mother and 96% vs 70%, for the neonate.3 Removing the fetus reduces oxygen consumption and removes the anatomical barrier to vena cava flow and optimal pulmonary mechanics. Importantly, maternal survival is also higher (72% vs 36%) when resuscitative hysterotomy is performed at the site of arrest instead of transfer to the operating theatre.3 Equipment necessary for Caesarean delivery (especially a scalpel) should be immediately available.
Team preparedness
Because this is a high-stakes/low-frequency event, maternal cardiac arrest response plans and plans to mobilise responders should be regularly reviewed by obstetricians, anaesthesiologists, neonatologists and labour and delivery nurses, with didactic and simulation sessions. It has been demonstrated in a simulated setting that in situ drills reduce the time to initiation of cardiopulmonary resuscitation (CPR) and resuscitative hysterotomy.8
Strong leadership and teamwork are essential. Team members should use closed-loop communication, acknowledging tasks assigned by the leader, announcing when the tasks have been completed, and should feel empowered to make suggestions to improve care. These situations are often hectic, and the leader should provide direction and strive to maintain team organisation. Once enough support arrives, the code leader should be in a position of oversight to ensure completion of tasks, identify the differential diagnosis and appropriate treatment.
Transoesophageal echocardiography
Where the equipment and expertise are available, transoesophageal echocardiography may be used to identify the cause of arrest and to assess the efficacy of the resuscitative efforts. It may help identify the presence or absence of gross pathology (spontaneous coronary artery dissection with wall motion abnormalities, aortic dissection, pericardial and pleural effusions, saddle pulmonary embolism, ventricular thrombus etc.) and may aid placement of the cannulae for extracorporeal membrane oxygenation (ECMO) cannulation. Echocardiography can also help assess the efficacy of chest compressions as the location of compression vector and return of myocardial function can be visualised. Acquisition of transthoracic or subcostal images is limited during resuscitation.
ECMO CPR
ECMO has been used to stabilise critically ill obstetric patients and extracorporeal CPR (ECPR) and used as rescue therapy in maternal cardiac arrest attributable to cardiopulmonary causes.9,10 Although there are few absolute contraindications in obstetric patients, caution is warranted in patients with massive haemorrhage or disseminated intravascular coagulopathy as extracorporeal circulation may activate coagulation factors and worsen thrombosis or coagulopathy. Obstetric patients at high risk of cardiopulmonary decompensation should be managed at hospitals with ECMO capabilities.
Return of spontaneous circulation
Usual post-resuscitation care should occur when return of spontaneous circulation is achieved. Patients may require further surgery to repair or close the resuscitative hysterotomy. Appropriate haemostasis, antibiotics and intensive care protocols should be followed including hypothermia where appropriate. Maternal hypothermia may worsen coagulopathy. Patients with return of awareness will require appropriate analgesia and sedation.6
Conclusions
In the rare occurrence of maternal cardiac arrest, prompt initiation of CPR, effective left uterine displacement, coordination of multidisciplinary provider response, early use of ECMO when available and appropriate, and early resuscitative hysterotomy improve both maternal and fetal survival. The addition of transoesophageal echocardiography may aid early recognition of the aetiology of cardiac arrest and assessment of the effectiveness of resuscitation.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Anne-Marie Madden BSc (Hons) MD FRCPC is a cardiothoracic anaesthesiologist at St-Paul's Hospital and clinical instructor at the University of British Columbia in Vancouver, Canada.
Marie-Louise Meng MD is an obstetric and cardiothoracic anaesthesiologist at Duke University Medical Center in Durham, NC, USA.
Matrix codes: 1B03, 2B05, 3B00
References
- 1.Soar J., Perkins G.D., Abbas G. European Resuscitation Council Guidelines for Resuscitation 2010 Section 8. Cardiac arrest in special circumstances: electrolyte abnormalities, poisoning, drowning, accidental hypothermia, hyperthermia, asthma, anaphylaxis, cardiac surgery, trauma, pregnancy, electrocution. Resuscitation. 2010;81:1400–1433. doi: 10.1016/j.resuscitation.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Mhyre J.M., Tsen L.C., Einav S., Kuklina E.V., Leffert L.R., Bateman B.T. Cardiac arrest during hospitalization for delivery in the United States, 1998–2011. Anesthesiology. 2014;120:810–818. doi: 10.1097/ALN.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckett V.A., Knight M., Sharpe P. The CAPS Study: incidence, management and outcomes of cardiac arrest in pregnancy in the UK: a prospective, descriptive study. BJOG. 2017;124:1374–1381. doi: 10.1111/1471-0528.14521. [DOI] [PubMed] [Google Scholar]
- 4.Alkema L., Chou D., Hogan D. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet. 2016;387:462–474. doi: 10.1016/S0140-6736(15)00838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creanga A.A., Syverson C., Seed K., Callaghan W.M. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366–373. doi: 10.1097/AOG.0000000000002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipman S., Cohen S., Einav S. The Society for Obstetric Anesthesia and Perinatology consensus statement on the management of cardiac arrest in pregnancy. Anesth Analg. 2014;118:1003–1016. doi: 10.1213/ANE.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 7.Vanden Hoek T.L., Morrison L.J., Shuster M. Part 12. Cardiac arrest in special situations: 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S829–S861. doi: 10.1161/CIRCULATIONAHA.110.971069. [DOI] [PubMed] [Google Scholar]
- 8.Fisher N., Eisen L.A., Bayya J.V. Improved performance of maternal–fetal medicine staff after maternal cardiac arrest simulation-based training. Am J Obstet Gynecol. 2011;205:239. doi: 10.1016/j.ajog.2011.06.012. e1–5. [DOI] [PubMed] [Google Scholar]
- 9.Sharma N.S., Wille K.M., Bellot S.C., Diaz-Guzman E. Modern use of extracorporeal life support in pregnancy and postpartum. Asaio J. 2015;61:110–114. doi: 10.1097/MAT.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 10.Agerstrand C., Abrams D., Biscotti M. Extracorporeal membrane oxygenation for cardiopulmonary failure during pregnancy and postpartum. Ann Thorac Surg. 2016;102:774–779. doi: 10.1016/j.athoracsur.2016.03.005. [DOI] [PubMed] [Google Scholar]