Key points.
-
•
Cardiac surgical patients deteriorate for a number of different reasons that often require thorough investigation including transoesophageal echocardiography to direct management.
-
•
Most cases of cardiac arrest following cardiac surgery are reversible.
-
•
Initial management of postoperative cardiac arrest should focus on immediate defibrillation and reversible causes before resternotomy within 5 minutes.
-
•
Avoidance of intravenous adrenaline is recommended.
-
•
Outcomes of cardiac arrest after cardiac surgery are favourable and result from a unified and protocolised management in such situations.
The incidence of cardiac arrest after adult cardiac surgery is low and reported as 0.7–2.9%.1, 2, 3 In contrast to traditional in-hospital arrests, survival rates are significantly higher following cardiac surgery (18% vs 79%),4 resulting from the higher incidence of reversible causes as primary precipitants. This informs the reasoning why management of cardiac arrest following cardiac surgery is different from conventional resuscitation, the key differences being avoidance of external chest compressions (ECC) and full dose adrenaline in favour of three-stacked shocks and resternotomy within 5 minutes of arrest.5, 6 Anaesthetists are key members of resuscitation teams, often leading arrest situations. However, application of traditional resuscitation methods in this patient group may cause unintended harm and worsen mortality.4 The European Resuscitation Council guidelines5 include a section on the resuscitation of patients after cardiac surgery, but despite this, knowledge of this special circumstance and integration within general resuscitation updates remains low.
This article aims to discuss the common causes for deterioration following cardiac surgery and will introduce the differences in the approach and management of the arrested postoperative cardiac surgical patient.
Common causes for the deterioration of the postoperative cardiac surgical patient
Many postoperative cardiac surgical patients follow a common pattern of correctable physiological abnormalities that are monitored and treated within cardiac intensive care units (CICUs). Deterioration following cardiac surgery often occurs in the immediate postoperative hours and is apparent as a deviation from the normal pattern of response, prompting immediate investigation and management to prevent further deterioration and cardiac arrest. The most common observation is hypotension, which is often multifactorial, and treatments targeting preload, contractility, and afterload can lead to stabilisation and further time for investigation and reassessment of response. However, markers of organ hypoperfusion such as increasing lactate, metabolic acidosis, oliguria, and confusion are important in identifying the deteriorating patient requiring immediate intervention. There are a number of causes for deterioration detailed below.
Hypovolaemia and bleeding
Hypovolaemia is common. Despite total body fluid increases by up to 30% following cardiopulmonary bypass (CPB), associated intravascular volume depletion is common due to capillary leakage, haemodilution, re-distribution, and polyuria. However, the most common cause is haemorrhage. This is associated with risk factors including: preoperative anticoagulation/antiplatelet treatments, inherited or acquired bleeding diathesis, emergency surgery, prolonged bypass times, and re-do surgery. The nature of the bleeding is often divided into ‘medical’ and ‘surgical’ bleeding.
‘Medical’ bleeding arises from coagulopathy that is often correctable and includes: residual heparin effects, platelet dysfunction (in number and activity), clotting factor deficiencies, and extensive fibrinolysis. Point of care testing and haematological analysis are useful in directing treatment as they help differentiate between patients requiring further protamine or clotting products. Cautious crystalloid resuscitation in bleeding patients is advised to prevent clotting factor dilution and reduction in blood oxygen carrying capacity. Red cell transfusion triggers are variable; however, a haemoglobin of 7–8 g dl−1 with a haematocrit of >0.25 is satisfactory.
‘Surgical’ bleeding is associated with larger blood loss (in the absence of a ‘medical’ cause) and often requires surgical re-exploration. Although most CICUs have local policies, chest drainage of >400 ml h−1 in the 1st hour, >200 ml h−1 in 2 consecutive hours or >100 ml h−1 in 4 consecutive hours should prompt consideration of surgical re-exploration. Common bleeding sites include sternal wire sites and anastomotic or side branch leaks from graft sites. Bleeding is often visible (i.e. high drain outputs), however, inappropriately positioned drains may hide increasing collections, particularly pericardial or pleural. A high index of suspicion must be maintained in any haemodynamically unstable patient with low drain output. These patients often require chest radiography and urgent transoesophageal echocardiography (TOE) as part of diagnostic assessment.
Low cardiac output state
Ventricular function will often fall following CPB, due to myocardial oedema, before returning to baseline within 24–72 hours, even in patients with normal preoperative function. The return to baseline can take longer with impaired preoperative biventricular function, prolonged surgical procedures or inadequate myocardial protection. Other causes of ventricular impairment include: metabolic dysfunction, ischaemia, reperfusion injury, and hypocalcaemia. In many cases, perioperative TOE will identify this, meaning patients with poor left ventricular (LV) function [and poor cardiac output (CO)] will require inotropic support or, in some cases, mechanical support with an intra-aortic balloon pump (IABP). Right ventricular (RV) failure accounts for approximately 20% of postoperative low CO state (LCOS). The causes of RV failure are similar but also include factors affecting RV afterload such as increased pulmonary vascular resistance and volume overload. In patients with either poor pre- or perioperative ventricular function, it is common to measure CO in the postoperative period to help direct treatment toward the failing ventricle. The institutional choice of device is variable, however, the use of the pulmonary artery catheter and non-invasive methods are common. Ventricular failure is associated with reduced cardiac indices and may present with hypotension. Patients are peripherally cool with dysrhythmias, high filling pressures and evidence of reduced end organ perfusion (e.g. low urine output). Metabolic disturbances are common, with rising lactate and base deficit. Treatment aims to remove precipitating factors, ensure adequate ventricular filling, using inotropes with cautious vasopressor use, mechanical ventricular support, and maintaining atrioventricular coordination through the use of dual chamber pacing and antiarrhythmics as appropriate. All patients are at risk of LCOS and any reduction or suspicion of ‘pump failure’ should be appropriately investigated by integration of echocardiography findings alongside clinical and biochemical data.
Graft and valve failure
Myocardial ischaemia following coronary revascularisation is a serious complication often masked in the immediate postoperative period by temporary epicardial pacing. Hallmark features include new ECG changes alongside a persistent LCOS. In such cases, early TOE can identify new regional wall motion abnormalities, in the territory supplied by the graft. The incidence of early graft dysfunction is low at 3% but, depending on the presentation, can be life threatening. Valve failure, although uncommon, can also present in the early hours following surgery and, along with graft failure, requires early diagnosis and involvement of the surgical team in deciding the most appropriate action.7
Tamponade
Cardiac tamponade is defined as the ‘rapid compression of the heart by an accumulation of fluid (often blood) within the pericardial sac that reduces ventricular filling and CO and is a surgical emergency. In the acute setting, early recognition and management influences patient survival.
Despite the ‘classical’ presentation of a falling blood pressure, reduced CO with rising filling pressures and tachycardia, tamponade can be difficult to diagnose as the ‘classical’ signs may be masked (e.g. a hypovolaemic, beta blocked patient with poor LV), therefore a high index of suspicion must be maintained. It is not uncommon for LCOS to be diagnosed as tamponade until proven otherwise, especially in high-risk cases. This is important, as the first presentation of a rapidly developing tamponade may be an arrhythmia or cardiac arrest.
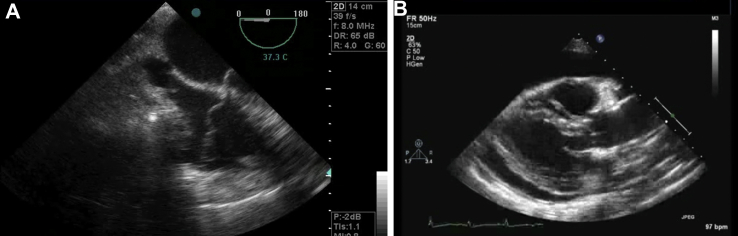
Echocardiography is useful in the diagnosis of tamponade. However, small volumes of blood [which can easily be missed on either transthoracic echocardiogram (TTE) or TOE] can cause significant compression and haemodynamic instability. Therefore, the absence of tamponade on either TTE or TOE should not preclude a return to theatre in the deteriorating patient with suspected tamponade. It should be noted that TTE is difficult in postoperative cardiac patients due to collection position, drain placements, and lung ventilation. In this context, TOE is a superior modality and should be used in cases of suspected tamponade because of its greater capability for anatomical cardiac imaging (Fig. 1A and B). However, the diagnosis of tamponade should remain clinical and focus on the prevention of cardiac arrest and its associated complications. The definitive treatment of tamponade is surgical drainage and maintenance of CO until this point.
Fig 1.
A, TOE demonstrating a cardiac tamponade (four-chamber view). Note the external compression of the right atrium (image top) leading to impaired venous return and cardiac output. If reading the pdf online, click on the image to view the video. B, A parasternal long-axis TTE image demonstrating a cardiac tamponade. Note the circumferential fluid surrounding and compressing the heart leading to impaired cardiac output. If reading the pdf online, click on the image to view the video.
Arrhythmias
The management of cardiac arrest arrhythmias is discussed later. Atrial fibrillation (AF) is the most common postoperative arrhythmia, despite the perioperative normalisation of potassium and magnesium.8 The management of AF postoperatively follows conventional guidelines and should be managed according to the degree of haemodynamic stability.6 Pharmacological cardioversion is often in line with local guidance and preference. An adjustment of pacing (if appropriate) is often required in such patients to exclude inappropriate atrial stimulation.
Vasodilation
Reduced systemic vascular resistance causing hypotension is another common finding in postoperative patients. The active rewarming of patients can reduce systemic vascular resistance leading to hypotension from vasodilation and relative hypovolaemia. Other common causes include: sepsis, anaphylaxis, adrenal insufficiency, the vasoplegic syndrome, and unopposed use of inodilator agents—further discussion of which is outside the scope of this article.
Management of cardiac arrest following cardiac surgery
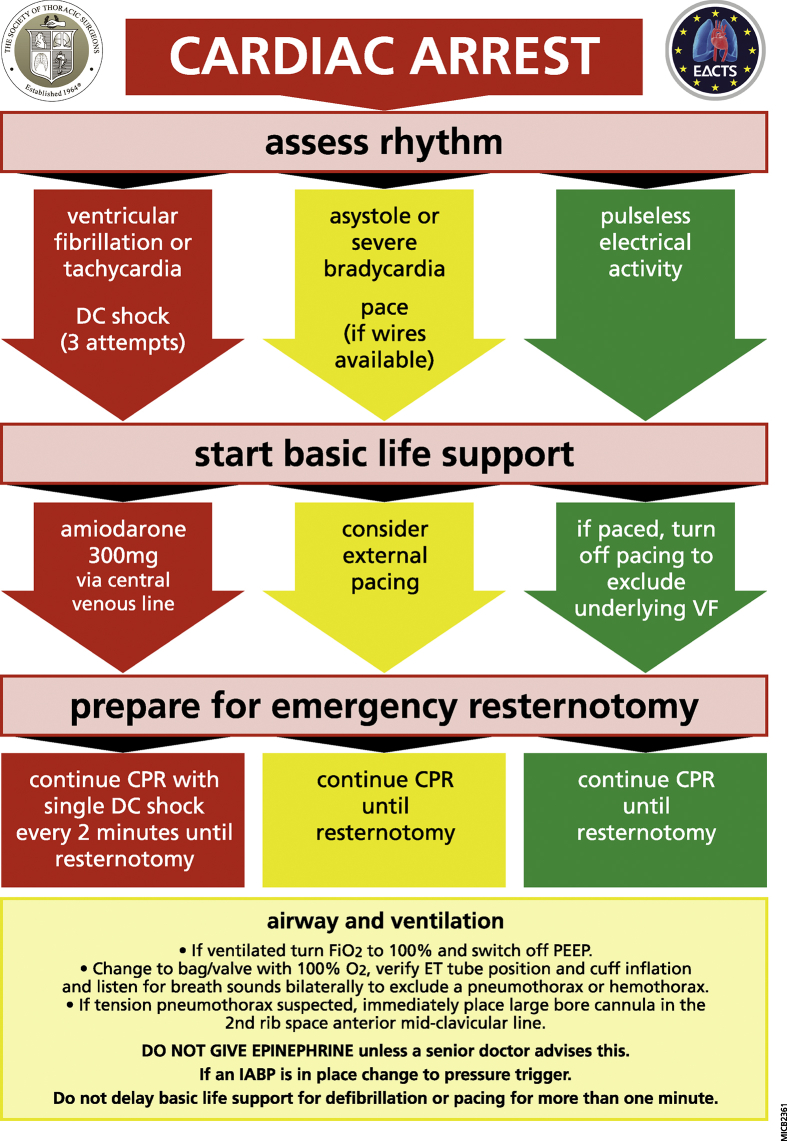
Resuscitation of the arrested postoperative cardiac surgical patient follows the principles of the chain of survival,6 however the priority is to correct reversible causes of arrest in a timely manner followed by immediate chest re-opening if these measures fail to preserve cerebral function. This is because the precipitants of arrest are commonly reversible and include: ventricular fibrillation (VF) in up to 25–50% of cases, with bleeding and tamponade accounting for a significant proportion.9 The Cardiac Advanced Life Support (CALS) protocol (Fig. 2) was designed for use in all CICUs for any cardiac surgical patient (excluding pulmonary surgery). However, it is vital that adoption of these protocols should be made on an institutional basis to ensure consistency of protocol delivery.
Fig 2.
The CALS algorithm for the management of postoperative cardiac arrest following cardiac surgery.
The CALS protocol
Patients arresting in CICU are monitored and often intubated. An absence of pulsatile arterial line with flat saturation, central venous and pulmonary artery pressure traces, and reduction in end tidal carbon dioxide all signify cardiac arrest and invite the first responder to activate the CALS protocol. In contrast to conventional resuscitation, and owing to potentially fatal complications of cardiac/graft injury, ECC can be delayed for up to 1 minute following identification of either VF or pulseless ventricular tachycardia (pVT) to allow for expeditious defibrillation10, 11 and to allow for institution of pacing in asystole or severe bradycardia. After this time, conventional ECC should be commenced. Pulseless electrical activity (PEA) arrests should receive immediate ECC in line with conventional published guidance.6
Traditional resuscitation supports defibrillation as soon as possible with further attempts after 2 minutes of ECC with subsequent attempts after further ECC cycles. In the cardiac surgical patient, efficacy of defibrillation reduces by 10% for every minute delay, in addition, success rates for immediate sequential shocks for VF or pVT decline from 78% with the first shock to 14% with the third, therefore immediate defibrillation with three sequential attempts at 150 Joules is advised.11 If unsuccessful, immediate resternotomy is advised; therefore, this should be anticipated and prepared for during the above attempts. During early resternotomy, a bolus of 300 mg of amiodarone should be administered with a further dose of 150 mg in refractory cases. Lidocaine at a dose of 1 mg kg−1 is a suitable alternative.12, 13
The initial arrest rhythm will respond to defibrillation in up to 50% of patients. Many patients arrest with a non-shockable rhythm resulting from tamponade, tension pneumothorax, or severe hypovolaemia (often secondary to bleeding). If immediate pacing fails to resolve an asystolic arrest (atropine is not recommended), or if the presenting rhythm is PEA, then immediate ECC should commence whilst preparing the patient for resternotomy. In the CICU, the effectiveness of ECC is confirmed by monitoring the arterial pressure trace with a target compression rate and depth to achieve a systolic impulse of > 60 mm Hg to maintain a mean perfusion pressure, preventing ventricular distension, LV wall stress, and ischaemia. An inability to achieve a systolic of >60 mm Hg during ECC should prompt a review of the adequacy of technique or whether ECC are ineffective due to an inadequate pre-load secondary to persistent hypovolaemia, cardiac tamponade or tension pneumothorax; emergency resternotomy will help address these problems.
Resternotomy and cardiac massage
In the situation of cardiac arrest refractory to immediate treatment, emergency resternotomy should occur within 5 minutes to facilitate internal cardiac massage or defibrillation (at 20 Joules). This occurs in up to 50% of postoperative cardiac arrests or in 2.7% of all patients undergoing cardiac surgery.9 The procedure should be performed seamlessly with simultaneous preparation occurring during initial resuscitation efforts by a minimum of two people.
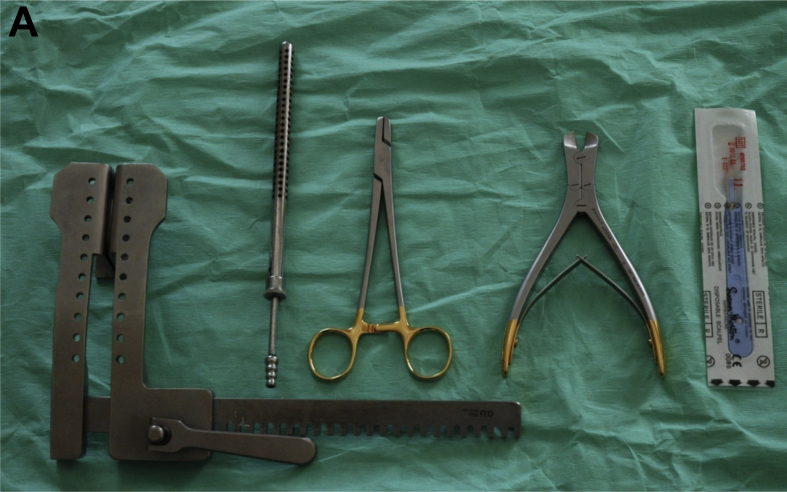
Due to time constraints, betadine and handwashing are not necessary if adhesive drapes with clear windows and closed-sleeve gowning techniques are adopted. Emergency reopening kits are simplified and easy to use. Instead of the 30 items found in surgical sternotomy packs, they contain five essential items to allow expeditious chest reopening (Fig. 3A). As soon as the decision is made for resternotomy, ECC will swap from non-sterile to sterile person following draping of the patient, continuing until chest reopening commences (online video 3). Ideally, a cardiac surgeon should perform the resternotomy, however, senior members of the CICU team are trained to reopen the chest during resuscitation situations (including anaesthetists). This is an integral part of resuscitation, therefore, unnecessary delays (including phone calls) should be avoided. At this point internal massage and defibrillation can be used as appropriate.
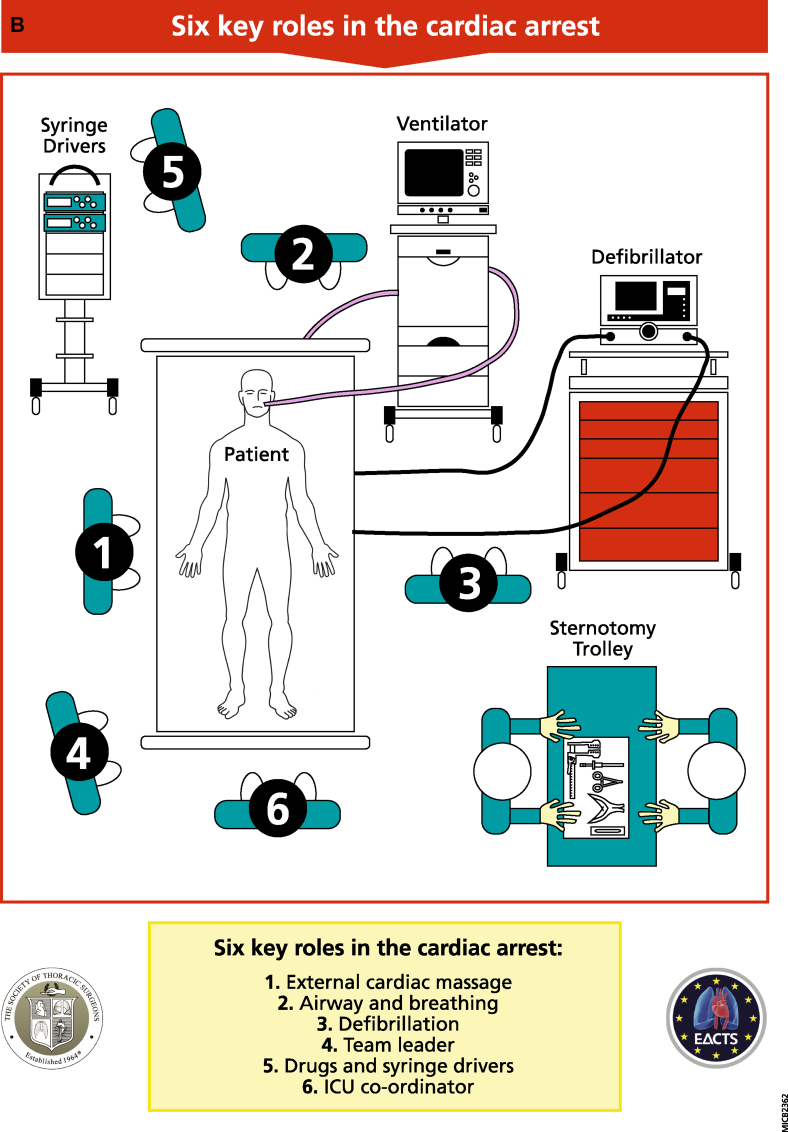
Fig 3.
A, The CALS re-opening kit. B, The six key roles during a cardiac arrest following cardiac surgery. Person 1: should perform external cardiac compressions at a rate of 100–120 beats min−1 whilst ensuring adequacy of compression depth by observing the arterial pressure. Person 2: should ensure adequate oxygen delivery, airway patency, ventilation, and exclusion of respiratory causes of arrest. Person 3: This person should connect the defibrillator, defibrillate as appropriate, and manage pacing as required. They are also responsible for internal defibrillation availability as required. Person 4: is the team leader. This person (often a senior clinician) coordinates the arrest process and ensures that the protocol is followed and resternotomy is anticipated and prepared for. Person 5: This individual stops all infusions after initial resuscitation and administers drugs and infusions as per direction from Person 4. Person 6: This role is coordination and is usually performed by a senior CICU nurse. This individual coordinates all peripheral activity as soon as the situation arises and ensures all equipment is ready and appropriate help is sought.
Supplementary videos relating to this process can be found at https://doi.org/10.1016/j.bjae.2017.11.002
The following are the supplementary data related to this article:
Team management during arrest
The CALS approach to the arrested patient clearly defines six key roles for clinical staff, shown in Fig. 3B. All members of clinical staff should be appropriately trained to fulfil any team role.
Airway management
Conventional airway management applies during CALS. Often, the patient is intubated, therefore confirmation of tube patency, lung compliance alongside 100% oxygen, and removal of positive end-expiratory pressure (PEEP) are required. In the absence of compromise, mechanical ventilation (with no PEEP) can be recommenced. In non-intubated patients, a bag-valve-mask and high flow oxygen via reservoir system are required until a definitive airway is secured. Capnography is essential. If airway pressures are high and tension pneumothorax suspected, it should be managed by conventional means.
Infusions and adrenaline
Many infusions in CICU contribute to vasodilation (e.g. sedatives and analgesics) and inadvertent flushing of these may precipitate cardiac arrest. During resuscitation, these infusions should cease. Awareness in the context of low or absent cerebral perfusion is unlikely; however, if concern exists, then it is acceptable to continue sedation, with other infusions commenced at the discretion of the attending clinician.
Intravenous adrenaline (1 mg) is commonly used during conventional resuscitation as studies demonstrated an increased rate of neurological recovery to discharge with no increase in survival.6, 14, 15 Despite no study conferring benefit or harm during resuscitation of the postoperative cardiac surgical patient, the risk of administering adrenaline in conventional doses is with profound hypertension, bleeding, or tearing of vessel anastomoses on return of spontaneous circulation (ROSC), which can precipitate catastrophic harm or further cardiac arrest.16 Adrenaline remains a useful drug in peri-arrest situations in smaller doses.
Pacing management
Disruption of intrinsic conduction is common following cardiac surgery. Many patients return from theatre with epicardial pacing leads (atrial, ventricular, or both). In situations of severe bradycardia, asystole, or PEA, these wires should be attached (if not already) and the pacemaker set to DDD at 80–100 beats min−1, with stimulation thresholds at maximum to ensure pacing. Some pacing boxes have an emergency button that activates asynchronous pacing, which is appropriate during resuscitation. In the absence of pacing wires, external transcutaneous pacing should be attempted, following ECC commencement.6, 9 A paced rhythm at the time of arrest may mask underlying VF; therefore, a pacing pause should be performed. During pacing failure, preparations should be made in anticipation of future treatments including: internal pacing, replacing wires, and new pacing boxes in order to reduce non-circulatory time.
IABP management
In CICUs, IABPs are triggered by either ECG or arterial line pressure. Cardiac arrest in the context of a stable ECG rhythm (e.g. PEA) may continue to trigger the IABP (if ECG trigger); thereby giving the impression of a CO by way of an IABP generated pressure trace. However, the loss of the cardiac component of the pressure trace will be evident (in addition to loss of other pulsatile traces discussed previously), suggesting cardiac arrest (this is also confirmed by pausing the IABP). In this situation, set the IABP to pressure trigger in a 1:1 ratio with maximal augmentation to enable the IABP to augment diastolic perfusion in line with ECC, thereby increasing coronary and cerebral perfusion. During resuscitation, the ECG trigger will be too irregular to use safely. In the absence of ECC or CO, the intrinsic trigger should be set at 100 beats min−1.
Extracorporeal support during cardiac arrest
If ROSC is not achieved after resternotomy, consideration may be given to the institution of extracorporeal membranous oxygenation-CPR (ECMO-CPR or E-CPR), if available within the institution. There is an emerging evidence base supporting the use of E-CPR in refractory cardiac arrest. Favourable outcomes are associated with patient selection, detailed standard operating procedures, and minimal time from arrest to E-CPR.17 Some estimates report that good neurological outcome can be achieved in 40–50% of E-CPR cases, however, all studies recognize the time from cardiac arrest to ECMO flow as the primary determinant of outcome with optimal times being under 30 minutes.17 It is common to have limited information availability during cardiac arrests, however, the patient benefits of cardiac surgery have been decided preoperatively, thereby indicating that temporary ECMO support is usually appropriate. Team decisions should also incorporate the reversibility for the arrest made on a case-by-case basis.
Cardiac arrest and re-opening outside the ICU
The success of the CALS protocol is due, in part, to the location, equipment, and team available within the CICU. The risk of delayed cardiac arrest is low, but possibilities do exist (e.g. delayed tamponade or bleeding following pacing wire removal). Ward-based resuscitation should initially be managed using defibrillation and/or pacing as described earlier, however adoption of standard advanced life support protocol6 should be used pending senior clinician decision for resternotomy as the injury risk increases beyond 10 days due to mediastinal adhesions. Internal cardiac massage is a superior resuscitation technique; however, resternotomy outside of CICUs is associated with poor survival.18 Local arrangements should be in place for resternotomy outside the ICU, most likely adopting conventional resuscitation whilst returning the patient to the CICU or theatre using the ‘scoop and run’ technique which has variable success.18
Summary
The incidence of cardiac arrest following cardiac surgery is low and associated with favourable outcomes if timely interventions are undertaken. Many factors precipitate patient deterioration, all of which necessitate investigation. In situations of cardiac arrest, the use of a universal structured approach improves outcome. Anaesthetists caring for postoperative cardiac surgical patients are strongly advised to familiarise themselves with such protocols or attend a suitable resuscitation course.19, 20
Declaration of interest
JD is a director of CALS-S UK Ltd.
MCQs
The associated MCQs (to support CME/CPD activity) can be accessed at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Dr Jonathan Brand BSc (Hons) MBChB (Hons) PGCertClinEd FHEA FRCA FFICM is a Consultant in cardiothoracic anaesthesia and critical care. His major interests include resuscitation, cerebral protection and anaesthesia for high risk cardiac and aortic surgery.
Dr Andrew McDonald MBChB FRCA is an advanced trainee in cardiothoracic anaesthesia and critical care. His professional interests include one lung ventilation, trauma, regional anaesthesia and preoperative optimisation.
Mr Joel Dunning BA (Hons) BMBCh (Hons) FRCS PhD is a Consultant thoracic surgeon, one of the founders of the CALS course and is a director of CALS-UK. His clinical interests include minimally invasive thoracic surgery in particular VATS and robotic surgery.
RCoA Matrix Codes: 1B04, 2C01, 3J02
Footnotes
Supplementary material related to this article can be found at https://doi.org/10.1016/j.bjae.2017.11.002.
Supplementary material
The following are the supplementary data related to this article:
References
- 1.Mackay J.H., Powell S.J., Osgathorp J., Rozario C.J. Six-year prospective audit of chest reopening after cardiac arrest. Eur J Cardiothorac Surg. 2002;22:421–425. doi: 10.1016/s1010-7940(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 2.Wahba A., Gotz W., Birnbaum D.E. Outcome of cardiopulmonary resuscitation following open heart surgery. Scand Cardiovasc J. 1997;31:147–149. doi: 10.3109/14017439709058084. [DOI] [PubMed] [Google Scholar]
- 3.Adam Z., Adam S., Everngam R.L. Resuscitation after cardiac surgery: results of an international survey. Eur J Cardiothorac Surg. 2009;36:29–34. doi: 10.1016/j.ejcts.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 4.Dunning J., Fabbri A., Kolh P.H. EACTS Clinical Guidelines Committee. Guideline for resuscitation in cardiac arrest after cardiac surgery. Eur J Cardiothorac Surg. 2009;36:3–28. doi: 10.1016/j.ejcts.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Truhlar A., Deakin C.D., Soar J. European Resuscitation Council guidelines for resuscitation 2015. Section 4: Cardiac arrest in special circumstances. Resuscitation. 2015;95:148–201. doi: 10.1016/j.resuscitation.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Resuscitation Guidelines 2015. Resuscitation Council UK. Available from: www.resus.org.uk/resuscitation-guidelines/ [Accessed 21 June 2017].
- 7.Force T., Hibberd P., Weeks G. Perioperative myocardial infarction after coronary artery bypass surgery. Clinical significance and approach to risk stratification. Circulation. 1990;82:903–912. doi: 10.1161/01.cir.82.3.903. [DOI] [PubMed] [Google Scholar]
- 8.Peretto G., Durante A., Rosario L., Cianflone D. Postoperative arrhythmias after cardiac surgery: incidence, risk factors, and therapeutic management. Cardiol Res Pract. 2014;2014:1–15. doi: 10.1155/2014/615987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunning J., Levine A., Ley J. The society of thoracic surgeons expert consensus for the resuscitation of patients who arrest after cardiac surgery. Ann Thorac Surg. 2017;103:1005–1020. doi: 10.1016/j.athoracsur.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Chan P.S., Krumholz H.M., Nichol G., Nallamothu B.K. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358:9–17. doi: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 11.Richardson L., Dissanayake A., Dunning J. What cardioversion protocol for ventricular fibrillation should be followed for patients who arrest shortly post-cardiac surgery? Interact Cardiovasc Thorac Surg. 2007;6:799–805. doi: 10.1510/icvts.2007.163899. [DOI] [PubMed] [Google Scholar]
- 12.Leeuwenburgh B.P., Versteegh M.I., Maas J.J., Dunning J. Should amiodarone or lidocaine be given to patients who arrest after cardiac surgery and fail to cardiovert from ventricular fibrillation? Interact Cardiovasc Thorac Surg. 2008;7:1148–1151. doi: 10.1510/icvts.2008.188656. [DOI] [PubMed] [Google Scholar]
- 13.Kudenchuk P.J., Brown S.P., Daya M. The Resuscitation Outcomes Consortium Investigators: amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374:1711–1722. doi: 10.1056/NEJMoa1514204. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs I.G., Finn J.C., Jelinek G.A., Oxer H.F., Thompson P.L. Effect of adrenaline on survival in out-of-hospital cardiac arrest: a randomised double-blind placebo-controlled trial. Resuscitation. 2011;82:1138–1143. doi: 10.1016/j.resuscitation.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Patanwala A.E., Slack M.K., Martin J.R., Basken R.L., Nolan P.E. Effect of epinephrine on survival after cardiac arrest: a systematic review and meta-analysis. Minerva Anestesiol. 2014;80:831–843. [PubMed] [Google Scholar]
- 16.Webb S.T. Caution in the administration of adrenaline in cardiac arrest following cardiac surgery. Resuscitation. 2008;78:101. doi: 10.1016/j.resuscitation.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Fagnoul D., Combes A., De Backer D. Extracorporeal cardiopulmonary resuscitation. Curr Opin Crit Care. 2014;20:259–265. doi: 10.1097/MCC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 18.Lees N.J., Powell S.J., Mackay J. Six-year prospective audit of ‘scoop and run’ for chest reopening after cardiac arrest in a cardiac surgical ward setting. Interact Cardiovasc Thorac Surg. 2012;15:816–824. doi: 10.1093/icvts/ivs343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Cardiac Surgery Advanced Life Support Course. Available from: http://www.csu-als.com [Accessed 21 June 2017].
- 20.The One Heart Course. Available from: http://www.oneheartcourse.co.uk [Accessed 21 June 2017].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.