Key points.
-
•
Pectus deformities (PD) are the most common chest wall deformities.
-
•
Most cases are idiopathic but can they be associated with other conditions.
-
•
Surgical treatment may be considered based on severity and associated cardiopulmonary symptoms, psychological concerns, or both; but treatment is invasive and the evidence for benefit is mixed.
-
•
Good postoperative analgesia is required for optimal recovery, but there is no single best regimen.
Learning objectives.
After reading this article you should be able to:
-
•
Understand the rationale for surgical treatment of PD.
-
•
Develop an approach to lung isolation and understand the role of intrapleural CO2 and associated physiological changes.
-
•
Discuss the multiple strategies that may be used for postoperative pain relief.
-
•
Describe the surgical techniques and recognise their potential complications.
Introduction
Chest wall deformity covers a variety of anomalies in the development of the chest wall. More than 95% of cases presenting for surgical correction of chest wall deformity are pectus excavatum (PE; also known as funnel chest or sunken chest) and pectus carinatum (PC; also known as pigeon chest).1 This article will focus on these conditions. Surgical correction of PD poses a unique set of challenges related to a generally young group of patients, the potential for concurrent conditions and major thoracic surgery.
Clinical presentation
The incidence of PD is estimated to be between one in 400 and one in 1000, of which 87% will be PE, 5% PC, and the remainder a combination of the two or other very rare deformities. PE and PC are more likely to present in males, with a male/female ratio of between 3:1 and 9:1.2 PD are predominantly idiopathic and isolated; however, 3–4% are manifestations of other conditions such as connective tissue disorders, most commonly Marfan's syndrome, and musculoskeletal disorders such as Poland syndrome. Many patients also have a cluster of ‘posture-related’ musculoskeletal issues.3
PE is defined as the posterior incursion of the sternum and of the adjacent costal cartilages (Fig 1). In contrast, PC is characterised by the protrusion of the anterior chest wall (Fig. 2, Fig. 3). The underlying pathogenesis of the disorders is poorly understood.
Fig. 1.
Example of pectus excavatum showing depression of the anterior chest wall.
Fig. 2.
Example of pectus carinatum demonstrating sternal protrusion and resulting convexity of the chest wall.
Fig. 3.
Chest CT showing right asymmetric PC - note there is no resultant impingement on the heart or lungs.
Although often described as congenital, it is typical for patients with PE to notice the sternal depression in the preteen and teen years as it worsens with growth. The deformity is permanent if left uncorrected.
Patients with PD may present with psychological distress about body image. Social withdrawal resulting from feelings of shame or bullying experiences may lead to isolation and behavioural changes. Body image dissatisfaction is a major predictor in quality of life measures and self-esteem. A study of 90 patients presenting for PD surgery showed that although rates of mental health disorders were not increased, there was a significant psychological impact. Patients with PC seemed to be at higher risk of mental distress than those with PE, possibly because it is more difficult to hide the chest wall deformity even under clothing. Body image was a major determinant for seeking corrective surgery especially for patients with PC.4 A minority of patients are symptomatic from PD (usually PE) and may present with breathlessness, palpitations, chest or back pain and presyncope or syncope. In those with PE this may be because of cardiac compression, lung compression or both. Surgical correction aims to enhance body image and reverse these symptoms. Before the decommissioning of some forms of pectus surgery by the NHS in February 2019, approximately 250–300 cases per year were performed in England and Wales by the NHS.
Selection of patients
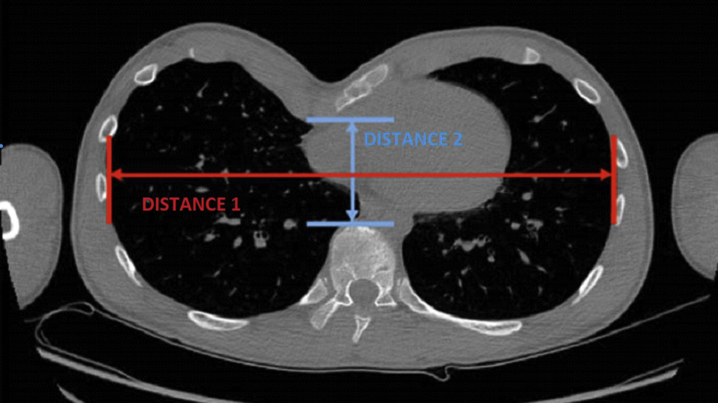
The severity of the deformity is assessed by history taking and physical examination. An objective measure routinely used to gauge the severity of PE is the Haller index (Fig 4). This is obtained from CT imaging by dividing the lateral diameter by the anteroposterior diameter. The condition is considered significant when Haller index is >3.25 (normal value 2.56). There is no similarly established index for PC, but there are some indices including a ‘reverse Haller index’ or a Haller index for carinatum. This is calculated by dividing the greatest AP diameter (depth of chest) by the transverse diameter, so the closest to a ratio of 1.0 or less the ‘rounder’ or more severe the PC.
Fig. 4.
Chest CT showing the Haller Index used to assess the severity of PE. The red line is the transverse chest wall distance (distance 1). The blue line is the anteroposterior distance and represents how close the back of the sternum gets to the front of the spinal or vertebral column (distance 2). Haller Index = distance 1/distance 2.
There is no consensus as to the age at which surgery should be offered, though it is unusual to offer corrective surgery to patients aged older than 40 yrs. Patients may present for correction as children as young as 12 yrs or as young adults. The significance of the functional or physical effects of PE is controversial and the evidence for the benefit of surgical treatment has been considered insufficient by NHS England (NHSE) in February 2019 to routinely commission surgery for PD.2 NHSE considered the psychological, social and behavioural outcomes across 26 studies but found serious weaknesses in the evidence. Although all of the studies reported statistically significant improved outcomes related to surgery, the investigators questioned the study designs and the functional significance and value of the outcomes measured. NHSE also considered the cardiorespiratory reserve, and functional and physical outcomes of surgery and either found the evidence base to be lacking or the studies to be poorly controlled. Investigators commented on the lack of standardisation of outcomes measures. There were no high-quality comparison studies made to a no intervention group. There was strong evidence of improvement in lung function as measured by lung function tests but no reference to functional status and an improvement in cardiac function. The significant limitations in the studies meant that the conclusion was that clinical effectiveness could not be inferred from the available evidence and was not weighed against the risks of surgery despite an 80–90% good to excellent anatomical outcome. It is likely through a formal clinical evaluation protocol that patients with demonstrable physiological abnormalities may be offered corrective surgery within the context of a trial. At the time of writing, none are in progress. Relative contraindications to surgical correction are complex congenital anomalies including primary cardiac and pulmonary impairment and neurodevelopmental disorders.
Surgical technique
There are two main surgical techniques for treating PD: the ‘open’ modified Ravitch operation and minimally invasive repair of PE (MIRPE) or Nuss procedure. The choice of procedure depends on multiple factors. Both procedures can be used for PE, but only the Ravitch procedure can be used for PC. A Cochrane review for the surgical treatment of PE found no evidence from RCTs to decide on the best surgical approach as there were no trials that met their inclusion criteria.5
The modified Ravitch procedure involves a chest incision, either a vertical midline sternal or transverse inframammary. Costal cartilage is cut and resected bilaterally and sternal osteotomies (small partial splits in sternum transversely) are performed to reposition the sternum. The sternum is then fixed to prevent displacement after appropriate positioning. One or more bars may be inserted to enable lifting of the sternum for PE. Typically, they are removed after 1 or 2 yrs.
The Nuss procedure is a video-assisted thoracoscopic approach. This approaches the defect using bilateral mid-axillary incisions and insertion of retrosternal metallic bars under direct vision, which are then fixed and stabilised to correct the defect. The bar or bars are usually removed within a few years of placement (Fig 5).
Fig. 5.
Typical chest X-ray appearance after bar placement (the blue circle shows the shift of the heart before and after bar insertion).
Preoperative assessment
As patients presenting for surgery vary widely, a thorough preoperative assessment should be undertaken with a focus on the cardiorespiratory systems and an assessment of any underlying genetic syndromes. A history of allergies, particularly metal allergies, should be sought. A case series of 1215 patients undergoing the Nuss procedure reported a 2.9% rate of allergy to the metal bar inserted. Three patients required further surgery as a result to remove the metalwork.
The preoperative assessment can be used as an opportunity for education and discussions about anaesthesia, the need for invasive monitoring and postoperative analgesia. Routine blood investigations (FBC and U&Es) and an ECG are needed. Chest imaging is part of the routine surgical workup. Imaging investigations should be examined for the presence of any chronic infections, the severity of the condition, and the position of the heart and great vessels for displacement or compression.
This period is also an opportunity for an assessment of body image dysmorphia. There is a poor correlation between objective physiological status and the perceived impact (mental quality of life and self-esteem) of PD in patients with the condition. This highlights the role of psychological assessment and the potential need for counselling and management of expectations for patients with exaggerated tendencies to body dysmorphia.
Cardiorespiratory investigations
Most patients do not require investigating beyond an ECG. For those who complain of palpitations, continuous ECG monitoring should be considered. Some degree of right ventricular compression is common but is not often clinically significant. In severe cases the heart can become displaced into the left hemithorax (Fig 6). The malposition can kink some of the great vessels leading to outflow obstruction, reducing stroke volume and so causing cardiovascular embarrassment particularly during exertion. A functional assessment of exercise tolerance is a useful measure to assess cardiopulmonary physiology. Echocardiography may be used to assess ventricular function and valvular insufficiency, and also detect any right ventricular compression. In symptomatic patients this may be used to support the case for surgical intervention. Mitral valve prolapse with mitral regurgitation is seen in 18–45% of patients with PE because of mechanical distortion of the mitral annulus.6 Pectus surgery resolves many of the cardiac abnormalities associated with the condition.7
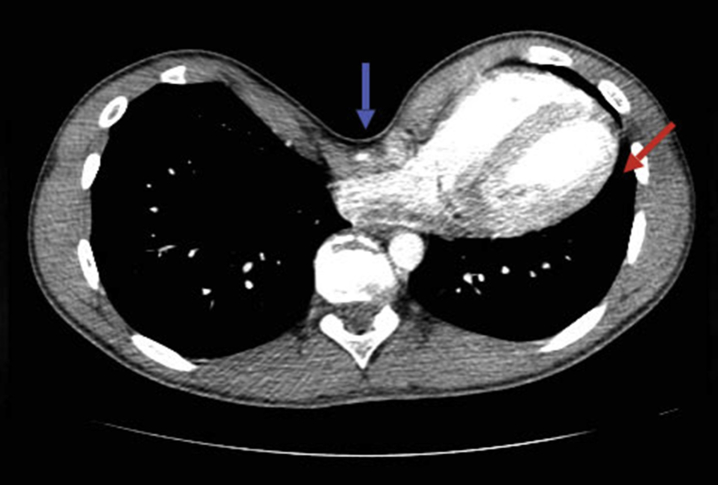
Fig. 6.
Chest CT showing significant sternal depression (blue arrow) and displacement of the heart and left lung (red arrow) in a patient with PE.
Pulmonary function tests are frequently performed on patients who present with PD as they often complain of respiratory symptoms, but these tests can be difficult to interpret. Results often appear normal or nearly normal, but may demonstrate a restrictive defect in some patients. Those with more severe deformities are at a higher risk of decreased pulmonary function with a restrictive pattern.8
Cardiopulmonary exercise testing (CPET) has been used to assess cardiopulmonary physiology with PD, with conflicting results.9 Malek and colleagues have conducted two meta-analyses examining the effects of surgical repair on cardiovascular and pulmonary function in patients with PE. The investigators found that cardiovascular function improved significantly after repair, but pulmonary function did not.10,11 Considerable differences in methodology have contributed to these results, and Malek and colleagues proposed specific strategies to be used for CPET in patients with PE so that results between studies could be compared effectively. Currently its use for PD is limited to research.
Preoperative preparation
Premedication with anxiolytic drugs should be considered on a case-by-case basis. A retrospective cohort study showed a statistically significant reduction in postoperative opioid requirement in patients who received premedication with lorazepam 0.02–0.1 mg kg−1 the evening before Nuss surgery.12 There is a body of evidence to support the use of gabapentin before operation for pain management after major surgery. A single dose of gabapentin has been shown to be effective in reducing postoperative pain and opioid use, and has been incorporated into protocols of some institutions for PD surgery.13
Management of anaesthesia
Standard monitoring is sufficient in most cases for induction of anaesthesia. Invasive arterial BP monitoring should be considered to monitor BP during critical surgical steps. Wide-bore i.v. access should be established before surgery because of the risk of intraoperative major haemorrhage. Central venous access is usually unnecessary, but can be considered in complex surgical repair, repeat thoracic surgery or if a patient's comorbidities warrant it. Perioperative transoesophageal echocardiography can be of value in patients with severe pectus deformity causing cardiac compression, both to monitor the manipulation of the right ventricle during bar insertion and assess for any valvular damage.
Conduct of anaesthesia
General anaesthesia, using either an inhalational technique or TIVA, is commonly supplemented with regional techniques (see below). Nitrous oxide should be avoided because of the risk of expanding pneumothoraces. There is some evidence to suggest that TIVA reduces post-thoracotomy chronic pain, but there are no studies specific to pectus surgery. The patient will require intraoperative neuromuscular blocking drugs to relax the diaphragmatic musculature. The choice of tracheal tube is dictated by surgical requirements. Ravitch surgery does not require lung isolation as it is an open procedure. The Nuss procedure benefits from lung isolation to facilitate the thoracoscopic approach. In addition to one lung ventilation or as an alternative to lung isolation, CO2 insufflation into the chest cavity can be used to generate a pneumothorax. Careful monitoring of physiological variables is required to avoid excessive intrapleural pressure and the potential for ‘tensioning’ and mediastinal shift.
At the end of the procedure, the lungs need to be re-expanded and the CO2 expelled. This can be achieved with reversal of lung isolation if a double lumen tube is being used, and several Valsalva manoeuvres together with the surgeon using a large-bore catheter placed in the pleural cavity to expel the gas into an underwater seal. A postoperative chest drain may not be required if there is no air leak at the end of the procedure.
Positioning
Patients are positioned supine with a bolster or gel pad placed behind the thoracic spine to improve surgical access. The brachial plexus is at particular risk when the arms are abducted during a Nuss repair. Vigilance is needed to ensure that when the arms are abducted beyond 90° they are repositioned as soon as possible. Alternative positioning techniques have been advocated involving abducting the arms but suspending the lower arms vertically using an arthroscopy sling and holding it in position using a right-angled suspension device attached to the operating table.14 Open repair does not always necessitate abducted arms in which case they may remain by the patients' sides.
Intraoperative complications in pectus surgery
The major potential intraoperative complications the anaesthetist needs to be aware of are cardiac, vascular or liver injury or perforation leading to life-threatening haemorrhage. Bar slippage and misplacement can also cause trauma, cardiac compression or right ventricular outflow tract obstruction which, if not recognised and corrected quickly, can lead to cardiovascular collapse.
A study looking solely at severe cardiovascular life-threatening events related to PE surgery suggests these are more common in minimally invasive techniques than open repair, but the numbers are small and the denominator for each type of surgery is unknown.15 Major complications such as these rarely require emergency cardiopulmonary bypass. Minor complications include pericardial tears, lung trauma, and pleural and pericardial effusions, which can be managed conservatively.
Postoperative pain management
Surgical repair of PD was initially treated via open surgery and hence has always been recognised as a source of severe pain. The Nuss repair was introduced later, but despite being a minimally invasive procedure pain is still a significant issue and may even be more significant with the Nuss repair than the Ravitch procedure.16 Good pain management is key to recovery, patients' satisfaction, supporting participation in physical therapy and reducing the duration of hospital stay.
Traditionally the standard analgesia protocol for pectus surgery included an epidural but there is now a wide variety of potential techniques. An American observational study across 14 institutions explored current paediatric analgesic practice for modified Nuss repair. Institutions used a wide variety of strategies including intrathecal morphine, epidural catheters, paravertebral catheters, wound catheters and no regional technique.10 Some of these will be discussed below.
Thoracic epidural anaesthesia
This remains the standard technique for perioperative analgesia in many institutions. An epidural catheter sited at T3–5 before surgery can provide analgesia for the intraoperative period and the first few days after surgery. Thoracic epidural anaesthesia (TEA) has the advantage of providing excellent quality analgesia and reducing the sympathetic response to surgery when working well. However, thoracic epidurals can be challenging to insert and have a significant failure rate of about 30% in clinical practice across all specialities. Incorrect placement accounts for half of failures, but active management of inadequate epidural analgesia, including resiting, results in a significantly improved success rate.17
Opioids such as fentanyl, diamorphine and morphine are commonly added to epidural infusions to improve the quality of analgesia. Clonidine has been studied in children having the Nuss procedure with evidence of a similar quality of analgesia to fentanyl but with fewer adverse effects. An RCT that compared thoracic epidural and i.v. PCA opioid found favourable pain scores on days 0 and 1 after operation for patients assigned to epidural analgesia. This equilibrated on day 2 but favoured the PCA arm thereafter until discharge. The mean duration for the epidural and PCA was 2.8 and 2.6 days, respectively.18
Paravertebral block
Paravertebral blocks are an established adjunct to thoracic surgical analgesia. These need to be performed bilaterally for pectus repair surgery, and a study in children undergoing the Nuss procedure showed similar analgesic benefit when compared with thoracic epidural analgesia.19 They can be inserted by the anaesthetist before operation or by the surgeon intraoperatively under direct vision. Paravertebral blocks have a lower incidence of adverse effects including urinary retention and hypotension.20
Intrathecal opioids
Intrathecal morphine can provide analgesia for up to 24 h. However, as further strong analgesia would be required thereafter its role is limited in this situation.
Chest wall blocks
Bilateral intercostal blocks from the second to sixth intercostal nerves have been used in combination with multimodal analgesia for pectus repair surgery. Studies in the paediatric population have demonstrated analgesic efficacy with reduced opioid consumption; however, large RCTs in adult patients are lacking.21
Although not used routinely, a number of novel peripheral nerve and fascial plane blocks have the potential to provide additional analgesic and opioid sparing effects in chest wall surgery. We have no experience with these novel blocks but have outlined the various options available. Bilateral parasternal blocks target the anterior intercostal nerves next to the sternum. This can be done either under ultrasound guidance or under direct vision by the surgeon, injecting local anaesthetic just lateral to the sternal border in the intercostal space.
Pectoralis blocks and the serratus plane block have become popular in breast surgery for anterior chest wall analgesia, and have the potential to provide additional analgesia in thoracic surgery. The evidence base for these blocks in PD surgery is currently limited to case reports.
Multimodal analgesia
A multimodal approach to analgesia maximises pain relief at lower analgesic doses of any individual drug thereby reducing the risk of adverse effects. Drugs used to complement the analgesic strategy may be paracetamol, NSAIDs, dexamethasone, ketamine, i.v. lidocaine, clonidine and magnesium.
When using a multimodal approach without regional anaesthesia, it is important to ensure the availability of opioid drugs for pain control. Opioids delivered via a PCA pump are recommended to allow patient control and comfort. The dose required in the first 24 h after operation can help guide opioid prescribing for the remainder of the stay and conversion onto an oral regimen. However, in our experience significant pain from these procedures lasts into the second and third postoperative day so care should be taken not to de-escalate parenteral opioids too early.
Surgical interventions for pain control
The two main types of surgical interventions described to help with postoperative pain control are cryoablation and intraoperative catheter placement.
Cryoablation is a novel surgical procedure that temporarily damages the intercostal nerves at multiple levels. It has been shown to provide analgesia for between days and weeks, but requires surgeons to learn a new technique and adds to duration of surgery. Cryoablation has been shown to reduce perioperative opioid use and reduce length of stay after a single intraoperative treatment after pectus repair in children, and may offer an alternative to regional analgesia.22 The long-term outcomes are not yet available, and there is a possibility of developing chronic pain.
Intraoperative catheter placement is most commonly done either via a subpleural route to help anaesthetise intercostal nerves, or subcutaneously to help with incisional pain and potentially anaesthetise intercostal nerves as well. The catheters are attached to elastomeric pumps containing local anaesthetics, which infuse over the course of several days.
Postoperative care
Patients should have a chest radiograph in recovery as residual pneumothorax is relatively common and if present, requires monitoring;, occasionally a neumothroax pmay require a chest drain as this may not be sited routinely during surgery.
Patients are usually cared for on the ward unless local policies dictate level 2 care to manage epidural analgesia. Rates of respiratory complications vary significantly in the literature but are quoted in the order of 5–10% of patients developing self-resolving atelectasis and pneumothorax and less than 1% developing pneumonia.23 It has been suggested that these risks can be reduced with an enhanced recovery programme incorporating components such as the use of high flow humidified oxygen, regular deep breathing and coughing exercises, good oral hygiene, incentive spirometry and early mobilisation. Early physiotherapy is key to enhancing recovery.
Long-term outcomes
Most studies report 80–90% good to excellent anatomical surgical outcomes. A small but long-term survey of 39 adult patients after PE repair with the Nuss procedure reported high levels of overall satisfaction with a self-reported improved social interaction (85%) and a general improvement in health and exercise tolerance (90%). Moderate to severe prolonged chest pain was the main complaint in this study. A greater proportion of adults require prolonged use of narcotic analgesia than paediatric patients (12.2% vs 3.6%, respectively).24
Conclusions
Surgery for pectus deformity encompasses a range of open and minimally invasive techniques that have the potential to cause major intraoperative complications and significant postoperative pain. It is normally performed as an adolescent or in early adult life so comorbidities are rare, but severe deformities have the potential to cause significant respiratory and cardiac compromise. The indications for surgery have become more limited in the UK as NHSE has decommissioned routine surgical repair of PD, despite overall high rates of satisfaction from surgical intervention, as the evidence base supporting surgery has many limitations. Good analgesic technique is critical to recovery. A wide variety of multimodal options and techniques are used across different centres as no single regimen has proven to be superior. Newer adjuncts such as cryoablation are being reported.
Declaration of interests
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) are accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Ian HuntMRCS FRCS is a cardiothoracic surgeon at St George's University Hospital and the medical director of The Pectus Clinic. He has published numerous articles and given many presentations at national and international meetings on topics in thoracic surgery. He has a particular interest in chest wall deformities including pectus or ‘congenital’ problems and acquired deformities including rib and chest wall injuries.
Mark EdsellFRCA is a consultant cardiothoracic anaesthetist at St George's University Hospital with an interest in anaesthesia for chest wall deformity surgery.
Tasmeen GhafoorFRCA was a specialty registrar in anaesthesia at Leeds Teaching Hospitals NHS Trust when this article was formulated. She has since gained a post as a consultant anaesthetist at Calderdale and Huddersfield NHS Trust.
Matrix codes: 1D02, 2A01, 3A01
References
- 1.Brochhausen C., Turial S., Müller F.K. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg. 2012;14:801–806. doi: 10.1093/icvts/ivs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NHS England . 2019. Clinical commissioning policy: surgery for pectus deformity.https://www.england.nhs.uk/wp-content/uploads/2019/02/1675-Policy_Surgery-for-pectus-deformity.pdf Available from: [Google Scholar]
- 3.Fraser S., Hunt I. Thoracic surgical correction of pectus excavatum: minimal and open approaches. In: Chavoin J.P., editor. Pectus excavatum and Poland syndrome surgery. Springer; Cham: 2019. pp. 99–114. [Google Scholar]
- 4.Steinmann C., Krille S., Mueller A., Weber P., Reingruber B., Martin A. Pectus excavatum and pectus carinatum patients suffer from lower quality of life and impaired body image: a control group comparison of psychological characteristics prior to surgical correction. Eur J Cardiothorac Surg. 2011;40:1138–1145. doi: 10.1016/j.ejcts.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira Carvalho P.E., da Silva M.V., Rodrigues O.R., Cataneo A.J. Surgical interventions for treating pectus excavatum. Cochrane Database Syst Rev. 2014;10:CD008889. doi: 10.1002/14651858.CD008889.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellyb R.E., Goretsky M.J., Obermeyer R. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg. 2010;252:1072–1081. doi: 10.1097/SLA.0b013e3181effdce. [DOI] [PubMed] [Google Scholar]
- 7.Coln E., Carrasco J., Coln D. Demonstrating relief of cardiac compression with the Nuss minimally invasive repair for pectus excavatum. J Pediatr Surg. 2006;41:683–686. doi: 10.1016/j.jpedsurg.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Lawson M.L., Mellins R.B., Paulson J.F. Increasing severity of pectus excavatum is associated with reduced pulmonary function. J Pediatr. 2011;159:256–261. doi: 10.1016/j.jpeds.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 9.Malek M.H., Coburn J.W. Strategies for cardiopulmonary exercise testing of pectus excavatum patients. Clinics. 2008;63:245–254. doi: 10.1590/s1807-59322008000200014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malek M.H., Berger D.E., Housh T.J., Marelich W.D., Coburn W.D., Beck T.W. Cardiovascular function following surgical repair of pectus excavatum: a metaanalysis. Chest. 2006;130:506–516. doi: 10.1378/chest.130.2.506. [DOI] [PubMed] [Google Scholar]
- 11.Malek M.H., Berger D.E., Marelich W.D., Coburn J.W., Beck T.W., Housh T.J. Pulmonary function following surgical repair of pectus excavatum: a meta-analysis. Eur J Cardiothorac Surg. 2006;30:637–643. doi: 10.1016/j.ejcts.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Ghionzoli M., Brandigi E., Messineo A., Messeri A. Pain and anxiety management in minimally invasive repair of pectus excavatum. Korean J Pain. 2012;25:267–271. doi: 10.3344/kjp.2012.25.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muhly W.T., Beltran R.J., Bielsky Perioperative management and in-hospital outcomes after minimally invasive repair of pectus excavatum: a multicenter registry report from the Society for Pediatric Anesthesia Improvement Network. Anesth Analg. 2019;128:315–327. doi: 10.1213/ANE.0000000000003829. [DOI] [PubMed] [Google Scholar]
- 14.Fox M.E., Bensard D.D., Roaten J.B., Hendrickson R.J. Positioning for the Nuss procedure: avoiding brachial plexus injury. Paediatr Anaesth. 2005;15:1067–1071. doi: 10.1111/j.1460-9592.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- 15.De Wolf J., Loobuyck V., Brian E., Benhamed L., Wurtz A. Life-threatening cardiovascular adverse events related to pectus excavatum surgery. Cardiovasc Disord Med. 2018;4 doi: 10.15761/CDM.1000191. [DOI] [Google Scholar]
- 16.Papic J.C., Finnell M.E., Howenstein A.M., Breckler F., Leys C.M. Postoperative opioid analgesic use after Nuss versus Ravitch pectus excavatum repair. J Pediatr Surg. 2014;49:919–923. doi: 10.1016/j.jpedsurg.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Hermanides J., Hollmann M.W., Stevens M.F., Lirk P. Failed epidural: causes and management. Br J Anaesth. 2012;109:144–154. doi: 10.1093/bja/aes214. [DOI] [PubMed] [Google Scholar]
- 18.St Peter S.D., Weesner K.A., Weissend E.E. Epidural vs patient-controlled analgesia for postoperative pain after pectus excavatum repair: a prospective, randomized trial. J Pediatr Surg. 2012;47:148–153. doi: 10.1016/j.jpedsurg.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Hall B.D.M., Boretsky K.R. A comparison of paravertebral nerve block catheters and thoracic epidural catheters for postoperative analgesia following the Nuss procedure for pectus excavatum repair. Paediatr Anaesth. 2014;24:516–520. doi: 10.1111/pan.12369. [DOI] [PubMed] [Google Scholar]
- 20.D’Ercole F., Arora H., Kumar P.A. Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth. 2018;32:915–927. doi: 10.1053/j.jvca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Lukosiene L., Rugyte D.C., Macas A., Kalibatiene L., Malcius D., Barauskas V. Postoperative pain management in pediatric patients undergoing minimally invasive repair of pectus excavatum: the role of intercostal block. J Pediatr Surg. 2013;48:2425–2430. doi: 10.1016/j.jpedsurg.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Sujka J., Benedict L.A., Fraser J.D., Aguayo P., Millspaugh D.L., St Peter S.D. Outcomes using cryoablation for postoperative pain control in children following minimally invasive pectus excavatum repair. J Laparoendosc Adv Surg Tech A. 2018;28:1383–1386. doi: 10.1089/lap.2018.0111. [DOI] [PubMed] [Google Scholar]
- 23.Choi S., Park H.J. Complications after pectus excavatum repair using pectus bars in adolescents and adults: risk comparisons between age and technique groups. Interact Cardiovasc Thorac Surg. 2017;25:606–612. doi: 10.1093/icvts/ivx162. [DOI] [PubMed] [Google Scholar]
- 24.Sacco Casamassima M.G., Gause C., Goldstein S.D. Patient satisfaction after minimally invasive repair of pectus excavatum in adults: long-term results of Nuss procedure in adults. Ann Thorac Surg. 2016;101:1338–1345. doi: 10.1016/j.athoracsur.2015.09.102. [DOI] [PubMed] [Google Scholar]