Learning objectives.
By reading this article, you should be able to:
-
•
Identify historical changes in patterns of maternal mortality related to airway management and aspiration of gastric contents.
-
•
Describe how to conduct a GA in obstetrics safely and safely manage a failed intubation to ensure good outcome for mother and baby.
-
•
Identify the types of cases in pregnant patients that may require GA, and the reasoning behind such decisions.
Key points.
-
•
Mortality from GA in obstetrics has decreased over the past 30 yrs.
-
•
Physiological changes of pregnancy should affect the approach to GA in parturients.
-
•
Desaturation during rapid sequence induction (RSI) in the parturient can be avoided by an effective preoxygenation technique, gentle bag-mask ventilation, and apnoeic oxygenation techniques.
-
•
Videolaryngoscopes should be used as first-line laryngoscope to maximise the success rate of intubation during RSI in obstetric practice.
-
•
Whilst GA is largely avoided in pregnant patients, it is the preferred mode of anaesthesia in certain situations.
General anaesthesia was once the primary anaesthetic technique used in obstetrics, both for vaginal deliveries and Caesarean section (CS). As the field of obstetric anaesthesia has advanced, the use of GA has been largely replaced by neuraxial techniques. The latest decennial survey on obstetric anaesthesia practices in the USA reported a reduction of the use of GA for CS from 35% in 1981 to less than 25% in 2011, with the majority of cases corresponding to emergency procedures.1 It is currently estimated that about 6% of CS still require GA and tracheal intubation.2
Failed tracheal intubation and the risk of aspiration and resulting aspiration pneumonitis have historically been the most dreaded complications of GA.3 Detailed guidelines for the management of difficult intubation in obstetrics have been developed.4 However, it remains important to remember that increased care should also be used when dealing with tracheal extubation and postoperative management. A review of maternal mortality from the state of Michigan (USA) reported eight anaesthesia-related deaths over an 18 yr period; five of these deaths resulted from airway obstruction or hypoventilation, and occurred during emergence or in the recovery unit.5
Whilst mortality associated with GA in obstetrics has decreased nearly 60% from 1979–1990 to 1991–2002, there are still reports of deaths associated with difficult intubation.6,7 Airway-related maternal mortality during obstetric GA is approximately 2.3 per 100,000 GAs for CS compared with one in 180,000 GAs for the general population; mortality after failed intubation is 1% in parturients. The incidence of front-of-neck airway access is also higher at 3.4 per 100,000 GAs for CS compared with two in 100,000 GAs for the general population.8 The consequences of a failed intubation in the obstetric patient may not only affect the mother, but also the fetus. A recent study found an increased rate of admission to a neonatal ICU after failed intubation of the mother.9
In the UK, the Confidential Enquiry into Maternal Deaths reports have shown that a substantial reduction in mortality related to GA and airway management is achievable; they recommend frequent practice and assessment of the skills needed to manage difficult intubation in obstetrics.10 Aspiration of gastric contents has been ratified as a rare event (two in 10,000 GAs), but identifying risk factors (e.g. obesity and known difficult airway) and adequately preparing (i.e. fasting and decompression of the stomach before anaesthesia in certain circumstances) are imperative in its prevention.8,11
Maternal considerations
Significant physiological changes of pregnancy should be taken into account when considering GA as the anaesthetic plan for a pregnant patient. The most pertinent to the anaesthetist are those changes involving the respiratory, cardiovascular, and gastrointestinal systems.
Because of the upward displacement of the diaphragm by the uterus and increase in transverse diameter of the thorax, functional residual capacity (FRC) is reduced 20% by term. Minute ventilation (MV) is increased by 50%. The combination of decreased FRC and increased MV accounts for rapid uptake of inhalational anaesthetic agents. Increased maternal resting metabolic rate and the increased metabolic demands of the fetoplacental unit cause an increase of 60% in oxygen consumption.12 The decreased FRC in addition to increased oxygen consumption explains the rapid oxygen desaturation of the pregnant patient during apnoea or airway obstruction. Anatomically, mucosal engorgement and laryngeal or pharyngeal oedema contribute to the increased difficulty of tracheal intubation and excessive risk of bleeding. Mallampati scores have been found to worsen during pregnancy and can further change during labour.13
Cardiac output increases by up to 50% at term, resulting from an increase in HR (10–20 beats min−1) and stroke volume (30–40%). Thus, induction of anaesthesia with i.v. anaesthetic agents is faster.
The gravid uterus displaces the stomach in a cephalad direction, causing increased intra-gastric pressure and loss of the protective effect of the diaphragm on the lower oesophageal sphincter (LOS) tone, as the oesophagus enters the thorax. Progesterone also decreases the tone of the LOS. It is common for pregnant women to have symptoms of acid reflux and regurgitation. Gastric emptying remains normal during pregnancy, but can be slowed by the onset of labour and the use of parenteral and neuraxial opioids.14 The risk of aspiration should be addressed when planning induction of GA in labouring parturients.
Fetal considerations
Exposing the developing fetus to GA raises concerns about the relationship between anaesthetic agents and the risk of teratogenesis and future behavioural impairment. One large retrospective study from Swedish birth registries compared more than 5,000 patients that had surgery during pregnancy, half of them under GA, with control subjects. They reported no increase in stillbirths or congenital abnormalities.15 None of the currently used anaesthetic agents, including propofol, opioids, neuromuscular blocking agents (NMBAs), and local anaesthetics, at standard concentrations, have been shown to have teratogenic effects on the fetus at any gestational age.16 Neuronal apoptosis after exposure to anaesthesia during the third trimester has been reported in animal studies. This has prompted questions about the effects of anaesthetic agents on neurocognitive development in humans, because of the inhibitory effect on γ-aminobutyric acid or N-methyl-D-aspartate receptors during this period of intense synaptogenesis. Nevertheless, there are no current human studies supporting differences on cognitive testing after a brief exposure to GA.17
Because the prime determinant of fetal perfusion is arterial pressure in the mother, it is critical to avoid maternal hypotension. Maintaining adequate maternal oxygenation to prevent fetal hypoxaemia is also fundamental when providing GA to the pregnant patient. Fetal monitoring during non-obstetric surgery or fetal procedures, in which this is feasible, provides useful information regarding the adequacy of perfusion and oxygenation.16 Before surgery, a plan is needed to clarify the purpose of fetal monitoring (delivery via CS or intrauterine fetal resuscitation) and ensure that trained personnel are available to expedite delivery should concerns over fetal HR arise.
Regarding neonatal outcomes after delivery, GA for CS has been historically linked with a slightly lower base deficit and higher umbilical artery pH when compared with neuraxial anaesthesia. This is partly explained by the effects of compression of the vena cava, maternal haemodynamic profile, and choice of vasopressor agent, rather than the technique itself.18
Obstetric airway
Causes of difficult airway in the obstetric patient
The incidence of failed intubation in obstetrics, one in 390–443, is higher than in non-obstetric patients.8 This higher incidence is often attributed to anatomical and physiological changes of pregnancy, training issues, and environmental and human factors. Because the use of GA for CS has diminished significantly over the years, real-life training opportunities have become less frequent. Multidisciplinary team simulation has been advocated to address the non-technical aspects of managing the obstetric airway.
General anaesthesia
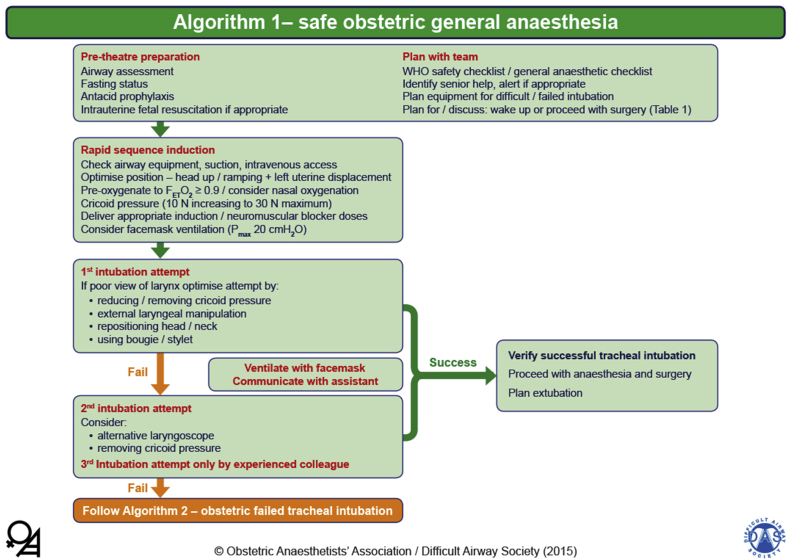
The recent publication of the Obstetric Anaesthetists' Association and Difficult Airway Society (OAA/DAS) obstetric difficult airway guidelines includes an algorithm for the ‘safe obstetric general anaesthetic’ (Fig. 1).4 There is emphasis on planning and preparation to include airway assessment; fasting and antacid prophylaxis; and, where appropriate, intrauterine fetal resuscitation. In addition to the use of the WHO surgical checklist, the use of a specific obstetric GA checklist is gaining popularity.
Fig 1.
OAA/DAS Obstetric Airway Guidelines, algorithm 1: safe obstetric general anaesthesia. Reproduced from Mushambi and colleagues, with permission from the Obstetric Anaesthetists' Association and Difficult Airway Society.4
Routine use of the head-up position during induction of GA is recommended as it improves airway manipulation and laryngoscopy in pregnant women with large breasts. A combination of the following measures is recommended to prevent desaturation during induction of anaesthesia and intubation: preoxygenation with a tight-fitting face mask for a minimum of 3 min to achieve an end-tidal oxygen level of 90%, mask ventilation before intubation (with maximum peak inspiratory airway pressure of 20 cmH2O), and apnoeic oxygenation via nasal cannulae at 5–15 L min−1. There is evidence in non-obstetric patients that high-flow humidified nasal oxygen (up to 60 L min−1) may be used for preoxygenation and to provide apnoeic oxygenation during rapid sequence induction (RSI). In pregnant women, recent work suggests that high-flow humidified oxygen may not be as effective as preoxygenation with a face mask, but these studies did not investigate the advantage during apnoeic oxygenation in this setting.19 The use of propofol for RSI in obstetric anaesthesia has several advantages, including familiarity, availability, reduced incidence of drug errors, and better suppression of airway reflexes when compared with thiopental. The 5th National Audit Project of the Royal College of Anaesthetists found a higher incidence of awareness during GA in obstetrics and in patients with unanticipated difficult airway.20 It is therefore recommended that additional doses of induction agent should always be available and be given if a difficult airway is encountered.
Because there are contentious issues related to intubating conditions, the relative risks of anaphylaxis, and the high cost of sugammadex, the choice of NMBA between suxamethonium and rocuronium (with sugammadex as backup) is still open to debate. There is no consensus whether short-acting opioids should be used routinely as part of induction of GA in obstetrics. However, in the presence of pre-eclampsia, maternal cardiac disease or neurological compromise, giving careful doses of opioids before induction can attenuate the hypertensive response to intubation and provide cardiovascular stability, and decrease the risk of intraoperative awareness.21 Provided that the anaesthetist is appropriately trained, there is now compelling evidence that videolaryngoscopes should be used as first-line devices, as they provide better laryngeal views, higher rates of successful tracheal intubation, and a better teaching tool than traditional direct laryngoscopes.22 There had been a suggestion that videolaryngoscopy may minimally increase time to intubation, but a recent study in obstetric patients found no differences in time to intubation between two different types of videolaryngoscopes and direct laryngoscopy.23 Videolaryngoscopes with a Macintosh-type blade have the added advantage of allowing both direct and indirect views. Cricoid pressure must be reduced or removed early if there is difficulty with the airway during the first attempt at laryngoscopy.
Difficult and failed tracheal intubation
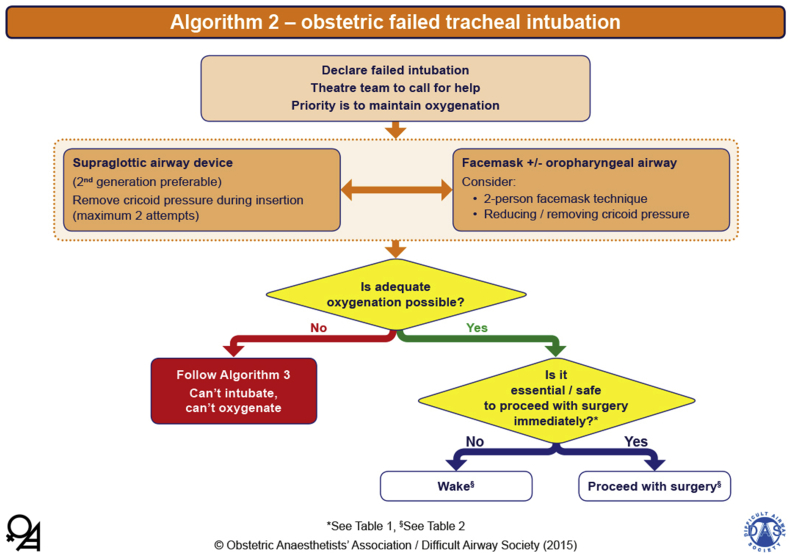
If the first attempt at intubation fails, oxygenation should continue with either face-mask ventilation or high-flow humidified nasal oxygen whilst communicating with the team. The second attempt should be by the most senior anaesthetist present, using a different laryngoscope. Removal of cricoid pressure should be considered. The recommended maximum number of attempts is two; a third attempt should only be done rarely and by a more senior and different anaesthetist. Airway swelling can develop very rapidly in a pregnant woman, and this can quickly escalate to a ‘cannot intubate/cannot oxygenate’ (CICO) situation.
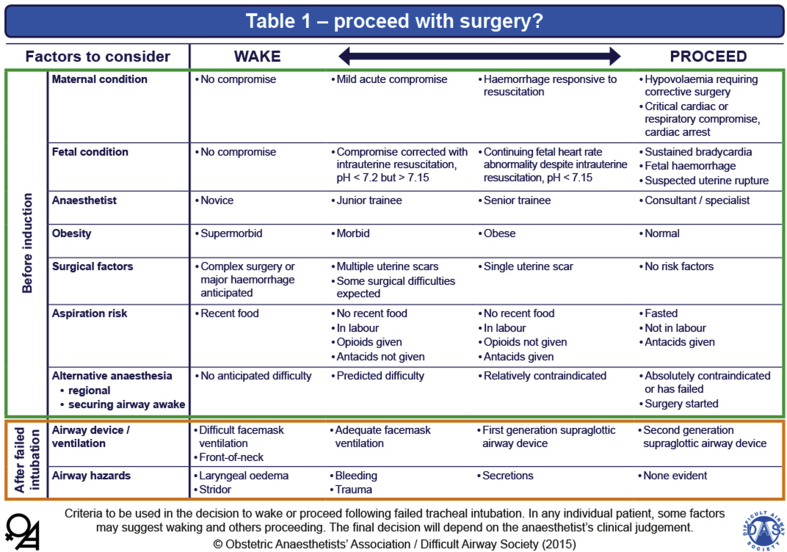
Once failed intubation has been declared, the priority is that oxygenation is established and maintained using either a face mask or second-generation supraglottic airway device (SAD), and the failed intubation algorithm should be followed (Fig. 2). Once oxygenation is established, the final decision needs to be made whether to proceed with the anaesthetic or wake the woman. This decision is influenced by several factors, the majority of which are present before the induction of GA, as outlined in Table 1 of the OAA/DAS guidelines (Fig. 3). It is important to recognise that the final decision to wake or proceed will invariably be made during a very stressful time. As a result, human factors and situational awareness will play a significant role in the final decision by the anaesthetist. Ultimately, the mother's well-being is the most important factor in this decision-making process.
Fig 2.
OAA/DAS Obstetric Airway Guidelines, algorithm 2: obstetric failed tracheal intubation. Reproduced from Mushambi and colleagues, with permission from the Obstetric Anaesthetists' Association and Difficult Airway Society.4
Fig 3.
OAA/DAS Obstetric Airway Guidelines, Table 1: proceed with surgery? Criteria to be used in the decision to wake or proceed after failed tracheal intubation. Reproduced from Mushambi and colleagues, with permission from the Obstetric Anaesthetists' Association and Difficult Airway Society.4
If the attempt to oxygenate the woman fails and a CICO scenario develops, this should be managed using surgical cricothyroidotomy.4 However, if cardiac arrest occurs before the baby is delivered, a perimortem CS should be carried out and completed within 5 min of the cardiac arrest.
Management after failed tracheal intubation
After a failed intubation and a decision to proceed with surgery, the anaesthetic needs to be conducted safely, ensuring good ventilation and appropriate depth of anaesthesia. The preferred technique is controlled ventilation, the use of NMBAs, a second-generation SAD as rescue airway device, and use of a volatile anaesthetic agent. The risk of regurgitation and pulmonary aspiration may be reduced by aspirating the gastric tube passed through the SAD and minimising fundal pressure at delivery.
In the event of a failed intubation, if the decision is to wake the woman, a plan for safe delivery must be formulated with the obstetric team. The anaesthetic plan may include regional anaesthesia or awake tracheal intubation. Awake tracheal intubation can be achieved with a flexible bronchoscope, a rigid direct or indirect laryngoscope, or a tracheostomy. Requirements for topical anaesthesia and sedation depend on the condition of the woman, who will be recovering from the effects of the i.v. anaesthetic agents. It is advisable to have two experienced anaesthetists for airway management at this stage.
The event should be well documented in the patient's notes and a critical incident form completed. The patient should be offered counselling and her general practitioner notified of the event to ensure that the information is communicated should the woman require GA in the future.
Special cases in obstetric anaesthesia
Despite a valid reluctance to use GA in the parturient, there are a number of situations, in which it is the preferred anaesthetic technique. As clinical practice changes, specifics of cases, in which GA is preferred, are likely to evolve.
Approximately 1–2% of pregnant women require non-obstetric surgery during their pregnancy.24 Whilst many of the most commonly performed procedures (appendicectomy, cholecystectomy, and ovarian procedures) could be done as either open (potentially under regional anaesthesia) or laparoscopically (under GA), studies support the use of the laparoscopic approach, as it has been associated with fewer maternal and fetal complications.25
Occasionally, pregnant patients may present for surgery, including CS, who refuse anything but GA. In these cases, a careful and thorough explanation of the risks and benefits of regional vs GA and an attempt to understand the patient's objection to neuraxial techniques are critical. Whilst swaying a patient may be difficult on the day of surgery, patients with a known opposition to or fear of regional anaesthesia would be well served by preoperative counselling. Practice changes to increase the usage of neuraxial anaesthesia for CS recommend a ‘simple 1-page explanation of neuraxial procedures, their benefits, and their rare complications’ given to the patient during a prenatal visit, to change patient attitudes.26
Operative delivery cases, in which significant bleeding is expected (placenta accreta spectrum [PAS], especially), have historically been considered as candidates for the use of GA as the primary anaesthetic. However, recent findings dispute the necessity of this approach. Even in cases of strongly suspected PAS, no association has been found between the degree of placental invasion and massive blood loss or large-volume transfusion.27 In those studies that have suggested a correlation between massive blood loss and preoperative diagnosis of PAS, there are numerous outliers with lower blood loss (800–1500 ml) in groups with significant placental invasion.28 Further, although it is a concern (and an argument for GA) that patients who do undergo massive transfusion are likely to develop some degree of coagulopathy either during or after surgery, literature in other circumstances suggests that it is safe to maintain an epidural catheter when a patient is in an anticoagulated state. There have been no reported cases of epidural haematoma in anticoagulated parturients receiving neuraxial procedures from 1952 to the present.29 In a case series of 129 patients with preoperatively suspected PAS, 122 patients safely received regional anaesthesia as their primary anaesthetic, and about 80% of the women who required hysterectomy were able to be safely maintained with regional anaesthesia.30 For these reasons, GA should not be considered a ‘requirement’ in patients scheduled for operative deliveries, in which there is an increased risk of massive haemorrhage.
Understanding when a neuraxial anaesthetic should be converted to GA is a critical skill for the anaesthetist. Despite an anaesthetist's best efforts, there will be occasional cases, where a neuraxial block is found to provide inadequate anaesthesia during surgery. When there is complaint of intraoperative pain, the anaesthetist should believe the patient and take action.31 These cases should be approached with humility and focus on the patient, assessing the nature of their pain and the sensory level. If re-dosing of a neuraxial catheter is not an option, supplementary analgesia with i.v. or inhaled adjuvants (opioids, ketamine, and nitrous oxide) may be needed. Conversion to GA may be necessary if analgesic dosing is insufficient.
It is important for the anaesthetist to understand the complexities of conversion to GA in the setting of massive haemorrhage or haemodynamic instability. A certain volume of blood loss, the degree of haemodynamic instability, and the need to place invasive monitors should not be absolute indications for conversion; rather, the whole clinical situation should be considered. Changes in the patient's mental status (especially progression to unarousable) are likely to correlate with a reduced ability to protect her airway and should be triggers to consider induction of GA. The anaesthetist in these cases must balance the risk, at any given time, of converting to what could be an ‘unnecessary’ GA vs the risk of not converting to GA and the patient requiring GA when in a very unstable condition.
In the burgeoning field of fetal anaesthesia, GA is often administered to the maternal patient for some of the reasons that it is avoided in most other pregnant patients. Fetal surgery encompasses an ever-growing number of intrauterine procedures on the fetus or placenta that usually take place during the mid-to late second trimester, although some fetal procedures may occur much later in the third trimester. These range from minor, lower-risk, and minimally invasive procedures (umbilical cord blood sampling and placement of thoracoamniotic or vesiculoamniotic shunts) to major, prolonged open-uterus surgeries (repair of fetal myelomeningocele, fetoscopic tracheal occlusion and ex utero intrapartum treatment [EXIT]).32 Whilst many of the minor fetal surgeries require little, if any maternally administered anaesthesia (they may be done under local or minimal sedation), many of the major fetal surgeries are performed under GA.
Unlike during operative delivery, where uterine relaxation is undesirable, it is a requirement during many fetal surgeries. Uterine relaxation during these cases allows better surgical exposure, maximises maternal blood flow through the uterus, and reduces the chances of uteroplacental separation. Uterine relaxation may be achieved with a combination of agents (nitroglycerine, indomethacin and magnesium) with volatile agents acting as the bedrock of the effort. In cases where postsurgical delivery is planned (EXIT procedure), uterine relaxation must be quickly reversed after delivery by converting the case to a total i.v. anaesthetic with the addition of uterotonics as needed.
Contrary to concerns at operative delivery of fetal transfer of maternally administered drugs, maternal-to-fetal transfer of certain agents is desirable during fetal surgery and is a reason for choosing GA in these cases (Table 1). It is unresolved whether a mid-gestation fetus can ‘feel’ pain. However, it can respond, both in a motor and an autonomic sense, to the noxious stimuli of surgery, which could make the surgery more difficult or even dangerous for the fetus.33 Sedative drugs and opioids (e.g. volatile anaesthetic agents, propofol, and remifentanil) given to the mother are known to cross the placenta and can blunt or ablate these responses. In some situations, certain drugs that either do not cross the placenta, such as neuromuscular blockers, which are needed in higher concentrations in the fetus, such as opioids, or which might be undesirable in the maternal circulation, such as sympathomimetics, can be given directly to the fetus to supplement the agents given to the mother.
Table 1.
Maternal-to-fetal transfer of drugs commonly used during GA. ∗Crosses to a limited extent (not clinically significant).
| Drug class | Examples | Crosses uteroplacental barrier? |
|---|---|---|
| Intravenous agents | Thiopental, propofol, ketamine | Yes |
| Inhalational agents | Isoflurane, sevoflurane, desflurane | Yes |
| Benzodiazepines | Midazolam, lorazepam | Yes |
| Opioids | Morphine, fentanyl, remifentanil | Yes |
| NMBAs | Vecuronium, rocuronium, suxamethonium | No |
| NMBA reversal agents | Neostigmine | Yes∗ |
| Sugammadex | Yes∗ | |
| Anticholinergic agents | Atropine | Yes |
| glycopyrrolate | Yes∗ |
Conclusions
Major advances in obstetric anaesthesia have resulted in improved maternal outcomes. Regional anaesthesia, with its high safety profile, is the most common method of providing anaesthesia to the parturient. However, in a small percentage of women, GA is the only option. Therefore, it is essential to take into account the physiological changes of pregnancy and administer a safe GA that minimises risks, such as failed intubation, aspiration and awareness.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Declaration of interests
MCM was chair and coauthor of the Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics, which have been referenced in this article. CD and LR declare that they have no conflicts of interest.
Biographies
Carlos Delgado MD is an assistant professor and the associate director of the Obstetric Anesthesia Division in the Department of Anesthesiology and Pain Medicine at the University of Washington, Seattle, WA, USA.
Laurence Ring MD is an assistant professor and the associate director of anesthesia at the Allen Hospital. He also serves as the director for the placenta accreta programme and as the director of fetal anaesthesia in the Department of Anesthesiology at Columbia University Medical Center, New York, NY, USA.
Mary Mushambi FRCA is a consultant anaesthetist, Difficult Airway Society professor of anaesthesia and airway management, and the assistant medical director at Leicester Royal Infirmary. She also serves as the chair of the Obstetric Anaesthetists' Association and Difficult Airway Society obstetric difficult airway guidelines group.
Matrix codes: 1C02, 2A09, 3B00
References
- 1.Traynor A.J., Aragon M., Ghosh D. Obstetric anesthesia workforce survey: a 30-year update. Anesth Analg. 2016;122:1939–1946. doi: 10.1213/ANE.0000000000001204. [DOI] [PubMed] [Google Scholar]
- 2.Juang J., Gabriel R.A., Dutton R.P., Palanisamy A., Urman R.D. Choice of anesthesia for cesarean delivery: an analysis of the national anesthesia clinical outcomes registry. Anesth Analg. 2017;124:1914–1917. doi: 10.1213/ANE.0000000000001677. [DOI] [PubMed] [Google Scholar]
- 3.Mendelson C.L. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191–205. doi: 10.1016/s0002-9378(16)39829-5. [DOI] [PubMed] [Google Scholar]
- 4.Mushambi M.C., Kinsella S.M., Popat M. Obstetric Anaesthetists’ Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia. 2015;70:1286–1306. doi: 10.1111/anae.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mhyre J.M., Riesner M.N., Polley L.S., Naughton N.N. A series of anesthesia-related maternal deaths in Michigan, 1985–2003. Anesthesiology. 2007;106:1096–1104. doi: 10.1097/01.anes.0000267592.34626.6b. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins J.L., Chang J., Palmer S.K., Gibbs C.P., Callaghan W.M. Anesthesia-related maternal mortality in the United States: 1979–2002. Obstet Gynecol. 2011;117:69–74. doi: 10.1097/AOG.0b013e31820093a9. [DOI] [PubMed] [Google Scholar]
- 7.Kovacheva V.P., Brovman E.Y., Greenberg P., Song E., Palanisamy A., Urman R.D. A contemporary analysis of medicolegal issues in obstetric anesthesia between 2005 and 2015. Anesth Analg. 2019;128:1199–1207. doi: 10.1213/ANE.0000000000003395. [DOI] [PubMed] [Google Scholar]
- 8.Kinsella S.M., Winton A.L., Mushambi M.C. Failed tracheal intubation during obstetric general anaesthesia: a literature review. Int J Obstet Anesth. 2015;24:356–374. doi: 10.1016/j.ijoa.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Quinn A.C., Milne D., Columb M., Gorton H., Knight M. Failed tracheal intubation in obstetric anaesthesia: 2 yr national case-control study in the UK. Br J Anaesth. 2013;110:74–80. doi: 10.1093/bja/aes320. [DOI] [PubMed] [Google Scholar]
- 10.Cantwell R., Clutton-Brock T., Cooper G. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl. 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 11.Knight M., Nair M., Tuffnell D., Shakespeare J., Kenyon S., Kurinczuk J.J. 2017. Saving lives, improving mothers’ care. Lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2013–15, Oxford. [Google Scholar]
- 12.Prowse C.M., Gaensler E.A. Respiratory and acid-base changes during pregnancy. Anesthesiology. 1965;26:381–392. doi: 10.1097/00000542-196507000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kodali B.S., Chandrasekhar S., Bulich L.N., Topulos G.P., Datta S. Airway changes during labor and delivery. Anesthesiology. 2008;108:357–362. doi: 10.1097/ALN.0b013e31816452d3. [DOI] [PubMed] [Google Scholar]
- 14.Carp H., Jayaram A., Stoll M. Ultrasound examination of the stomach contents of parturients. Anesth Analg. 1992;74:683–687. doi: 10.1213/00000539-199205000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Mazze R.I., Kallen B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol. 1989;161:1178–1185. doi: 10.1016/0002-9378(89)90659-5. [DOI] [PubMed] [Google Scholar]
- 16.ACOG Committee opinion no 775: nonobstetric surgery during pregnancy. Obstet Gynecol. 2019;133:e285–e286. doi: 10.1097/AOG.0000000000003174. [DOI] [PubMed] [Google Scholar]
- 17.Jevtovic-Todorovic V. Exposure of developing brain to general anesthesia: what is the animal evidence? Anesthesiology. 2018;128:832–839. doi: 10.1097/ALN.0000000000002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds F., Seed P.T. Anaesthesia for Caesarean section and neonatal acid-base status: a meta-analysis. Anaesthesia. 2005;60:636–653. doi: 10.1111/j.1365-2044.2005.04223.x. [DOI] [PubMed] [Google Scholar]
- 19.Shippam W., Preston R., Douglas J., Taylor J., Albert A., Chau A. High-flow nasal oxygen vs. standard flow-rate facemask pre-oxygenation in pregnant patients: a randomised physiological study. Anaesthesia. 2019;74:450–456. doi: 10.1111/anae.14567. [DOI] [PubMed] [Google Scholar]
- 20.Pandit J.J., Andrade J., Bogod D.G. The 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: summary of main findings and risk factors. Anaesthesia. 2014;69:1089–1101. doi: 10.1111/anae.12826. [DOI] [PubMed] [Google Scholar]
- 21.Devroe S., Van de Velde M., Rex S. General anesthesia for caesarean section. Curr Opin Anaesthesiol. 2015;28:240–246. doi: 10.1097/ACO.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 22.Zaouter C., Calderon J., Hemmerling T.M. Videolaryngoscopy as a new standard of care. Br J Anaesth. 2015;114:181–183. doi: 10.1093/bja/aeu266. [DOI] [PubMed] [Google Scholar]
- 23.Blajic I., Hodzovic I., Lucovnik M., Mekis D., Novak-Jankovic V., Stopar Pintaric T. A randomised comparison of C-MAC™ and King Vision® videolaryngoscopes with direct laryngoscopy in 180 obstetric patients. Int J Obstet Anesth. 2019;39:35–41. doi: 10.1016/j.ijoa.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Tolcher M.C., Fisher W.E., Clark S.L. Nonobstetric surgery during pregnancy. Obstet Gynecol. 2018;132:395–403. doi: 10.1097/AOG.0000000000002748. [DOI] [PubMed] [Google Scholar]
- 25.Pearl J.P., Price R.R., Tonkin A.E., Richardson W.S., Stefanidis D. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767–3782. doi: 10.1007/s00464-017-5637-3. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins J.L. Excess in moderation: general anesthesia for cesarean delivery. Anesth Analg. 2015;120:1175–1177. doi: 10.1213/ANE.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 27.Wright J.D., Pri-Paz S., Herzog T.J. Predictors of massive blood loss in women with placenta accreta. Am J Obstet Gynecol. 2011;205:38. doi: 10.1016/j.ajog.2011.01.040. e1–6. [DOI] [PubMed] [Google Scholar]
- 28.Chong Y., Zhang A., Wang Y., Chen Y., Zhao Y. An ultrasonic scoring system to predict the prognosis of placenta accreta: a prospective cohort study. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leffert L., Butwick A., Carvalho B. The Society for Obstetric Anesthesia and Perinatology consensus statement on the anesthetic management of pregnant and postpartum women receiving thromboprophylaxis or higher dose anticoagulants. Anesth Analg. 2018;126:928–944. doi: 10.1213/ANE.0000000000002530. [DOI] [PubMed] [Google Scholar]
- 30.Markley J.C., Farber M.K., Perlman N.C., Carusi D.A. Neuraxial anesthesia during Cesarean delivery for placenta previa with suspected morbidly adherent placenta: a retrospective analysis. Anesth Analg. 2018;127:930–938. doi: 10.1213/ANE.0000000000003314. [DOI] [PubMed] [Google Scholar]
- 31.Bogod D. Pain during caesarean section. BJOG. 2016;123:753. doi: 10.1111/1471-0528.13845. [DOI] [PubMed] [Google Scholar]
- 32.Ring L.E., Ginosar Y. Anesthesia for fetal surgery and fetal procedures. Clin Perinatol. 2019;46:801–816. doi: 10.1016/j.clp.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Lee S.J., Ralston H.J., Drey E.A., Partridge J.C., Rosen M.A. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947–954. doi: 10.1001/jama.294.8.947. [DOI] [PubMed] [Google Scholar]