Learning objectives.
By reading this article, you should be able to:
-
•
Describe the consequences of failure of an intrathecal drug delivery system.
-
•
Explain the diagnostic procedures available to determine the cause of failure.
-
•
Describe the management of intrathecal drug delivery system failure.
Key points.
-
•
Device failures and human errors related to intrathecal drug delivery systems can lead to fatal complications as a result of withdrawal or overdose.
-
•
Health professionals often fail to recognise the complications of intrathecal drug delivery failure because of a lack of awareness.
-
•
Opioid-induced intrathecal granulomas can cause spinal cord compression and radicular symptoms.
-
•
Non-pathogenic microorganisms can cause meningitis with minor clinical symptoms.
Introduction
Intrathecal drug delivery is an invasive treatment for the management of therapy-resistant pain, spasticity and dystonia not controlled by oral drug therapy. This form of delivery is based on the premise that effective treatment can be achieved by administering a low volume of drugs near to the site of action, thus reducing the incidence and severity of adverse effects. High systemic doses of drugs are required to achieve the same concentrations. On-label drugs used include morphine, baclofen and ziconotide, but the use of off-label drugs such as hydromorphone, clonidine, fentanyl and mixtures of different drugs are used frequently. Errors and treatment failures can have life-threatening consequences. Health professionals are often unaware of potential complications of intrathecal drug delivery and their management because of a lack of exposure to these systems. This article aims to provide an overview of the management of complications and diagnostic procedures for troubleshooting drug delivery devices.
Drug delivery device-related complications
Device-related complications can be caused by malfunction of the pump or catheter, which will lead to therapy failure.
Intrathecal catheter-related complications
Problems associated with intrathecal catheters are migration, laceration, occlusion or disconnection.1 The Ascenda intrathecal catheter (Medtronic Inc., Minneapolis, MN, USA) seems to perform significantly better than earlier models.2
Pump-related complications
Pump failure is uncommon despite the high implantation rate.3 Failures of the Synchromed II (Medtronic Inc.) pump are rare. Pump stall caused by rotor corrosion can occur in earlier pump generations that are still in use. Programmable pumps have an electric power source, the failure of which can result in pump failure. Low battery life can result in pump failure, but this is indicated by the programmer device.
MRI-related complications
Programmable pumps can fail temporarily during use of MRI because of rotor stall induced by the magnetic fields. A pump should automatically restart after the termination of the scan, but it can sometimes take a few hours to do so. A reboot can be attempted by programming a bolus delivery; an emergency pump replacement must be performed should this process fail.
The use of MRI in patients with the programmable Prometra pump system (Flowonix Medical Inc., Mount Olive, NJ, USA) is complicated. The device is MRI-compatible after its medication reservoir has been entirely emptied and refilled with a 0.9% NaCl solution and the system programmed at a zero-flow rate. These steps are required because the magnetic field opens the pump valves, which results in an immediate discharge of the contents of the drug reservoir, the inner tubing and the catheter into the patient, thus causing an overdose. Removing the medication from the entire system is not easy and can only be entrusted to experienced clinicians, who may not be always accessible. Fixed-rate delivery devices operate mechanically and are powered by a gas pressure chamber surrounding a flexible inner reservoir, both made of titanium. An increase in temperature induced by MRI temporarily increases the delivery rate of drug. In our view, the potential complications of termination of therapy outweigh the risk posed by the transient increase in delivery rate, particularly in patients receiving intrathecal baclofen (ITB) therapy.
Human factors
Human factors include refill schedule mistakes, miscalculations of the dead space of the inner pump tubing or catheter content, programming mistakes and incorrect refill and access port procedures. Such errors can be mitigated by creating a prescription system in advance and adhering to it. Drug admixtures and pump programme prescriptions should be double-checked by competent clinicians. Refill procedures should only be performed by clinicians who are familiar with the technique required, and aware of the symptoms and signs of overdose or failure of therapy and the significance of neurological signs and symptoms. Suitable arrangements must be in place for 24/7 medical cover because of the risk of life-threating complications. For reasons of safety, dose changes in an outpatient setting should not exceed more than 10–15% of the daily dose.
Complications
Early referral to a specialist centre is essential as treatment options in a non-specialist setting are limited to supportive measures.4
Overdose
An overdose is usually the result of incorrect pump programming, pump failure, or, in the case of the Prometra pump, failure to empty the reservoir before MRI. Rarely is an overdose a consequence of an error related to preparation of the drug solution.
Intrathecal baclofen or opioid overdose
Intrathecal baclofen overdose is characterised by ascending hypotonia, hypotension, hypothermia, nausea, vomiting, respiratory depression, seizures and decreased conscious level from somnolence to coma.5 Intrathecal analgesic overdose of opioids can present with nausea, vomiting, hyperalgesia, double vision, nystagmus, itching, myoclonus, respiratory depression, seizures and somnolence to coma and even death.6
Intrathecal clonidine overdose
Overdose of clonidine can present as severe hypertension, confusion, unconsciousness, profuse sweating, dysarthria and respiratory depression.7
Intrathecal ziconotide overdose
Overdose with intrathecal ziconotide is relatively common, even during dose titration, because of its narrow therapeutic window and the delayed onset and offset of analgesia.8 The drug is cleared from the CSF relatively quickly but remains bound within tissues.9 Adverse effects include dizziness, confusion, memory impairment, ataxia, abnormal gait, somnolence, asthenia, headache, nausea, vomiting and diarrhoea. Less frequent adverse effects include postural hypotension, impaired vertebral expression, abnormal thought processes, dry mouth, anxiety, peripheral oedema, nystagmus and elevated creatine phosphokinase.9 The adverse outcomes of ziconotide are reversible within 4 days to 2 weeks.9,10
Management of overdose
The management of an overdose includes supportive treatment, with close monitoring. In severe cases, lumbar puncture with the aspiration of 30 ml CSF and reinjection of the same volume of saline should be considered.
Intravenous naloxone is required for the management of an opioid overdose.6 To prevent damage to the pump, a programmable pump that has been stopped should be restarted within 48 h. Reducing the flow rate may not be adequate to manage symptoms of overdose. In such cases, the reservoir should be emptied and rinsed several times with saline to ensure that it is entirely free of the highly concentrated drug. The next step is to fill the reservoir with the medication at a lower concentration. The refill is followed by emptying the catheter via a catheter access port (CAP) puncture, programming for a bolus volume of the calculated catheter content and repeat aspiration of the catheter. If the aspirated content is less than the inner pump tubing volume, the programming and the aspiration will have to be repeated. As the final step, the inner tubing and catheter should be filled by bolus pump programming. In a fixed flow device, emptying the internal tubing is impossible. The only solution is then several CAP punctures with aspiration for 24 h.
Withdrawal
Sudden termination of intrathecal drug delivery as a consequence of catheter issues, pump issues or human factors such as programming errors or manipulation of pump by the patient can result in drug withdrawal phenomena. Within several to 48 h, abrupt interruption can present varied symptoms, which can result in misdiagnosis and life-threatening delay in treatment. Ziconotide can be discontinued abruptly without withdrawal effects.9
Intrathecal baclofen withdrawal
Abrupt ITB interruption presents within several hours or up to 2 days with mild exacerbation of spasticity, fever, excessive sweating and pruritus.1 Diagnosis of withdrawal may not be suspected by clinicians unfamiliar with this condition. Neuropsychiatric symptoms such as hallucinations, disorientation and psychosis can occur. Hypotension, tachycardia and seizures may develop. In the most severe cases, hyperthermia, rhabdomyolysis, disseminated intravascular coagulation and potentially fatal multiorgan failure can occur.11
Intrathecal opioid withdrawal
Symptoms of intrathecal opioid withdrawal symptoms include lacrimation or rhinorrhoea, piloerection (‘goose flesh’), myalgia, diarrhoea, nausea/vomiting, anxiety, pupillary dilation and photophobia, insomnia, autonomic hyperactivity (yawning, tachypnoea, hyperreflexia, tachycardia, sweating, hypertension), and hyperthermia.12 Intrathecal clonidine withdrawal, which has also been reported in ITB,13 manifests in the form of hypertension and leads to rapid onset of a sympathomimetic crisis with associated Takotsubo-type cardiomyopathy.14 In the absence of obstructive coronary artery lesions, this syndrome, also known as left ventricular apical ballooning syndrome and stress-induced cardiomyopathy, is typically characterised by transient systolic dysfunction of the apical and mid-segments of the left ventricle. Patients often present with symptoms and signs of acute coronary syndrome.
Management of drug withdrawal
Supportive treatment with close monitoring is required. Oral baclofen in combination with intravenous benzodiazepines is required for management of ITB withdrawal.4,11
Intravenous supplementation may be required in patients with symptoms of opioid or clonidine withdrawal. Externalised intrathecal catheters can be used if supplementation therapy is not sufficient. The disadvantage of this approach is that a larger volume needs to be delivered by an external device. In patients with pump explantation caused by infection, withdrawal can be overcome by the temporary use of the explanted pump as an external device, whereby the original spinal catheter is externalised with replacement of the proximal catheter part.15
Cerebrospinal fluid leak
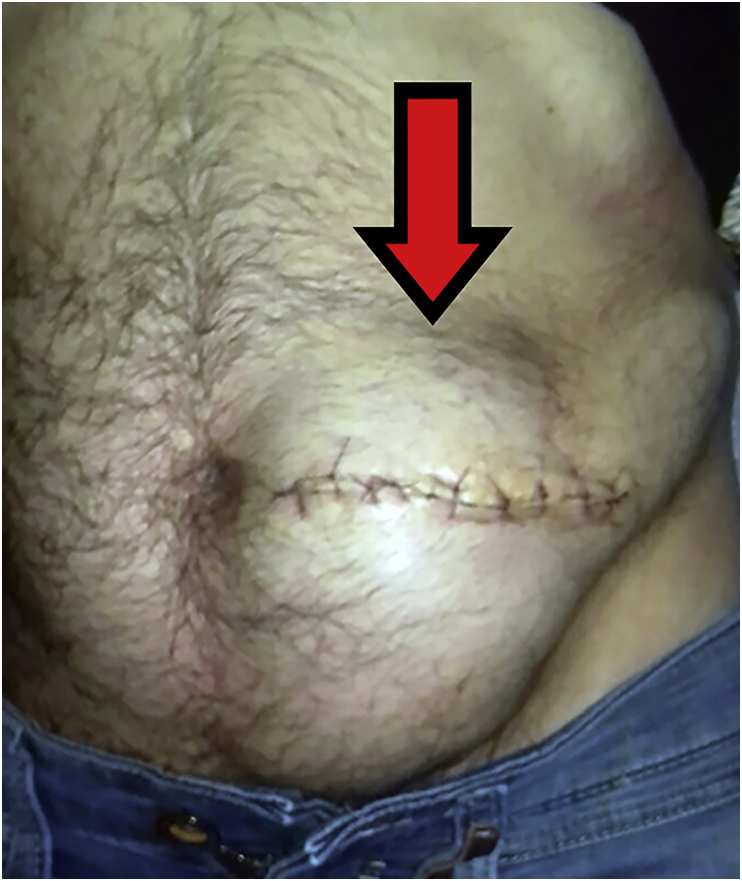
Cerebrospinal fluid leaks can be caused by persistent dural opening at the catheter insertion site, catheter–pump disconnection, a damaged catheter–pump connector, a perforated or sheared catheter, or an interruption of the catheter–catheter connector. Older catheter systems that are still in use have a sutured catheter–pump fixation. The suture can cut the connector, leading to CSF leak. A severe CSF leak can result in withdrawal syndrome, but this outcome is unusual with a minor leak. Limited leakage is detected based on treatment failure, spinal headache, or inadvertently during the percutaneous refill procedure (Fig. 1). More pronounced leaks lead to local CSF accumulation outside the spinal canal and are recognised by dorsal swelling, ventral swelling, or both, but visible swelling may be absent.
Fig 1.
Overlying catheter above the refill membrane.
In some cases, the passage of CSF along the catheter to the pump pocket leads to abdominal swelling (Fig. 2). This swelling should be differentiated from a seroma, haematoma, or abscess. Cerebrospinal fluid can be confirmed by puncturing the distension, followed by examination of the fluid for beta-2 transferrin.
Fig 2.
Dorsal dura leakage leading to abdominal pocket protrusion.
A severe CSF leak is associated with intracranial hypovolaemia, which can cause a subdural haematoma. Typically, a CSF leak is characterised by postural headache, but this is often absent. Nevertheless, a severe CSF leak should be considered an emergency. The origin of a leak is determined by CAP CT myelography or 111Indium-diethylenetriamine-penta-acetic-acid (111In-DTPA) scintigraphy.
Management of CSF leak
The treatment for CSF leakage is to identify the cause and, if it is device-related, to perform a surgical restoration. In contrast to dural leaks as result of lumbar puncture, treatment with bed rest only is frequently insufficient when a leakage is related to inserted intrathecal catheters. In such cases, one or more homologous blood patches and bed rest are then the treatment of choice. Cerebrospinal fluid accumulation in the pump pocket being passed through along the catheter is treated by percutaneous fluid aspiration. The needle is introduced above the pump and remains in contact with it to prevent accidental catheter puncture.
Granuloma formation
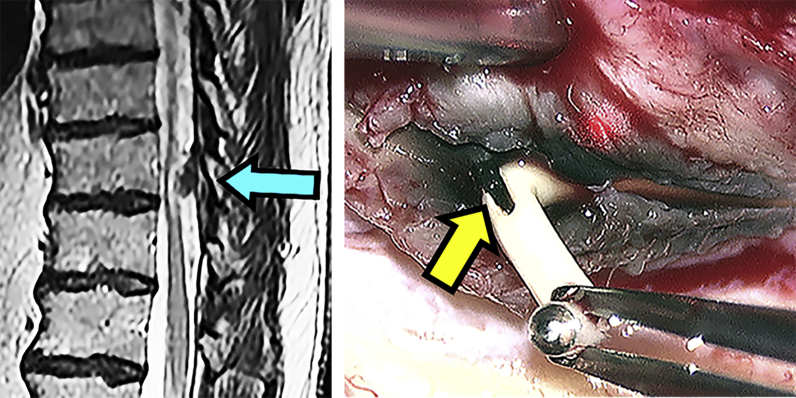
Granuloma formation at the thoracic catheter tip is an uncommon complication of intrathecal opioid administration and is rare with ITB.16,17 An association between granuloma and the dose of the intrathecal medication remains unclear.18 Opioid-induced granulomas can cause spinal cord compression and radicular pain in the thoracic or lumbar regions. There is exacerbation of pain despite dose increments, as drugs are unable to reach the target neural tissue. Diagnosis is based on MRI showing an abnormal tissue formation at the catheter tip (Fig. 3).
Fig 3.
A granuloma (blue arrow). Tremendous in vivo in growth with an abnormal black colouring (yellow arrow). The patient complained of exacerbation of pain in his legs without neurological signs. The causes can easily be overlooked when MRI is limited to the lumbar area.
Management of granuloma formation
Catheter replacement at another level or termination of the infusion with monitoring may be appropriate, as the mass could resolve on its own.16 Surgical removal of the granuloma is indicated in cases that involve neurological deficits.
Obstruction of CSF flow
Cerebrospinal fluid flow obstruction can be caused by focal arachnoiditis with fibroconnective adhesions, which will result in accumulation of the infused medication. The accumulation gives rise to a high local concentration, which can lead to a vicious cycle of chronic arachnoiditis.
Management of CSF flow obstruction
In cases of limited CSF flow obstruction, catheter replacement can be attempted. However, multiple revisions are not indicated. Preliminary results show that restoration of the CSF flow by microsurgical adhesiolysis or percutaneous balloon fenestration appears to solve the problem.19
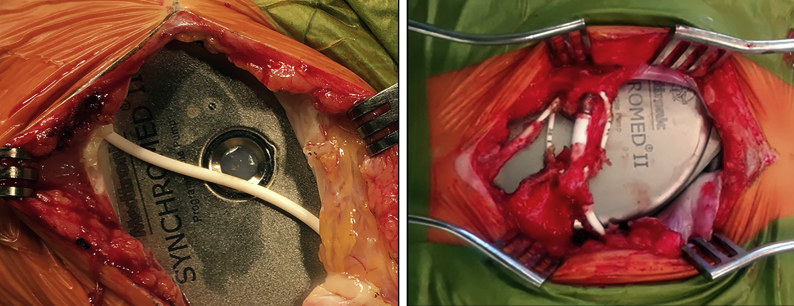
Infection
Implantable device infections can be attributed to the surgical procedure or the refilling of the percutaneous pump reservoir. They can be caused indirectly via haematogenous seeding from a distant source. The clinical manifestations of local infection include localised pain, protrusion, erythema and fever. In a progressive infection with pus formation, the swelling is more pronounced and the skin has a shiny appearance. A striking observation is that a device infection is not invariably accompanied by fever. The causative microorganisms can be identified on cultures of the local pus, blood and CSF.
Pump pocket infection can spread to the CNS, leading to meningitis.20 Isolated meningitis can occur even should the pump pocket appear to be normal. The clinical symptoms of meningitis are highly dependent on the causative microorganism. Symptoms such as fever, headache, stiff neck, vomiting and change in consciousness occur inconsistently. There is a risk of missing the diagnosis of meningitis. Staphylococcus aureus meningitis presents with high-grade fever. In contrast, Staphylococcus capitis meningitis can be missed, as it does not present with high-grade fever.
Treatment of infection
Superficial infection is treated with oral antibiotics.21 In more severe infections, explantation of the implanted device is necessary to mitigate the risk of meningitis.20 In patients receiving ITB or intrathecal clonidine, the dose should be reduced gradually over 5 days, as sudden termination can lead to a potentially life-threatening withdrawal syndrome. Patients with cerebral damage or a spinal cord injury at the T6 level or higher who are implanted with an ITB device are at risk of autonomic dysreflexia upon sudden withdrawal. Broad-spectrum antibiotics such as meropenem and vancomycin should be given until the causative microorganism is identified.
Anecdotal information suggests that pump explantation may not always be necessary.22, 23, 24 System salvage should be considered in infection with a non-pathogenic microorganism, minor clinical symptoms and in patients at high risk of sympathetic crisis.22,24 Antibiotic treatment with meropenem and vancomycin delivered intravenously combined with vancomycin in the pump and oral rifampicin is an option.24 Successful pump salvage by wrapping the pump in two gentamicin-impregnated collagen sheets has been described in a patient with Staphyloccocus aureus infection of the pocket site.23 The patient had previously experienced life-threatening autonomic dysreflexia after ITB termination, which precluded temporary discontinuation of ITB. Major concerns of this method are the potential for the developing antibiotic resistance with long-term gentamicin administration and the risk of recurrence.
Diagnosis of intrathecal drug delivery system failure
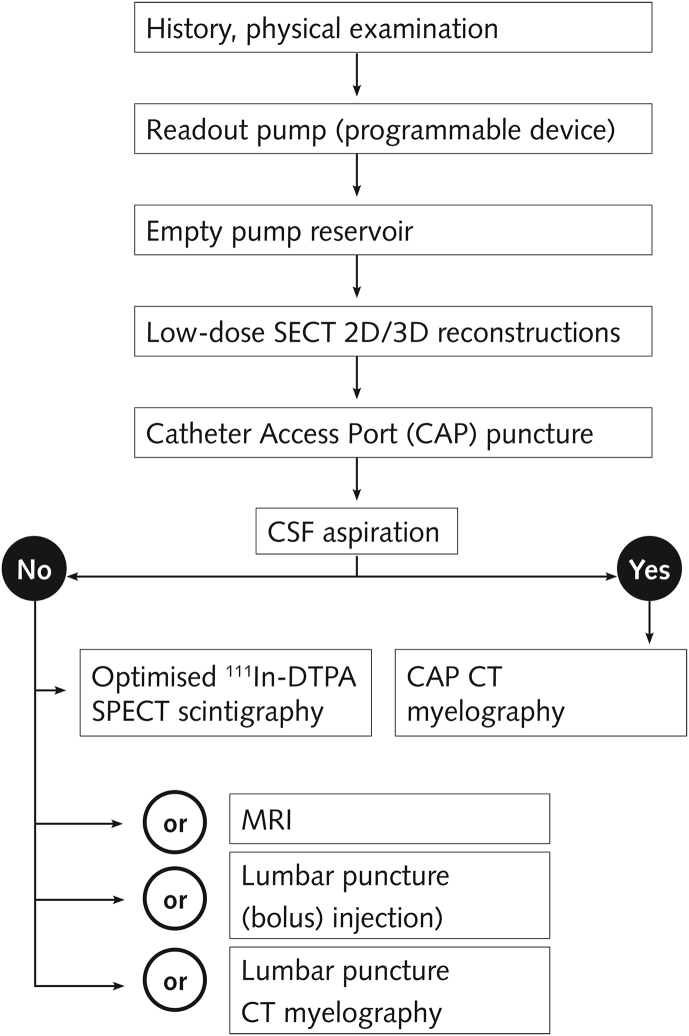
We use an algorithm in our institution to determine the cause of intrathecal drug delivery failure (Fig. 4). The primary diagnostic steps are estimating the amount of fluid in the pump reservoir and a low-dose single-energy CT (SECT) scan.25 Computed tomography with post-processing two-dimensional (2-D) and 3-D reconstructions allows visualisation of the entire drug delivery pathway as opposed to plain radiography (Fig. 5). Catheter access port myelography can be used should CT fail to determine the cause. Fluid should be aspirated from the CAP before injection of contrast material to prevent overdose, as the catheter is filled with a high concentration of drugs. The highly viscous contrast material should then be flushed with normal saline. The catheter should subsequently be refilled with a precisely calculated amount equaling to the catheter volume by either pump bolus or manual injection.
Fig 4.
Overview (aetiology, diagnostic algorithm) of troubleshooting an intrathecal drug delivery system. CSF, cerebral spinal fluid; SECT, single-energy CT; DECT, twin-beam dual-energy CT; 111In-DTPA SPECT, 111indium-diethylene-triamine-penta-acetic-acid single-photon emission CT.
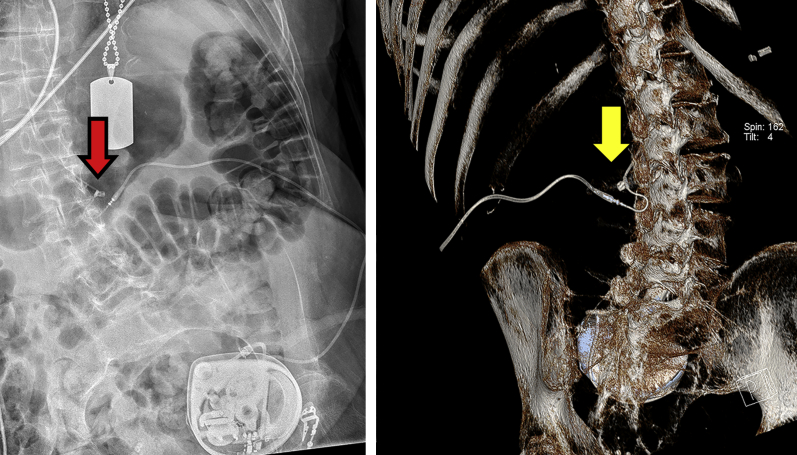
Fig 5.
Improved visualisation of the catheter. Plain radiography indicated a possible kinked catheter (red arrow). DECT with 3D volume rendering revealed a regular curved catheter (yellow arrow) with no obstruction.
Information concerning a catheter's diameter and length is essential in calculating its volume. This information should be recorded in the clinical records and in the memory of the programmable pump during implantation. By combining CAP myelography with CT and 2- and 3-D reconstructions, failures associated with drug delivery can be identified. It is crucial to not limit the CT field of view to the spine but rather to extend it to the abdominal region where the pump is implanted. Contrast material should not be injected if it is not possible to aspirate fluid via the CAP, as there will be a risk of overdose. Non-contrast CT can be used to identify an abnormal catheter position. In addition, lumbar puncture CT myelography can be considered if in doubt. 111In-DTPA scintigraphy can be useful when CAP CT myelography fails to identify the cause of failure, and when fluid cannot be aspirated via the CAP or if dynamic information regarding catheter flow and the spread of intrathecal medications is required. It can also help to identify minor leakages. 111In-DTPA scintigraphy can be enhanced by adding single-photon emission CT (SPECT) and by standardising the flow rate in programmable pump devices. Magnetic resonance imaging is of limited value in intrathecal drug delivery system failure and is used to exclude granuloma, syringomyelia or intrathecal obstructions.
Summary
Although intrathecal drug administration using an implanted pump system has been used in clinical practice for many decades, there is limited awareness of its applications, complications associated with its use and management of complications. Early diagnosis and management of withdrawal and overdose is essential as they can be potentially life threatening. Early involvement of clinicians with experience in managing these devices is essential.
Declaration of Interest
Dr. Delhaas reports personal fees in the past from Medtronic Inc, as a consultant , outside the submitted work; Prof. Huygen reports grants and personal fees from ABBOTT, personal fees from Grunenthal, outside the submitted work; In addition, Prof. Huygen has a patent 2022004 the Netherlands pending.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Elmar Delhaas MDis an anaesthesist, senior interventional pain physician and researcher at the Centre for Pain Medicine and Department of Radiology & Nuclear Medicine, Erasmus University Medical Centre. He has more than 30 yrs of experience in treatment using intrathecal drug delivery systems.
Frank Huygen MD PhDis an anaesthesist and head of the Centre for Pain Management at Erasmus University Medical Centre. He is also involved in treatment using intrathecal drug delivery systems.
Matrix codes: 1H02, 2E03, 3E00
References
- 1.Delhaas E.M., Harhangi B.S., Frankema S.P.G., Huygen F., van der Lugt A. Plain radiography in patients treated with intrathecal drug delivery using an implantable pump device. Insight Imag. 2017;8:499–511. doi: 10.1007/s13244-017-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motta F., Antonello C.E. Comparison between an Ascenda and a silicone catheter in intrathecal baclofen therapy in pediatric patients: analysis of complications. J Neurosurg Pediatr. 2016;18:493–498. doi: 10.3171/2016.4.PEDS15646. [DOI] [PubMed] [Google Scholar]
- 3.Stetkarova I., Yablon S.A., Kofler M., Stokic D.S. Procedure- and device-related complications of intrathecal baclofen administration for management of adult muscle hypertonia: a review. Neurorehabil Neural Repair. 2010;24:609–619. doi: 10.1177/1545968310363585. [DOI] [PubMed] [Google Scholar]
- 4.Coffey R.J., Edgar T.S., Francisco G.E. Abrupt withdrawal from intrathecal baclofen: recognition and management of a potentially life-threatening syndrome. Arch Phys Med Rehabil. 2002;83:735–741. doi: 10.1053/apmr.2002.32820. [DOI] [PubMed] [Google Scholar]
- 5.Watve S.V., Sivan M., Raza W.A., Jamil F.F. Management of acute overdose or withdrawal state in intrathecal baclofen therapy. Spinal Cord. 2012;50:107–111. doi: 10.1038/sc.2011.112. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz A., Sogut A., Kilinc M., Sogut A.G. Successful treatment of intrathecal morphine overdose. Neurol India. 2003;51:410–411. [PubMed] [Google Scholar]
- 7.Perruchoud C., Bovy M., Durrer A. Severe hypertension following accidental clonidine overdose during the refilling of an implanted intrathecal drug delivery system. Neuromodulation. 2012;15:31–34. doi: 10.1111/j.1525-1403.2011.00392.x. discussion 34. [DOI] [PubMed] [Google Scholar]
- 8.Wermeling D.P. Ziconotide, an intrathecally administered N-type calcium channel antagonist for the treatment of chronic pain. Pharmacotherapy. 2005;25:1084–1094. doi: 10.1592/phco.2005.25.8.1084. [DOI] [PubMed] [Google Scholar]
- 9.Penn R.D., Paice J.A. Adverse effects associated with the intrathecal administration of ziconotide. Pain. 2000;85:291–296. doi: 10.1016/s0304-3959(99)00254-7. [DOI] [PubMed] [Google Scholar]
- 10.Pope J.E., Deer T.R. Intrathecal pharmacology update: novel dosing strategy for intrathecal monotherapy ziconotide on efficacy and sustainability. Neuromodulation. 2015;18:414–420. doi: 10.1111/ner.12274. [DOI] [PubMed] [Google Scholar]
- 11.Stetkarova I., Brabec K., Vasko P., Menel L. Intrathecal baclofen in spinal spasticity: frequency and severity of withdrawal syndrome. Pain Phys. 2015;18:E633–E641. [PubMed] [Google Scholar]
- 12.WHO Guidelines . WHO; Geneva: 2009. Clinical guidelines for withdrawal management and treatment of drug dependence in closed settings. [PubMed] [Google Scholar]
- 13.Levy J., De Brier G., Hugeron C., Lansaman T., Bensmail D. Takotsubo cardiomyopathy as a reversible complication of intrathecal baclofen withdrawal. Ann Phys Rehabil Med. 2016;59:340–342. doi: 10.1016/j.rehab.2016.07.384. [DOI] [PubMed] [Google Scholar]
- 14.Lee H.M., Ruggoo V., Graudins A. Intrathecal clonidine pump failure causing acute withdrawal syndrome with 'stress-induced' cardiomyopathy. J Med Toxicol. 2016;12:134–138. doi: 10.1007/s13181-015-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang R.S., Sukul V., Collison C., Prusik J., Pilitsis J.G. A novel approach to avoid baclofen withdrawal when faced with infected baclofen pumps. Neuromodulation. 2018;22:834–838. doi: 10.1111/ner.12873. [DOI] [PubMed] [Google Scholar]
- 16.Miele V.J., Price K.O., Bloomfield S., Hogg J., Bailes J.E. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251–261. doi: 10.1016/j.ejpain.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Deer T.R., Raso L.J., Coffey R.J., Allen J.W. Intrathecal baclofen and catheter tip inflammatory mass lesions (granulomas): a reevaluation of case reports and imaging findings in light of experimental, clinicopathological, and radiological evidence. Pain Med. 2008;9:391–395. doi: 10.1111/j.1526-4637.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- 18.Deer T.R., Pope J.E., Hayek S.M. The Polyanalgesic Consensus Conference (PACC): recommendations for intrathecal drug delivery: guidance for improving safety and mitigating risks. Neuromodulation. 2017;20:155–176. doi: 10.1111/ner.12579. [DOI] [PubMed] [Google Scholar]
- 19.Delhaas E.M., Harhangi B.S., van Doormaal P.J. Restoration of rostral cerebrospinal fluid flow to solve treatment failure caused by obstruction in long-term intrathecal baclofen administration. J Spin Cord Med. 2019:1–10. doi: 10.1080/10790268.2019.1646476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malheiro L., Gomes A., Barbosa P., Santos L., Sarmento A. Infectious complications of intrathecal drug administration systems for spasticity and chronic pain: 145 patients from a tertiary care center. Neuromodulation. 2015;18:421–427. doi: 10.1111/ner.12265. [DOI] [PubMed] [Google Scholar]
- 21.Dickey M.P., Rice M., Kinnett D.G. Infectious complications of intrathecal baclofen pump devices in a pediatric population. Pediatr Infect Dis J. 2013;32:715–722. doi: 10.1097/INF.0b013e318287f02a. [DOI] [PubMed] [Google Scholar]
- 22.Rovlias A., Dimitrios P., Nikolaos P., Alexandros B. Intrathecal baclofen pump infection treated by adjunct intrareservoir teicoplanin instillation. Surg Neurol Internat. 2017;8:38. doi: 10.4103/sni.sni_418_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peerdeman S.M., De Groot V., Feller R.E. In situ treatment of an infected intrathecal baclofen pump implant with gentamicin-impregnated collagen fleece: technical note. J Neurosurg. 2010;112:1308–1310. doi: 10.3171/2009.8.JNS081692. [DOI] [PubMed] [Google Scholar]
- 24.Zed P.J., Stiver H., Devonshire V., Jewesson P.J. Continuous intrathecal pump infusion of baclofen with antibiotic drugs for treatment of pump-associated meningitis: case report. J Neurosurg. 2000;92:347–349. doi: 10.3171/jns.2000.92.2.0347. [DOI] [PubMed] [Google Scholar]
- 25.Delhaas E.M., van der Lugt A. Low-dose CT with two- and three-dimensional postprocessing as an alternative to plain radiography for intarthecal catheter visualization: a phantom pilot study. Neuromodulation. 2019;22:818–822. doi: 10.1111/ner.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]