Key points.
-
•
Oxygen metabolism generates harmful reactive oxygen species, which are countered in all cells by ubiquitous antioxidant enzymes and molecules.
-
•
Oxygen toxicity primarily affects lung tissue because it is exposed to the highest Po2, but clinical effects in humans still take more than 24 h to develop.
-
•
Physiological effects of hyperoxia include inhibition of hypoxic pulmonary vasoconstriction, promotion of atelectasis, and systemic vasoconstriction.
-
•
Adverse clinical effects of hyperoxia occur, including increased mortality, in intensive care, acute medicine, and perioperative medicine.
-
•
Inhaled oxygen must be prescribed, and the dose titrated to target oxygen saturation or arterial Po2.
Learning objectives.
By reading this article, you should be able to:
-
•
Describe the biochemical and pathophysiological changes by which hyperoxia damages biological systems.
-
•
Explain both the beneficial and harmful effects of hyperoxia in clinical practice.
-
•
Describe strategies to reduce the likelihood of hyperoxia causing harm.
This paper aims to update readers on the basic science and adverse clinical effects of hyperoxia, and then review current evidence regarding when hyperoxia may be clinically indicated and how to avoid its inadvertent occurrence. Hyperoxia describes any situation when cells, tissues, or organs are exposed to oxygen at a higher than normal partial pressure. Although there is no accepted clinical definition of hyperoxia, or indeed normoxia, in this article, and in keeping with most people's understanding, hyperoxia can be defined as an arterial Po2 value greater than the normal for that patient's age when breathing air. Hyperoxia may be ‘normobaric’ when breathing a high inspired concentration at one atmosphere absolute pressure, or ‘hyperbaric’ when breathing at high atmospheric pressure. In the latter situation, inspired oxygen concentration may be normal (0.21) or lower but still result in an inspired Po2 greater than that when breathing air at normal atmospheric pressure.
Oxygen transport between inspired gas and the mitochondria is almost entirely by diffusion down a partial pressure gradient, the only exception being mass transport around the vasculature of oxygen bound to haemoglobin in the blood. The highest Po2 will therefore always be in lung tissue, in particular in the alveolus where cells are directly exposed to the gas without the protective layer of airway lining fluid and mucous seen in the conducting airways.
Alveolar Po2 (Pao2) can be derived from a basic form of the alveolar gas equation:
| Pao2=Pio2–Paco2/RER, |
where Pio2 is the inspired partial pressure of oxygen, Paco2 is the alveolar partial pressure of carbon dioxide, and RER is the respiratory exchange ratio.
This equation shows that only a lowered Paco2, for example from hyperventilation, or an increased Pio2 from breathing extra oxygen can cause hyperoxia. In practice, hyperventilation breathing air can raise arterial Po2 to a maximum of around 16 kPa—a further increase can only be achieved by increasing the inspired oxygen concentration, increasing the ambient pressure, or both.1
Hyperoxia could also describe a higher than normal blood oxygen content, for example in a patient with polycythaemia and a normal Pao2, but most clinicians reserve the term for situations where Pao2 is increased.2
How can oxygen be harmful?
Pathophysiology of oxygen toxicity
Many animals have existed in an atmosphere containing oxygen for millions of years, evolving complex biological systems to utilise the oxygen to efficiently generate energy. However, this use of such a chemically reactive molecule as oxygen has come at a cost and has required the development of systems to prevent its harmful effects. This section outlines the biochemistry of oxygen toxicity.
Reactive oxygen species
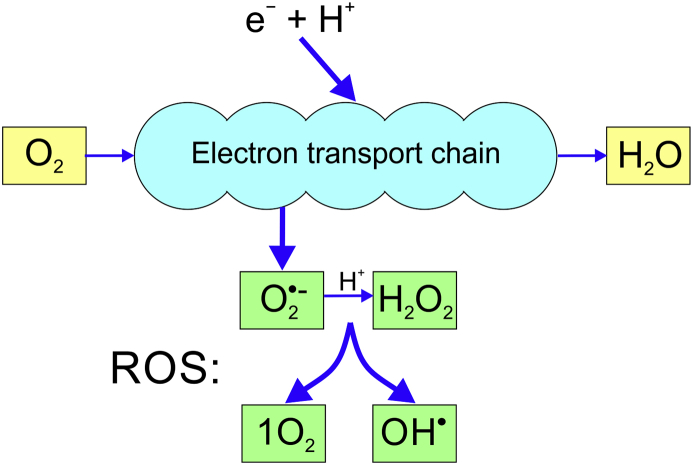
During cellular metabolism, elemental oxygen is reduced to water by the transport of electrons along a chain of enzymes in the mitochondria. As a result, hydrogen ions (protons) are also forced to move along the chain and across the mitochondrial membrane against their concentration gradient. This process, called chemiosmosis, involves electron donor molecules generated from the citric acid cycle modifying the electrical charge of the mitochondrial enzymes until this forces the positively charged protons across the mitochondrial membrane. At the end of the mitochondrial chain the protons reach a high enough energy level to combine with molecular oxygen. Adenosine triphosphate production is the result. Not all the high energy electrons and hydrogen ions form water, with ‘leakage’ from the enzyme chain leading to a variety of other molecules being generated (Fig 1), which are collectively referred to as reactive oxygen species (ROS). ROS were previously known as oxygen free radicals, being so named as they have one or more unpaired electrons, and are therefore ‘free’ to interact with surrounding molecules, making them unstable and highly reactive.
Fig 1.
The reduction of oxygen to water in the mitochondria, with four alternative reactions that can occur to generate the most abundant reactive oxygen species (ROS).
The two most abundant ROS are the superoxide anion (O2•−) produced by reduction of molecular oxygen without the addition of any hydrogen ions and hydrogen peroxide (H2O2) formed by the reduction of the superoxide anion (Fig. 1).
Although the superoxide anion and hydrogen peroxide themselves have direct toxic effects, their interactions produce even more damaging ROS molecules. The Fenton or Haber–Weiss reaction results in the formation of two extremely reactive species, the hydroxyl radical (OH•) and singlet oxygen (1O2, a high-energy, short-lived form of oxygen in which all electrons are paired), alongside the harmless hydroxyl ion:
| O2•− + H2O2 → OH− + OH• + 1O2 |
The interaction between superoxide anion and hydrogen peroxide is the source of much of the oxygen-related damage to biological systems, because of continuously produced, toxic hydroxyl radicals. The hydroxyl radical is extremely unstable, immediately reacting with any molecule nearby, removing its electrons and thus generating a chain reaction for further ROS formation.
Biological effects of ROS and antioxidants
In all cells a balance exists between oxidant and antioxidant molecular activity which is highly regulated to ensure proper function. The development of defences against hyperoxic damage is a central theme of animal evolution.3 This balance between the detrimental effects of the ROS and the antioxidants that counter them is known as the redox state of the cell. Aside from their beneficial roles in the regulation of phagocytes and possibly in the killing of microorganisms, most effects of ROS are harmful.
ROS exert harmful effects on biological structures by multiple mechanisms. Examples are damaging DNA or RNA, impairing DNA repair systems, affecting transcription or by lipid peroxidation, the last of which directly damages cell membrane structure. They may also interfere with protein function by direct oxidation of amino acids or oxidative deactivation of enzymes.
Antioxidants are ubiquitous molecules found in all cells that use oxygen, and the more common ones are shown in Table 1. Antioxidants can exert their effects by various mechanisms, including prevention of ROS formation in mitochondria or scavenging and rapidly inactivating ROS molecules. It is impossible to underestimate the importance of these systems. It is estimated that almost 2% of oxygen consumed in mitochondria forms ROS molecules that have to be inactivated, and that the balance between ROS and antioxidants is a key component of the ageing process.
Table 1.
Classifications of cellular antioxidants. GSH, glutathione; SOD, superoxide dismutase.
| Enzymatic | Non-enzymatic |
|---|---|
| SOD Catalase GSH peroxidase |
GSH Ascorbic acid Vitamin E Beta-carotene Uric acid |
| Endogenous | Exogenous (dietary) |
| SOD Catalase GSH peroxidase Uric acid Bilirubin |
Ascorbic acid Vitamin E Beta-carotene |
Hyperoxia will increase ROS formation, therefore overwhelming the antioxidant mechanisms’ capacity, tipping the balance toward cellular oxidative stress, with the potential for tissue damage. Exposure time and Po2 ultimately determine the cumulative oxygen 'dose'.
Clinical oxygen toxicity
As previously discussed, oxygen toxicity can be subdivided into inhalation of oxygen-enriched gas at normal pressure (normobaric hyperoxia) or inhaling oxygen at raised atmospheric pressure (hyperbaric hyperoxia) when the Po2 achieved can be extremely high.
Normobaric hyperoxia
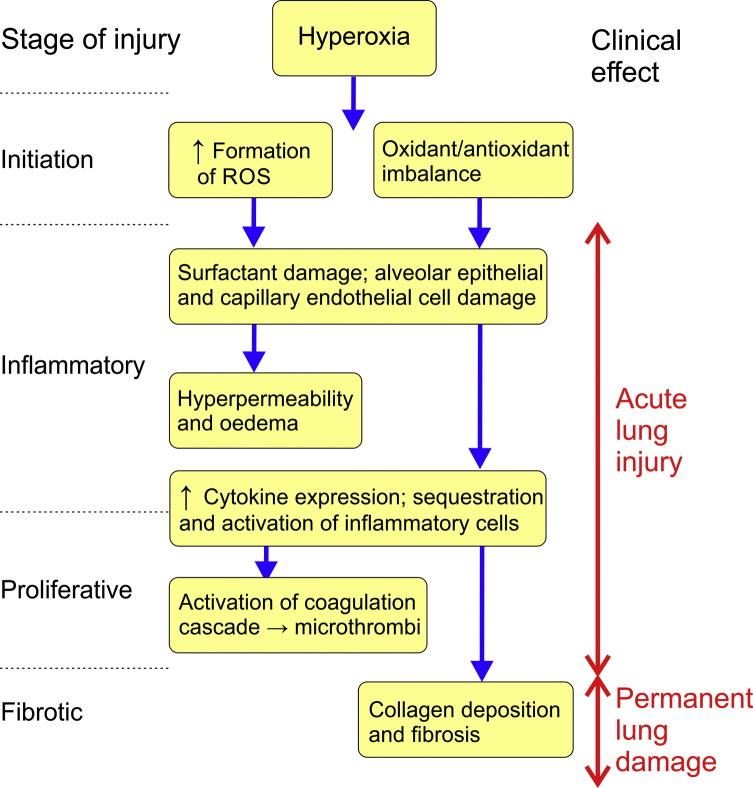
The lung is the organ most susceptible to oxygen toxicity, as in pulmonary tissue the Po2 is always the highest in the body. After exposure to a high concentration of oxygen (i.e. Fio2 >0.6 for ≥24 h) damage occurs to respiratory epithelium of the tracheobronchial tree, manifesting clinically as retrosternal burning or tightness, chest pain, dyspnoea, and breathing becoming painful. Reduction in vital capacity is the first measurable change in lung function, occurring after approximately 24 h; as oxygen exposure continues a range of pathological changes occur (Fig 2), ultimately leading to acute lung injury (ALI). Clinically, the patient may become tachypnoeic and progressively more hypoxaemic until respiratory failure ensues. If continued exposure to oxygen occurs, changes to the lungs can become irreversible. The oxygen ‘dose’ that human lungs can tolerate is unknown, but early space vehicles, which used 100% oxygen at low pressure, exposed astronauts to a Po2 value of 34 kPa for several days without ill effects.
Fig 2.
Summary of the pathophysiology of pulmonary oxygen toxicity. ROS, reactive oxygen species.
Pulmonary toxicity develops in four stages, which overlap based on their severity and degree of reversibility. Initiation is characterised by an increase in ROS concentrations and a reduction in antioxidant levels, followed by an inflammatory stage during which there is destruction of the pulmonary lining and migration of inflammatory mediators. Later changes, including cellular hypertrophy, increased secretions from alveolar type II cells and increased monocytes constitute a proliferative phase. Finally, the fibrotic stage occurs, involving collagen deposition and thickening of the interstitial space. These lung changes are irreversible. Compared with most other animals, humans are relatively resistant to pulmonary oxygen toxicity, probably because we lack the ability to produce ascorbic acid in our cells so have instead evolved better enzymatic antioxidant systems (Table 1).
Management of pulmonary toxicity is supportive, often involving an increased Fio2 and assisted ventilation, which may then further aggravate the changes associated with toxicity. Clinical diagnosis of oxygen toxicity can be difficult. Other causes of ALI with indistinguishable features often present alongside hyperoxic ALI such as sepsis or pneumonia. Lung biopsy may show changes consistent with the diagnosis but is normally used primarily to exclude other causes of the ALI.
Hyperbaric hyperoxia
When exposed to oxygen at high barometric pressure (>2 atm absolute), central nervous system toxicity can occur. This begins with non-specific symptoms such as nausea, headache, dizziness, muscle twitching, visual disturbances, irritability and disorientation, but can progress to convulsions (the Paul–Bert effect). Oceanic divers are therefore more likely to experience symptoms of CNS toxicity. The mechanism by which these effects occur is poorly understood but is believed to be a result of interactions between gamma-amino butyric acid and nitric oxide.
Other physiological effects of hyperoxia
Aside from direct oxygen toxicity, breathing high concentrations of oxygen has other physiological effects. Most of the studies of these effects used 100% oxygen at normal atmospheric pressure.
Hypoxic pulmonary vasoconstriction
This vital reflex of the pulmonary circulation serves to match regional ventilation and perfusion ( and ). It is highly variable between individuals, and becomes important only when lung pathology leads to areas of lung with low / ratios. Under these circumstances local vasoconstriction in affected areas reduces blood flow and decreases shunt. Administration of supplemental oxygen will abolish hypoxic pulmonary vasoconstriction (HPV) in these areas within a few minutes, but will also better oxygenate the blood passing through these regions, hopefully improving arterial Po2. These changes may have adverse effects on carbon dioxide elimination as described below.
Control of breathing
The use of oxygen to treat respiratory failure, particularly that caused by exacerbations of chronic obstructive pulmonary disease (COPD), has long been known to be associated with development of hypercapnia in some patients. Traditional teaching has been that this results from abolishing hypoxic ventilatory drive, but evidence for this is lacking with studies having failed to find changes in minute ventilation in most patients when oxygen is administered. Altered / relationships caused by impaired HPV is a more likely explanation, with inhaled oxygen quickly leading to increased perfusion of hypoxic lung regions, diverting blood away from better-ventilated regions, increasing alveolar dead space.
Absorption atelectasis
This term describes the situation in which inhalation of high oxygen concentrations results in collapse in areas of the lung with low ratios. The rapid absorption of oxygen from the alveoli distal to almost closed or briefly closed bronchioles reduces the alveolar pressure and accelerates collapse. The ability of 100% oxygen to exacerbate atelectasis has been demonstrated in awake volunteers breathing close to residual volume and in patients during anaesthesia.
Vascular effects
Hyperoxia, including breathing 100% oxygen at normal atmospheric pressure, causes systemic vasoconstriction within minutes, most easily demonstrated with forearm plethysmography, an effect which is abolished by large doses of vitamin C, suggesting that ROS are involved in the mechanism. The effect also occurs in critical organs such as the cerebral and coronary circulations, and there is a suggestion from one study of a greater effect in diseased arteries already under oxidative stress.4
Therapeutic use of hyperoxia
Oxygen is used most commonly in clinical practice in the treatment of hypoxaemia. However, there are some circumstances where oxygen is used in a non-hypoxaemic patient.
Normobaric hyperoxia
Hyperoxia may be of benefit in the following conditions.2
Carbon monoxide poisoning
Patients with CO poisoning may have normal Pao2 values, but a greatly reduced amount of oxygen bound to haemoglobin as this has been displaced by the higher binding affinity of CO. The half-life of CO reduces from approximately 300 min when breathing air to 90 min when breathing high concentrations of oxygen via a reservoir mask. In patients with CO poisoning who have mental impairment or are comatose, safe delivery of high oxygen concentrations is likely to require tracheal intubation and artificial ventilation with 100% oxygen. The half-life of CO is further reduced to approximately 20 min under hyperbaric conditions (3 atm) but there is conflicting evidence about its benefit in clinical practice. A Cochrane review in 2011 of six randomised controlled trials of the use of hyperbaric oxygen in carbon monoxide poisoning concluded that existing randomised trials did not establish whether its use reduced the incidence of adverse neurological outcomes.5
Pneumothorax
Inhalation of high concentration oxygen increases the rate of absorption of collections of gas in the body. Lowering PN2 in blood greatly reduces the total partial pressure of dissolved gases in venous blood, therefore increasing the rate at which gases diffuse down their partial pressure gradient from the gas collection into the blood. Animal studies have confirmed that breathing supplemental oxygen does lead to a faster resolution of a pneumothorax; however, above a Fio2 of 0.6 evidence of toxicity also occurred. British Thoracic Society (BTS) guidance on the management of pneumothorax advocates the use of high concentration oxygen (reservoir mask at 15 L min−1 aiming for 100% saturation) in all patients without COPD who require hospital admission because of a moderate-sized pneumothorax that does not require drainage.2
Cluster headache
Relief from the severe pain associated with cluster headaches has been widely reported and treatment with at least 12 L min−1 via a reservoir mask and availability of home oxygen is recommended.2
Postoperative complications
Many early studies demonstrated that hyperoxia (Fio2 of 0.8 in the perioperative period) reduced postoperative nausea and vomiting (PONV) but randomised controlled trials have shown conflicting results. A systematic review and meta-analysis of 22 randomised controlled trials showed no overall benefit from hyperoxia in reducing PONV.6 The only significant beneficial effect was seen in a specific subgroup of patients who received only inhalation anaesthesia and no prophylactic antiemetics.6
There is similarly conflicting evidence of the ability of hyperoxia to reduce the incidence of surgical site infections (SSIs), which is believed to occur by increasing tissue Po2 within wounds to enhance neutrophil capacity. One meta-analysis6 did demonstrate that a high perioperative oxygen reduced the risk of an SSI, whereas another group concluded that there was no overall benefit except within subgroups undergoing general anaesthesia and colorectal surgery.7 In 2016 the WHO recommended using hyperoxia in all patients whose trachea is intubated, to reduce the incidence of SSI.8 This blanket recommendation, with no acknowledgment of potential adverse effects, has been criticised.9 More recent studies have failed to demonstrate a benefit of oxygen.10
Hyperoxia has also been shown to improve anastomotic integrity in experimental animal models and in patients undergoing colorectal and gastric surgery, but study populations were very small and further research is required in this area.2
Hyperbaric hyperoxia
The use of hyperbaric oxygen in decompression sickness is well established and is beyond the scope of this review. Its use for CO poisoning is described above.
Hyperbaric hyperoxia has also been used to improve the healing of open wounds or for the treatment of anaerobic infections, presumably by direct oxygenation of the tissues and again from purported improvement of neutrophil killing of microorganisms. A Cochrane review of five randomised controlled trials of its effect on wound healing found hyperbaric oxygen reduced amputation risk in patients with chronic diabetic foot ulcers, but concerns regarding the size and quality of the studies were raised.11
Strategies to avoid unintentional hyperoxia in clinical practice
Treat supplemental oxygen as a drug
In medicine, particularly in anaesthesia and critical care, the use of oxygen is widespread in the treatment or prevention of hypoxaemia. Often it will be the quickest and simplest way to prevent hypoxic tissue damage. Unfortunately, as a result of this widespread belief in the universal efficacy of inhaled oxygen, hyperoxia is often simply accepted rather than trying to maintain normoxia.
Oxygen is arguably the most widely used drug in acute medicine and anaesthesia. In the most recent, and largest to date, BTS oxygen audit in 2015, 14% of hospital patients were receiving supplemental oxygen at the time of the audit.12 Despite showing improvements from results in 2011, the audit still demonstrated areas of poor practice for oxygen prescribing, administration, and monitoring in that:
-
•
Forty-three percent of patients on oxygen had no valid prescription for it.
-
•
Only 53% had a target Spo2 range prescribed despite 70% of audited hospitals having a policy requiring a range to be set.
-
•
Sixty-nine percent of patients with a range prescribed had Spo2 values within the required range; 9% had Spo2 values below and 22% above.
-
•
Nine percent of patients receiving oxygen were identified as being at risk of type 2 respiratory failure because Spo2 values exceeded their target.
If referring to any other prescribed drug these results would be totally unacceptable, for example on any given day 6000 patients were receiving oxygen without any written order.12 Perhaps it is still widely assumed that using oxygen in the acutely ill patient cannot be harmful despite the growing body of evidence suggesting otherwise.
Use oxygen rationally in clinical practice
A summary of current recommendations for the use of oxygen in common acute medical conditions is shown in Table 2.
Table 2.
Summary of potential consequences of hyperoxia in medical emergencies and recommendations for its avoidance. CPR, cardiopulmonary resuscitation; MI, myocardial infarction.
| Medical condition | Potential consequences | Recommendations |
|---|---|---|
| Resuscitation and ventilation in neonates | Retinopathy Bronchopulmonary dysplasia |
Use of air for resuscitation, and if needed, judicious use of supplementary oxygen guided by pulse oximetry.13 |
| Respiratory failure | Hypercapnia Ventilatory support Acute lung injury |
Target Spo2 to 94–98% or 88–92% if patient is considered at risk of hypercapnia2: • COPD with previous hypercapnic respiratory failure • Cystic fibrosis or bronchiectasis • Morbid obesity (BMI >40 kg m−2) • Musculoskeletal disorders associated with respiratory muscle weakness • Therapy with opioids or benzodiazepines. |
| Myocardial Infarction | Increased infarct size Recurrent MI Arrhythmias |
Oxygen should only be administered if there is evidence of hypoxia, but there is no agreement on the absolute definition of hypoxia in this situation. The routine use of oxygen in patients with suspected myocardial infarction and Spo2 ≥90% does not reduce mortality at 1 yr, which suggests that this may be a suitable target.14 |
| Stroke | Increased disability Mortality |
Patients who have had a stroke should receive supplemental oxygen only if their Spo2 decreases below 95%. The most recent trial suggests no benefit from oxygen if Spo2 is ≥93%.15 |
| Adult resuscitation | Neurological outcome Survival |
Highest possible oxygen concentration during CPR. With return of spontaneous circulation titrate oxygen to Spo2 values of 94–98%. |
Whereas in the past almost any unwell patient would have been administered high flow oxygen, Table 2 shows that in most circumstances inspired oxygen should now be titrated to oxygen saturation. Only medical emergencies or the critically ill should receive 15 L min−1 via a non-rebreathe mask.2 A recent guideline has made a strong recommendation that acutely ill medical inpatients should have target oxygen saturations of no more than 96%.16 Under most circumstances measurement of hyperoxia can be difficult as the usual monitor of hypoxaemia, a pulse oximeter, provides little quantitatively useful information about Pao2 when above about 97%, although a reading of 100% does of course suggest hyperoxia. However, in intensive care, and often in theatre, measurement of arterial blood gases is feasible, and inspired oxygen can then be even more accurately titrated to avoid hyperoxia.
Critical care
There is no evidence of benefit from supranormal oxygen delivery in critically ill patients.2 A systematic review and meta-analysis of 19 cohort studies concluded hyperoxaemia was associated with increased in-hospital mortality in critically ill patients, but caution was advised again in the interpretation of the results because of the heterogeneity of studies included.17 A recent large (14 441 patients) observational study again found a U-shaped relationship between mean Pao2 whilst in the ICU and mortality, with both the degree and duration of hyperoxia adversely affecting outcome.18 The ideal oxygen level to maintain in a critically ill patient remains uncertain, but the most recent data suggests that ‘conventional’ levels of 9–13 kPa or oxygen saturations of 94–98%, are normal values.19
Perioperative oxygen
Anaesthetists use hyperoxia deliberately more than any other speciality, and this remains a vital safety strategy for periods in any anaesthetic where the ability to deliver oxygen to the patient is threatened, for example before induction, tracheal intubation or extubation. Some of the adverse effects of hyperoxia described above are not relevant under these circumstances as artificial ventilation is in place and atelectasis can be treated with recruitment manoeuvres. Outside of these well-defined and short periods of time when 100% oxygen is administered, anaesthetists should adopt a rational approach to oxygen administration to avoid hyperoxia. It has been suggested that perioperative oxygen levels could be controlled more precisely, involving administration of oxygen to a defined target Pao2 of 8–10 kPa or Sao2 of 88–92%.3
This approach has implications in some patients not just for the immediate perioperative period, but also into the postoperative period. A recent large hospital-based registry study found significant effects of intraoperative Fio2 on the frequency of developing a postoperative pulmonary complication with an odds ratio of 2 when using 79% inspired oxygen compared with 31%.20 The authors suggest that absorption atelectasis forming during anaesthesia and surgery can become the focus for a postoperative lung infection, and that intraoperative oxidative stress in patients with pre-existing lung disease will worsen their postoperative lung function. Another observational study found that patients having surgery for cancer had worse survival up to 3 yrs after operation if they received 80% compared with 30% inspired oxygen intraoperatively. The same effect was not seen in non-cancer surgery, suggesting a role for oxygen in the survival of metastatic cells seeded at the time of surgery, potentially resulting from oxygen's effects on neovascularisation or growth factors such as erythropoeitin.21
In the postoperative period the most common reason for oxygen administration is alongside an opioid patient-controlled analgesia system (PCA), the rationale being to prevent hypoxaemia in the event of respiratory depression. Hyperoxia has been found to have an additive effect on opioid-induced respiratory depression in healthy volunteers on a remifentanil infusion.22 Those on 50% inspired oxygen (compared with air) showed a steeper reduction in minute ventilation, an increase in end-tidal CO2 and a higher incidence of apnoeic episodes. As with other areas, more evidence to support or refute the routine use of oxygen after surgery is still required. In the absence of large randomised controlled trials on the effects of hyperoxia in patients using a PCA, current guidelines suggest oxygen should be used to correct rather than prevent hypoxia.2
Patients previously exposed to bleomycin
Oxygen is known to be potentially harmful to patients who have been treated with bleomycin, a chemotherapy agent used to treat germ cell tumours, squamous cell carcinoma, and non-Hodgkin's lymphoma. Bleomycin can cause pulmonary toxicity and dose-related progressive pulmonary fibrosis even when breathing air at atmospheric pressure. High concentrations of oxygen can then cause further damage, even if given years after the initial bleomycin. Bleomycin acts as a chemotherapeutic agent by damaging DNA, an action mediated by facilitating ROS production, and this DNA damage provides a potential mechanism by which long-term oxygen sensitivity may occur even after treatment is completed.
In patients with known bleomycin lung injury, it is recommended to only administer oxygen if Spo2 decreases below 85% and to reduce or stop oxygen if Spo2 values reach 88%.2 Patients with possible bleomycin lung injury should have oxygen titrated to target Spo2 values of 88–92%.2
Conclusions
Oxygen remains the best treatment for any patient with hypoxaemia, but evidence continues to accumulate of a lack of benefit of oxygen in many conditions not associated with hypoxaemia, and of harm from hyperoxia. With the now widespread availability of pulse oximetry, routine care should be titration of oxygen therapy to target oxygen saturations. Recent audits have continued to demonstrate the overuse of oxygen in hospitals, with many patients receiving oxygen without it having been prescribed or at inappropriate doses. Greater awareness is needed in all staff groups of the risks and benefits of using oxygen, including in ICUs and operating theatres.
Declaration of interest
The authors declare that they have no conflict of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Andy Lumb FRCA is consultant anaesthetist at St James's University Hospital and honorary clinical associate professor at the University of Leeds, specialising in thoracic anaesthesia. His research focuses on respiratory physiology. He teaches at all levels and lectures at postgraduate meetings both in the UK and abroad. He has authored many chapters, reviews and editorials, and four editions of Nunn's Applied Respiratory Physiology. He is an editorial board member of BJA Education and an associate editor of the BJA.
Liz Horncastle FRCA is a specialty registrar in anaesthesia at St James's University Hospital, Leeds, UK.
Matrix codes: 1A01, 2C02, 3C00
References
- 1.Lumb A. 8th Edn. Elsevier; Edinburgh: 2017. Nunn’s applied respiratory physiology. [Google Scholar]
- 2.British Thoracic Society Emergency Oxygen Guideline Development Group BTS guidelines for oxygen use for adults in healthcare and emergency settings. Thorax. 2017;72 i1–90. [Google Scholar]
- 3.Martin D.S., Grocott M.P. Oxygen therapy in anaesthesia: the yin and yang of O2. Br J Anaesth. 2013;111:867–871. doi: 10.1093/bja/aet291. [DOI] [PubMed] [Google Scholar]
- 4.McNulty P.H., Robertson B.J., Tulli M.A. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol. 2007;102 doi: 10.1152/japplphysiol.00595.2006. 2040–5. [DOI] [PubMed] [Google Scholar]
- 5.Buckley N.A., Juurlink D.N., Isbister G. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev. 2011:CD002041. doi: 10.1002/14651858.CD002041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hovaguimian F., Lysakowski C., Elia N. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomised controlled trials. Anesthesiol. 2013;119:303–316. doi: 10.1097/ALN.0b013e31829aaff4. [DOI] [PubMed] [Google Scholar]
- 7.Togioka B., Galvagno S., Sumida S. The role of perioperative high inspired oxygen therapy in reducing surgical site infection: a meta-analysis. Anesth Analg. 2012;114:334–342. doi: 10.1213/ANE.0b013e31823fada8. [DOI] [PubMed] [Google Scholar]
- 8.Allegranzi B., Zayed B., Bischoff P., the WHO Guidelines Development Group New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e288–e303. doi: 10.1016/S1473-3099(16)30402-9. [DOI] [PubMed] [Google Scholar]
- 9.Ball L., Lumb A.B., Pelosi P. Intraoperative fraction of inspired oxygen: bringing back the focus on patients’ outcome. Br J Anaesth. 2017;119:16–18. doi: 10.1093/bja/aex176. [DOI] [PubMed] [Google Scholar]
- 10.Kurz A., Kopyeva T., Suliman I. Supplemental oxygen and surgical-site infections: an alternating intervention controlled trial. Br J Anaesth. 2018;120:117–126. doi: 10.1016/j.bja.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Kranke P., Bennett M., Roeckl-Wiedmann I. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004:CD004123. doi: 10.1002/14651858.CD004123.pub2. [DOI] [PubMed] [Google Scholar]
- 12.O’Driscoll R. British Thoracic Society emergency oxygen audit report national audit period: 15 August–1 November 2015. https://www.brit-thoracic.org.uk/document-library/audit-and-quality-improvement/audit-reports/bts-emergency-oxygen-audit-report-2015/(accessed 15 October 2018).
- 13.Wyllie J., Ainsworth S., Tinnion R. Resuscitation Council (UK); 2015. Resuscitation and support of transition of babies at birth.https://www.resus.org.uk/resuscitation-guidelines/resuscitation-and-support-of-transition-of-babies-at-birth/ guidelines. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann R., James S.K., Jernberg T. For the DETO2X-SWEDEHEART investigators. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. 2017;377:1240–1249. doi: 10.1056/NEJMoa1706222. [DOI] [PubMed] [Google Scholar]
- 15.Roffe C., Nevalle T., Sim J. Effect of routine low-dose oxygen supplementation on death and disability in adults with acute stroke: the Stroke Oxygen Study randomised clinical trial. JAMA. 2017;318:1125–1135. doi: 10.1001/jama.2017.11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siemieniuk R.A.C., Chu D.K., Kim L.H.-Y. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. Br Med J. 2018;363:k4169. doi: 10.1136/bmj.k4169. [DOI] [PubMed] [Google Scholar]
- 17.Helmerhorst H.J., Roos-Blom M.J., van Westerloo D.J. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508–1519. doi: 10.1097/CCM.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 18.Helmerhorst H.J.F., Arts D.L., Schultz M.J. Metrics of arterial hyperoxia and associated outcomes in critical care. Crit Care Med. 2017;45:187–195. doi: 10.1097/CCM.0000000000002084. [DOI] [PubMed] [Google Scholar]
- 19.Girardis M., Busani S., Damiani E. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. The oxygen-ICU randomised clinical trial. JAMA. 2016;316:1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 20.Staehr-Rye A.K., Mayhoff C.S., Scheffenbichler F.T. High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. Br J Anaesth. 2017;119:140–149. doi: 10.1093/bja/aex128. [DOI] [PubMed] [Google Scholar]
- 21.Meyhoff C.S., Jorgensen L.N., Wetterslev J., Christensen K.B., Rasmussen L.S., the PROXI Trial Group Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomised clinical trial. Anesth Analg. 2012;115:849–854. doi: 10.1213/ANE.0b013e3182652a51. [DOI] [PubMed] [Google Scholar]
- 22.Niesters M., Mahajan R.P., Aarts L. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth. 2013;110:837–841. doi: 10.1093/bja/aes494. [DOI] [PubMed] [Google Scholar]