Learning objectives.
By reading this article you should be able to:
-
•
Explain the physiological basis of the four general causes of systemic hypoxia: hypoxic, circulatory, anaemic and histotoxic hypoxia.
-
•
Describe the mechanism underlying chemotransduction in the carotid bodies.
-
•
Discuss how the respiratory system increases alveolar oxygenation and how the cardiovascular system maintains oxygen delivery.
-
•
Identify how hypoxia can affect the fetus and how the fetus defends itself against periods of hypoxia.
Key points.
-
•
Tissue hypoxia can result from the inability to transfer oxygen from the atmosphere to the mitochondria by obstruction at any point in the oxygen cascade.
-
•
Hypoxaemia, low oxygen tension in arterial blood, is detected by the carotid body.
-
•
The response to hypoxia is multisystem involving coordinated respiratory, cardiovascular, metabolic and cerebrovascular responses.
-
•
Fetal oxygenation occurs via the placenta. The strategy in fetal life therefore focuses on redistribution of the oxygenated blood away from the periphery and towards the brain, and reducing oxygen consumption.
The evolution of mammals, and indeed humans, has taken hundreds of millions of years. Although we commonly associate water with the potential for life, it has really been water coupled with increases in atmospheric oxygen that has allowed development of species with high aerobic demand.1,2 As a species, this evolutionary entanglement with oxygen has been a great asset, but maybe as individuals it also exposes a significant weakness. We are so dependent on oxygen that only minutes of decreased oxygen delivery to our brain can lead to significant life-altering hypoxic injury. Consequently, the respiratory and cardiovascular systems are essentially slaves to oxygen uptake from the atmosphere and delivery to our tissues. Interruptions in this flow can rapidly lead to a state of tissue hypoxia. Therefore, mammals have evolved a complex multisystem response to hypoxia. In this article, we examine how mammals detect hypoxic states and then explore in detail the respiratory, cardiovascular, metabolic and cerebrovascular responses in the adult. We will then consider how the fetus withstands such insults and consider the transition in the response to hypoxia from fetal to postnatal life.
Classical hypoxic states
Atmospheric pressure is approximately 101 kPa (760 mmHg), of which 21% is oxygen, giving a partial pressure of about 21 kPa (160 mmHg). Upon inspiration, humidification of air at body temperature lowers oxygen partial pressure to 19.8 kPa (149 mmHg) and addition of alveolar CO2 further decreases it to approximately 14 kPa (105 mmHg) in the alveoli. Arterial partial pressure of oxygen (Pao2) is 13 kPa (100 mmHg), and there is a significant diffusion gradient down to the mitochondrial level where Po2 may be only 1–2 kPa. Tissue hypoxia is caused by any obstacle in this oxygen cascade, the journey of molecular oxygen from the atmosphere to the point of being an electron acceptor within the mitochondria (Table 1). Historically, there are four broad classifications for hypoxic states as first described by Barcroft. First, hypoxaemic hypoxia is where oxygen transport to and/or across the alveolus may become impaired. Second, circulatory or stagnant hypoxia exists where blood may be sufficiently oxygenated but transport to the tissues is prevented. This is often referred to as a state of shock and may arise through severe hypovolaemia, cardiac failure, circulatory obstruction (e.g. massive pulmonary embolism) or failure of distribution (e.g. septic or anaphylactic shock). Sickle cell disease may also lead to tissue hypoxia through sickling in the microcirculation. Third, anaemic hypoxia exists where there is an inability to carry oxygen, for example severe iron or significant folate deficiency, bleeding or haemoglobinopathies. Finally, histotoxic hypoxia is where oxygen is delivered to end organs, but they are unable to utilise it, often because of electron transport chain dysfunction or inhibition, as can be seen in severe sepsis, cyanide and carbon monoxide poisoning. Although carbon monoxide does lead to a degree of anaemic hypoxia through generation of carboxyhaemoglobin, the role of inhibition of haem flavoproteins in mitochondria is often overlooked.
Table 1.
General causes of systemic hypoxia
| Cause of hypoxia | Impairment | Examples |
|---|---|---|
| Hypoxaemic | Gas transport to, or across, the alveoli | Low atmospheric oxygen tension (e.g. altitude) Obstructive lung disease (e.g. asthma, chronic obstructive pulmonary disease) Restrictive lung disease (e.g. fibrosis) Pulmonary oedema Pulmonary consolidation Acute respiratory distress syndrome |
| Circulatory/stagnant | Transport of oxygen from the alveoli to tissues | Hypovolaemia Cardiogenic shock Distributive shock (e.g. septic, anaphylactic) Obstructive shock (e.g. cardiac tamponade, tension pneumothorax, massive pulmonary embolism) |
| Anaemic | Oxygen carrying capacity of blood | Low haemoglobin concentration (e.g. iron deficiency, folate deficiency) Genetic haemoglobinopathies (e.g. thalassaemias) |
| Histotoxic | Oxygen utilisation at cellular level | Cellular dysfunction (e.g. sepsis) Cyanide (mitochondrial complex IV inhibitor) Carbon monoxide poisoning |
Oxygen delivery can be calculated for the whole body or to the individual organ systems as a product of oxygen content and blood flow. As oxygen content is the sum of dissolved oxygen and that bound to haemoglobin, total oxygen delivery can be calculated as:
| (1) |
where o2 is oxygen delivery (ml min−1); Pao2 is the partial pressure of oxygen (kPa); Sao2 is arterial oxygen saturation as a percentage; [Hb] is haemoglobin content (g dl−1); 0.023 is the solubility of oxygen (in ml dl−1 kPa−1); 1.34 is Hüfner's constant, the oxygen carrying capacity of saturated haemoglobin (ml g−1); and blood flow (i.e. cardiac output) in dl min−1.3 In the adult o2 ≈ 1000 ml min−1. Oxygen consumption at rest is 250 ml min−1; therefore, approximately 25% of available oxygen is extracted (i.e. approximately 25% of that delivered) giving mixed venous blood oxygen saturations of approximately 75%. From the equation above it can be seen how hypoxaemic hypoxia (via reduced Pao2 and Sao2), stagnant hypoxia (via reduced blood flow) and anaemic hypoxia (via reduced [Hb]) may cause tissue hypoxia, as they all conspire to reduce oxygen delivery. In contrast, in histotoxic hypoxia, there is no deficit in oxygen delivery, but rather a failure of the tissue to consume oxygen, and consequently the venous oxygen saturation increases.
The adult response to acute hypoxia
Chemoreception
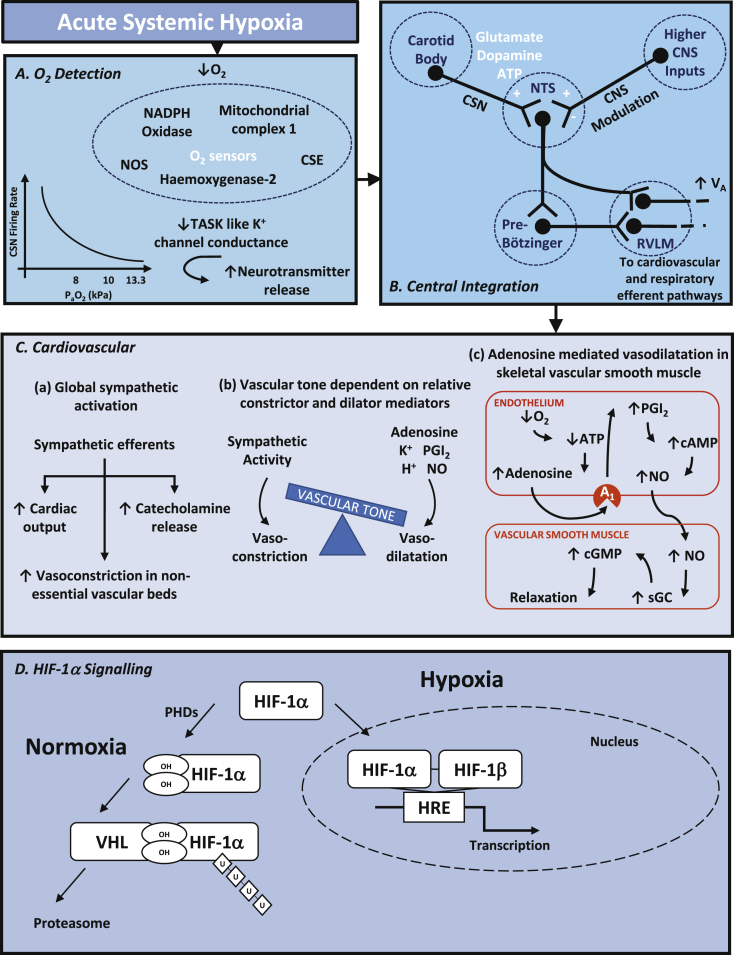
Located at the bifurcation of the common carotid artery, and identified by Heymans in 1926, the carotid bodies are the main arterial blood oxygen sensors. Type 1 glomus cells within the carotid body respond to decreases in oxygen tension by releasing neurotransmitter onto the carotid sinus nerve (CSN), a branch of the glossopharyngeal nerve.4 In addition to the carotid bodies, the aortic bodies, found on the arch of the aorta, contribute to oxygen sensing in the systemic circulation and relay afferent signals via sensory fibres within the vagus nerve. Both the carotid and aortic bodies have a low, if any, basal firing rate under normoxic conditions (Pao2 >13.3 kPa or 100 mmHg). As oxygen tension decreases below 13.3 kPa, firing rates increase within seconds, and in the range of 8.0–10.5 kPa (60–80 mmHg), this increase is hyperbolic.5 Interestingly, this point at which CSN firing rates increases rapidly corresponds to the point in the oxygen–haemoglobin dissociation curve at which oxygen unloading becomes significant. This means that the chemoreceptors are finely tuned to respond to changes in Pao2 that are physiologically important.
Significant insights into the cellular mechanism underlying chemotransduction within the carotid body came in the 1980s when Lopez-Barneo and colleagues showed that hypoxia leads to closure of acid-sensitive K+ ion channels, now referred to as TASK-like K+ channels, and hence depolarisation and release of neurotransmitter.6 The precise mechanisms of O2 sensing are complex and may include reactive oxygen species (ROS) production by mitochondria or NADPH (nicotinamide adenine dinucleotide phosphate) oxidase under hypoxic conditions and/or the gaseous neurotransmitters carbon monoxide and hydrogen sulphide. Interestingly, general anaesthetic agents significantly suppress this response in the carotid body. In vitro, both halothane and sevoflurane suppress the increase in intracellular Ca2+ seen in response to hypoxia, more so halothane.7
The carotid body is uniquely developed to detect only Pao2 and therefore hypoxaemic hypoxia. It is not sensitive to anaemic or circulatory hypoxia. It achieves this because of a high blood flow relative to its size and therefore to its metabolic rate. Consider the well-known Fick relationship as applied to oxygen delivery and consumption of the carotid body:
| (2) |
where o2 is oxygen uptake, is blood flow, and Sao2 and Svo2 are the arterial and venous haemoglobin saturations in blood supplying the carotid body. Cells in a tissue are generally considered to be in equilibrium with the venous end of a capillary, and so the oxygen tension sensed by the carotid body glomus cells will be related to the term Svo2. By rearranging the Fick equation, we obtain:
| (3) |
Inspection of this equation reveals that if blood flow is very large compared to oxygen consumption, then the term on the far right of the equation tends to zero, and so conveniently, the blood flow and haemoglobin terms disappear. As a result, Svo2 ≈ Sao2 and Pvo2 ≈ Pao2. Hence, carotid body glomus cells ‘sense’ only Pao2. They are insensitive to the haemoglobin concentration or the blood flow. By contrast, consider the kidneys, which detect (and provide an erythropoetic response to) not only chronic hypoxaemic hypoxia but also chronic anaemic hypoxia. Importantly though, they need to be insensitive to stagnant conditions, circulatory conditions, or both. From equation (3), it is clear how kidney erythropoietin- producing cells (which detect Svo2) will be sensitive to hypoxaemic hypoxia, as a decrease in Sao2 will produce a decrease in Svo2. But how is it able to distinguish anaemic hypoxia from stagnant hypoxia when the carotid bodies do not? The answer lies in the metabolic uniqueness of the kidney. Whereas in every other tissue a change in metabolic rate generally drives the resulting change in perfusion (to various degrees of completeness), the kidney is different in that its perfusion drives its metabolic rate. Increased perfusion drives increased glomerular filtration rate which necessitates increased tubular metabolic activity. Consequently, o2 and are directly proportional to each other in the kidney and therefore the terms o2 and in equation 3 cancel out, leaving only the term . Hence, the Po2 of the erythropoietin producing cells is dependent on both Pao2 and [Hb], but not blood flow.
Central pathways regulating respiratory and cardiovascular responses to hypoxia
Afferent signals from the carotid and aortic bodies travel via the CSN and vagus nerve respectively, synapsing at the nucleus of the solitary tract (NTS) in the medulla of the brainstem. In the NTS, afferent nerve endings release the excitatory neurotransmitter glutamate onto postsynaptic NMDA receptors, exciting interneurones. From the NTS, there are several projections of neurones to respiratory and autonomic centres.8 Activation of the pre-Bötzinger complex by the NTS, a key site of respiratory pattern generation, results in increased ventilatory drive.
Respiratory response
In the adult, the hypoxic ventilatory response aims to increase alveolar oxygenation, specifically the partial pressure of oxygen in alveolar gas (Pao2). This is achieved through increases in both tidal volume (VT) and ventilation rate.8 Under isocapnic conditions, where Paco2 is maintained experimentally by supplementing CO2 in inspired gas, this response is greater than poikilocapnic conditions, where Paco2 can vary with alveolar ventilation. Either way the initial ventilatory response is followed by a decrease in ventilation, termed hypoxic ventilatory decline, typically over a period of 30 min in most models. If hypoxia is sustained for hours to days (i.e. becomes chronic, e.g. ascent to high altitude), there is a further increase in ventilatory drive. Classically, it was taught that this is attributable to central resetting of CO2-sensitive chemoreceptors. However, this view is now disputed, and tissue pH may remain alkalotic even when ventilation increases. Instead alterations in carotid sinus sensitivity to hypoxia and alterations in central nervous system gain are now thought to contribute to this response.8
At the alveolar level, the partial pressure of O2 (Pao2) is dependent on the mass balance of oxygen, that is Pao2 is determined by O2 delivery and removal from the alveoli, and can be represented as follows:
| (4) |
where Pio2 is the partial pressure of oxygen in inspired gas, o2 is oxygen uptake from alveolar gas, and A is the alveolar ventilation rate. Given that R, the respiratory exchange ratio, is defined as , and co2=Paco2×A, equation (4) can be rewritten by substitution as the more familiar alveolar gas equation:
| (5) |
This is useful to estimate Pao2 from inspired O2, which can be directly controlled, and alveolar CO2, which can be measured from end tidal CO2. In turn, the difference between alveolar and arterial Po2 (the A–a gradient) can be estimated and used as indication of a diffusion defect or ventilation/perfusion mismatch.
Cardiovascular response
In the adult, acute hypoxia leads to cardiovascular responses that aim to maintain oxygen to essential tissues. The response is complex with a centrally driven neural and endocrine response that sees sympathetic activation with increases in sympathetic nerve activity, heart rate, and systolic and diastolic blood pressure in humans.9 In animal models, plasma adrenaline and noradrenaline increase and contribute to cardiovascular responses, particularly the tachycardia,10 and there is vasoconstriction in cutaneous, renal and mesenteric vascular beds.11 This central sympathetic activation is somewhat opposed in some tissue beds by the direct action of hypoxia where vasodilatation predominates, for example the brain, heart and skeletal muscle, allowing increased blood flow, albeit poorly oxygenated, to be preferentially streamed to these organs to aid survival.12 Interestingly, by experimentally controlling respiratory movements to prevent hyperventilation, and therefore activation of lung vagal stretch receptors, a bradycardic response may occur. This suggests that the ventilatory response to acute hypoxia is secondary to the primary effects of hypoxia on carotid body cardiovascular reflex activation.13
Chemoreceptor activation of the NTS leads to activation of cardiovascular autonomic nuclei within the brainstem. The rostral ventrolateral medulla (RVLM) is activated by interneurones from the NTS and the pre-Botzinger complex, which itself receives inputs from the NTS.14 The RVLM represents a pre-sympathetic centre with activation leading to increased cardiovascular efferent sympathetic activity via the intermediolateral cell column (IML). This is seen as activation of effector sympathetic fibres to the heart and vasculature. Within the heart, cardiac sympathetic nerves release noradrenaline onto β1 adrenergic receptors and activate G-proteins and adenyl cyclase in turn, leading to positive chronotropic and inotropic effects to increase cardiac output.
There is redistribution of cardiac output with increased vascular conductance in cerebral, adrenal and skeletal muscle with maintained or increased resistance in the renal, gut and splenic vascular beds.15 The resultant vasomotor activity is dependent upon the relative contributions of sympathetic nervous activity, which has vasoconstrictor action, and local tissue responses which mostly have dilator effects.16 Hypoxia-induced vasodilatation of arterioles supplying skeletal muscle has been closely investigated, particularly in animal models. The systemic response to hypoxia includes a sympathetic activation with noradrenaline release from sympathetic nerve endings onto α1 receptors, normally resulting in vasoconstriction. However, in skeletal muscle, the release of local vasodilator influences opposes and overcomes this in a process termed sympatholysis.16 Current experimental work suggests that about 50% of local vasodilator effects are driven by adenosine acting on the A1 receptor on the vascular endothelium, leading to release of nitric oxide (NO). Under hypoxic conditions, the regeneration of adenosine triphosphate (ATP) is reduced, and levels of adenosine diphosphate (ADP), adenosine monophosphate (AMP), and adenosine are increased. Adenosine released from endothelial cells acts in an autocrine fashion on purinergic receptors, promoting vasodilatation via NO-dependent pathways.17 Furthermore, endothelial contributions to hypoxic vasodilatation of vascular smooth muscle come from mediators including ATP and endothelium-dependent hyperpolarisation factors, which include K+, H2O2, eicosanoid derivatives and gasotransmitters (e.g. H2S, CO).16 Hence, local tissue hypoxia drives vasodilatation and promotes an increase in vascular conductance, thereby maintaining blood flow and the delivery of oxygen to where it is most needed.
Cerebrovascular response
It has long been known that during periods of cerebral hypoxia, oxygen delivery is maintained primarily by vasodilatation leading to increases in cerebral blood flow (CBF).18 In humans, basal CBF of approximately 0.5 ml g−1 min−1 can almost double once cerebral oxygen tension lowers below 6.6 kPa (50 mmHg). This effect is more profound in areas regulating autonomic functions, including the brainstem and mid-brain, relative to the cerebral cortex. Opposing hypoxic cerebrovascular dilatation are the influences of hypocapnia and alkalosis, which may arise because of respiratory compensation to isolated hypoxia. In contrast, asphyxia, that is hypoxia in the presence of hypercapnia and reduced pH, augments cerebral vasodilatation and therefore CBF.
Cerebral hypoxic vasodilatation has three intertwined pathways that arise from an increase in the abundance of deoxygenated haemoglobin, leading to NO generation and vascular smooth muscle relaxation.19 First, deoxyhaemoglobin can convert circulating nitrite (NO2–) to NO. Second, NO, or S-nitrosothiol (SNO) groups produced in the lungs can bind to haemoglobin in the oxygenated state allowing them to be transported to distant sites. Upon arrival in hypoxic tissues, including the brain, SNO is liberated when haemoglobin transforms to the deoxygenated state. Finally, hypoxia induces ATP release from red blood cells (RBCs), which acts via purinergic receptors to promote vasodilatation.
Intracellular oxygen sensing
In addition to this classical description of oxygen sensing, work in the past 20–30 yrs, driven independently by the laboratories of Kaelin, Ratcliffe and Semenza, has elucidated the mechanisms by which every cell can sense hypoxia, including the central role of hypoxia inducible factors (HIFs).20 This work has led to the joint award of the 2019 Nobel Prize for Physiology or Medicine to the three researchers. The key molecular switch to upregulating these genes is HIF-1α (Fig. 1D). HIF-1α, acting together with HIF-1β, acts as a transcription factor on nuclear hypoxia response elements promoting transcription of target genes. Under normoxic conditions, prolyl hydroxylases add hydroxyl groups to proline residues on HIF-1α making it a target for the tumour suppressor VHL (von Hippel–Lindau protein) to bind. The VHL complex catalyses ubiquitination (addition of ubiquitin groups), which then marks HIF-1α for proteolysis by the proteasome. The key is that the action of prolyl hydroxylase is dependent upon oxygen, and therefore hypoxia prevents the destruction of HIF-1α. Under hypoxic conditions, HIF-1α accumulates and may activate transcription of genes promoting erythropoiesis, angiogenesis and cell survival.
Fig. 1.
General overview of (A) O2 detection, (B) central integration, (C) cardiovascular and (D) HIF-1α signalling in response to hypoxia. ATP, adenosine triphosphate; cGMP, cyclic guanosine monophosphate; CSE, cystathionine gamma lyase; CNS, central nervous system; CSN, carotid sinus nerve; HIF, hypoxia inducible factor; HRE, hypoxia response element; K+, potassium ion; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; NO, nitric oxide; NOS, nitric oxide synthase; NTS, nucleus of the solitary tract; Po2, arterial oxygen partial pressure; PGI2, prostaglandin I2; PKG, protein kinase G; RVLM, rostral ventrolateral medulla; sGC, soluble guanylyl cyclase; TASK, acid sensitive K+ channel; U, ubiquitin; VHL, von Hippel–Lindau protein; ↑, increase; ↓, decrease.
The fetal response to acute hypoxia
The potential for a period of oxygen deprivation in the fetus is substantial with the fetus being relatively hypoxic compared with the mother even under normal circumstances. Oxygen tension in human umbilical venous blood returning from the placenta at term is approximately 4 kPa (30 mmHg), fetal blood haemoglobin saturation is about 50%, and oxygen content about 100 ml L−1, with about a 60% extraction ratio by the fetal circulation.21 Therefore, the degree to which the fetus can compensate during periods of reduced oxygenation by increasing oxygen extraction alone is limited, and a whole body response to hypoxia is required. This response to hypoxia during fetal life is well characterised, and it involves coordinated neural, endocrine, and metabolic mechanisms that aid fetal survival. This fetal response to hypoxia is well conserved across species, from reptiles to birds and mammals, including non-human primates and the human fetus.22 In contrast to the adult, when acute hypoxia triggers both ventilatory and cardiovascular responses, acute fetal hypoxia inhibits fetal breathing movements and the compensatory mechanisms are primarily dependent on cardiovascular responses.22
In the fetal period, acute hypoxia leads to redistribution of the cardiac output away from the periphery and towards the cerebral, coronary, and umbilical circulations. This is the so-called fetal brain-sparing response.22 In contrast to the adult, acute hypoxia also triggers bradycardia rather than an increase in heart rate.22 The fetal carotid body-mediated bradycardia and peripheral vasoconstriction are therefore good examples of the primary carotid chemoreflex responses to acute hypoxia, uncomplicated by ventilatory influences. They are therefore similar to the adult cardiovascular responses to acute hypoxia when hyperventilation is prevented.12,13 Fetal bradycardia reduces myocardial oxygen consumption; it prolongs end diastolic filling time and thereby volume, helping to maintain cardiac output. In addition, fetal bradycardia reduces the speed of blood flow through the microcirculation, maximising gaseous exchange in the myocardial and cerebral vascular beds.22 As in the adult, hypoxia is sensed by the carotid arterial chemoreceptors and information is relayed to the fetal brain via the glossopharyngeal nerve.22 In turn, there is activation of both the sympathetic and parasympathetic arms of the autonomic nervous system. The neural component of the sympathetic nervous system drives α1 adrenoreceptor-mediated vasoconstriction of the peripheral circulation. This leads to increased peripheral vascular resistance and reduced blood flow in peripheral circulations. In contrast, carotid vascular resistance is decreased to direct a greater proportion of blood flow to the brain.23 Vagal activation of the heart leads to the profound fetal bradycardia,23 that is recognised clinically as a fetal deceleration by cardiotocography. It is important to note that the bradycardia is not an arterial baroreflex in response to the peripheral vasoconstriction-induced increase in fetal arterial blood pressure. If the vasoconstriction is blocked with phentolamine, an α1-adrenergic selective antagonist, bradycardia still occurs in the absence of any hypertension.23 If the period of hypoxia is prolonged, severe, or both, the fetus will release a vast array of hormones into the fetal circulation to augment the redistribution of blood flow. For example, fetal plasma adrenaline and noradrenaline, cortisol, angiotensin II, arginine vasopressin, and neuropeptide Y all increase in response to acute hypoxia to maintain the fetal peripheral vasoconstrictor response. Towards the end of pregnancy and associated with the prepartum surge in fetal plasma glucocorticoids, the gain of the fetal cardiovascular response increases, in part because of greater endocrine responses to hypoxia.22 Hence, the bradycardia becomes more sustained and the magnitude of the peripheral vasoconstrictor response to acute hypoxia increases in the late gestation fetus. Recently, it has also become appreciated that there is a hypoxic response at the level of the vasculature itself. Here, there is a dynamic local relationship between the potent vasodilator NO and ROS generation, for example the superoxide anion (•O2–). Hypoxia-induced increases in the •O2–/NO ratio in the fetus potentiate the peripheral vasoconstriction and redistribution of blood flow away from peripheral circulations and towards the brain.22 The fetus also instigates metabolic responses leading to increases in the circulating concentrations of glucose and lactate. The fetal hyperglycaemia during acute hypoxia results from a decrease in glucose uptake and utilisation by peripheral tissues and an increase in hepatic glucose production. The fetal lactic acidaemia arises from anaerobic metabolism of glucose in hypoxic fetal tissues, particularly in the hind limb where blood flow and oxygen delivery are markedly declined.
During postnatal life, there is a transition from the fetal to adult cardiovascular responses to hypoxia. Indeed, in paediatric patients it is often observed that hypoxia leads to bradycardia.24 The primary fetal cardiovascular response remaining into postnatal life has been attributed to increased rates of survival in drowning children compared with adults, as the facial immersion reflex is more pronounced and strongly linked to the fetal cardiovascular response.25 This response, along with better outcomes, is often cited as a reason to continue prolonged resuscitation of paediatric patients submerged in cold water. However, this primary cardiovascular chemoreflex response has also been hypothesised to contribute to the pathogenesis of sudden infant death syndrome.26 It is noted that in normal sleeping human neonates, hypoxia leads to an increase in a state of arousal that is associated with a biphasic response in ventilation. First, there is an increase in ventilation before a reduction in ventilatory rate towards baseline. This increase in arousal and ventilation gives the neonate the chance to respond to hypoxic challenges.27 With advancing postnatal life, there is a change in this cardiorespiratory response. The ventilatory response is no longer biphasic, primarily leading to hyperventilation and the development of the tachycardic response to acute hypoxia, which helps maintain cardiac output and oxygenation, as shown not only in animal studies but also human neonates.27 In some groups of neonates, for example premature, smoke-exposed or born at high altitude, this initial increase in ventilation in response to acute hypoxia may not occur, putting them at risk of persistent bradycardia and cardiovascular collapse. It has been suggested that failure of activation of the primary ventilatory response to acute hypoxia in the newborn may be associated with structural differences and alterations in serotonin signalling in the brainstem.28
Conclusions
In summary, hypoxia represents a significant threat to pre- and postnatal life. We have seen how as mammals we are well set up to detect hypoxaemia rapidly through activation of the carotid and aortic bodies. The adult physiological compensatory responses to acute hypoxia are determined by our ability to hyperventilate, increasing oxygen uptake and matching this with activation of cardiovascular responses designed to increase cardiac output and thereby drive the newly oxygenated blood to hypoxia-sensitive tissues. In marked contrast, in fetal life, because of our inability to breathe in utero to increase oxygenation, we have evolved compensatory responses that are contingent on the fetal cardiovascular system alone. These focus on reducing oxygen consumption by triggering bradycardia and making best use of the finite oxygen supply, by redistributing the cardiac output away from peripheral circulations and towards the fetal brain. In the newborn, there is a halfway house, both in terms of cardiovascular and respiratory compensatory responses, making the neonate vulnerable to hypoxia until the cardiorespiratory responses fully mature.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Andrew Kane MRCP PhD is a specialty registrar in anaesthesia in the Northern School of Anaesthesia and Intensive Care Medicine with research interests in hypoxia and cardiovascular control.
Elke Kothmann FRCA CertMedEd is a consultant anaesthetist who has research interests in perioperative medicine and prehabilitation for major surgery.
Dino Giussani PhD ScD FRCOG is professor of developmental cardiovascular physiology and medicine at the University of Cambridge. His research interests include fetal brain sparing during acute hypoxia and investigating how adverse conditions during pregnancy can programme disease in offspring in later life.
Matrix codes: 1A01, 2B05, 3J03
References
- 1.Berger M.M., Grocott M.P.W. Facing acute hypoxia: from the mountains to critical care medicine. Br J Anaesth. 2017;118:283–286. doi: 10.1093/bja/aew407. [DOI] [PubMed] [Google Scholar]
- 2.Lane N. Oxford University Press; Oxford: 2002. Oxygen. [Google Scholar]
- 3.McLellan S.A., Walsh T.S. Oxygen delivery and haemoglobin. Contin Educ Anaes Crit Care Pain. 2004;4:123–126. [Google Scholar]
- 4.Prabhakar N.R., Semenza G.L. Oxygen sensing and homeostasis. Physiology. 2015;30:340–348. doi: 10.1152/physiol.00022.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhakar N.R., Peers C. Gasotransmitter regulation of ion channels: a key step in O2 sensing by the carotid body. Physiology. 2014;29:49–57. doi: 10.1152/physiol.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Barneo J., Lopez-Lopez J., Urena J., Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 7.Pandit J., Buckler K. Differential effects of halothane and sevoflurane on hypoxia-induced intracellular calcium transients of neonatal rat carotid body type I cells. Br J Anaesth. 2009;103:701–710. doi: 10.1093/bja/aep223. [DOI] [PubMed] [Google Scholar]
- 8.Pamenter M.E., Powell F.L. Time domains of the hypoxic ventilatory response and their molecular basis. Compreh Physiol. 2016;6:1345–1385. doi: 10.1002/cphy.c150026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie A., Skatrud J.B., Puleo D.S., Morgan B.J. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol. 2001;91:1555–1562. doi: 10.1152/jappl.2001.91.4.1555. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.C., Werner J.C., Downing S.E. Adrenal contribution to cardiac responses elicited by acute hypoxia in piglets. Am J Physiol Heart Circ Physiol. 1980;239:H751–H755. doi: 10.1152/ajpheart.1980.239.6.H751. [DOI] [PubMed] [Google Scholar]
- 11.Marshall J.M. Analysis of cardiovascular responses evoked following changes in peripheral chemoreceptor activity in the rat. J Physiol. 1987;394:393–414. doi: 10.1113/jphysiol.1987.sp016877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall J.M. The integrated response to hypoxia: from circulation to cells. Exp Physiol. 1999;84:449–470. [PubMed] [Google Scholar]
- 13.Angell-James J.E., Daly M.D.B. The effects of artificial lung inflation on reflexly induced bradycardia associated with apnoea in the dog. J Physiol. 1978;274:349–366. doi: 10.1113/jphysiol.1978.sp012152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dampney R.A.L. Central neural control of the cardiovascular system: current perspectives. Adv Physiol Educ. 2016;40:283–296. doi: 10.1152/advan.00027.2016. [DOI] [PubMed] [Google Scholar]
- 15.Marshall J.M., Metcalfe J.D. Effects of systemic hypoxia on the distribution of cardiac output in the rat. J Physiol. 1990;426:335–353. doi: 10.1113/jphysiol.1990.sp018141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall J.M. Interactions between local dilator and sympathetic vasoconstrictor influences in skeletal muscle in acute and chronic hypoxia. Expl Physiol. 2015;100:1400–1411. doi: 10.1113/EP085139. [DOI] [PubMed] [Google Scholar]
- 17.Ray C.J., Abbas M.R., Coney A.M., Marshall J.M. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol. 2002;544:195–209. doi: 10.1113/jphysiol.2002.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen P.J., Alexander S.C., Smith T.C., Reivich M., Wollman H. Effects of hypoxia and normocarbia on cerebral blood flow and metabolism in conscious man. J Appl Physiol. 1967;23:183–189. doi: 10.1152/jappl.1967.23.2.183. [DOI] [PubMed] [Google Scholar]
- 19.Hoiland R.L., Bain A.R., Rieger M.G., Bailey D.M., Ainslie P.N. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. Am J Physiol Regul Integ Compar Physiol. 2016;310:R398–R413. doi: 10.1152/ajpregu.00270.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaelin W.G., Jr., Ratcliffe P.J., Semenza G.L. Pathways for oxygen regulation and homeostasis: the 2016 Albert Lasker basic medical research award. JAMA. 2016;316:1252–1253. doi: 10.1001/jama.2016.12386. [DOI] [PubMed] [Google Scholar]
- 21.Postigo L., Heredia G., Illsley N.P. Where the O2 goes to: preservation of human fetal oxygen delivery and consumption at high altitude. J Physiol. 2009;587:693–708. doi: 10.1113/jphysiol.2008.163634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giussani D.A. The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol. 2016;594:1215–1230. doi: 10.1113/JP271099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giussani D.A., Spencer J.A., Moore P.J., Bennet L., Hanson M.A. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones P., Dauger S., Peters M.J. Bradycardia during critical care intubation: mechanisms, significance and atropine. Arch Dis Child. 2011;97:139–144. doi: 10.1136/adc.2010.210518. [DOI] [PubMed] [Google Scholar]
- 25.Nitta M., Kitamura T., Iwami T. Out-of-hospital cardiac arrest due to drowning among children and adults from the Utstein Osaka Project. Resuscitation. 2013;84:1568–1573. doi: 10.1016/j.resuscitation.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darnall R.A. The carotid body and arousal in the fetus and neonate. Respir Physiol Neurobiol. 2013;185:132–143. doi: 10.1016/j.resp.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bissonnette J.M. Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integ Compar Physiol. 2000;278:R1391–R1400. doi: 10.1152/ajpregu.2000.278.6.R1391. [DOI] [PubMed] [Google Scholar]
- 28.Kinney H.C. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Develop Psychobiol. 2009;51:223–233. doi: 10.1002/dev.20367. [DOI] [PubMed] [Google Scholar]