Abstract
Objective
Germline BRCA1-2 pathogenic variants (gBRCApv) increase the risk of pancreatic cancer and predict for response to platinating agents and poly(ADP-ribose) polymerase inhibitors. Data on worldwide gBRCApv incidence among pancreatic ductal adenocarcinoma (PDAC) patients are sparse and describe a remarkable geographic heterogeneity. The aim of this study is to analyze the epidemiology of gBRCApv in Italian patients.
Materials and methods
Patients of any age with pancreatic adenocarcinoma, screened within 3 months from diagnosis for gBRCApv in Italian oncologic centers systematically performing tests without any selection. For the purposes of our analysis, breast, ovarian, pancreas, and prostate cancer in a patient's family history was considered as potentially BRCA-associated. Patients or disease characteristics were examined using the χ2 test or Fisher's exact test for qualitative variables and the Student's t-test or Mann–Whitney test for continuous variables, as appropriate.
Results
Between June 2015 and May 2020, 939 patients were tested by 14 Italian centers; 492 (52%) males, median age 62 years (range 28-87), 569 (61%) metastatic, 273 (29%) with a family history of potentially BRCA-associated cancers. gBRCA1-2pv were found in 76 patients (8.1%; 9.1% in metastatic; 6.4% in non-metastatic). The gBRCA2/gBRCA1 ratio was 5.4 : 1. Patients with gBRCApv were younger compared with wild-type (59 versus 62 years, P = 0.01). The gBRCApv rate was 17.1% among patients <40 years old, 10.4% among patients 41-50 years old, 9.2% among patients 51-60 years old, 6.7% among patients aged 61-70 years, and 6.2% among patients >70 years old (none out of 94 patients >73 years old). gBRCApv frequency in 845 patients <74 years old was 9%. Patients with/without a family history of potentially BRCA-associated tumors had 14%/6% mutations.
Conclusion
Based on our findings of a gBRCApv incidence higher than expected in a real-life series of Italian patients with incident PDAC, we recommend screening all PDAC patients <74 years old, regardless of family history and stage, due to the therapeutic implications and cancer risk prevention in patients' relatives.
Key words: germline BRCA, epidemiology, pancreatic cancer genetics, familial cancer
Highlights
-
•
This is the largest case series of incident PDAC patients screened for germline BRCA1-2 pathologic variants (gBRCApv).
-
•
The incidence of gBRCA1-2pv was 8.1% in the whole population; 9.1% in metastatic patients; 6.4% in non-metastatic patients.
-
•
No gBRCA1-2pv was observed over the age of 73.
-
•
These data suggest screening all PDAC patients <74 years old, independently from disease stage.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest cancers, with a 5-year overall survival (OS) rate of approximately 9%.1 Landmark whole genome sequencing studies revealed the existence of a distinct subpopulation of PDAC with highly unstable genomes, due to mutations in DNA damage repair (DDR) genes.2,3 Among more than 450 proteins involved in the DDR mechanism, BRCA1 and 2 are the best known due to their crucial role in homologous recombination (HR) double-strand DNA break repair.4 Recently, germinal BRCA1-2 pathogenic variants (gBRCA1-2pv) have been found to be associated with an increased risk of developing PDAC. Approximately 3%–10% of unselected individuals with PDAC have a positive family history of pancreatic cancer, and approximately 10%–20% of pancreatic adenocarcinomas are thought to be due to a heritable cause.5,6
The prevalence of loss of function gBRCA1-2pv ranges from 5% in unselected PDAC case series to 15%-20% in familial PC.7, 8, 9, 10 A worldwide screening of 2206 patients with metastatic PDAC from 12 countries in the POLO trial revealed a mutation rate of 6%, with remarkable geographic variability.11
The identification of gBRCA1-2pv PDAC patients has a key clinical relevance both for treatment and prevention. In fact, loss of BRCA1-2 function and HR deficiency confer sensitivity to DNA damage-inducing drugs, particularly those determining cytotoxic DNA crosslinks that interfere with DNA replication. Metastatic PDAC patients whose tumors carry pathogenic variants of DDR genes may derive greater benefit with platinum-based chemotherapy and poly(ADP-ribose) polymerase inhibitors.12, 13, 14 Although supported by a low level of evidence, exquisite sensitivity of BRCA1-2-mutant tumors to platinum compounds has been validated in multiple preclinical and clinical studies.15,16 In addition, the international randomized, placebo-controlled phase III POLO trial demonstrated that maintenance olaparib significantly prolongs progression-free survival in metastatic PDAC patients with a gBRCApv whose disease had not progressed during first-line platinum-based chemotherapy.13 Also, germline BRCA testing of patients affected by PDAC might allow the identification of carrier family members, who are at increased risk for breast, ovary, pancreatic, and prostate cancers17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 and might be enrolled in focused screening programs.
The information about the epidemiology of gBRCA1-2pv in Italy is limited to the 250 metastatic PDAC patients collected in the POLO trial, while data about frequency and distribution of gBRCA1-2pv in a larger Italian population independent of age, stage, family history, or any other selection criterion are lacking. The present study is aimed at fulfilling this information gap, allowing scientific societies and regulatory authorities to better modulate guidelines for genetic testing.
Materials and methods
The promoting institution of this study retrieved a list of gastrointestinal oncologists distributed in all Italian regions. Two simple straight questions were e-mailed: (i) whether they did or did not screen their PDAC patients for gBRCA1-2pv as a part of routine clinical practice, and in cases of a positive response, (ii) whether they selected patients to be screened based on age, family history of BRCA-related tumors, fitness to receive platinum-containing chemotherapy, and/or other criteria. Responding oncologists subsequently received the study proposal and were invited to participate requiring the completion of a specific case report form.
The study aimed to determine the prevalence of gBRCA1-2pv in a large, unselected, real life-based series of Italian patients with incident PDAC.
Patients of any age were eligible for the analysis if they had a pathologic diagnosis of PDAC, irrespective of the type of recommended treatment. To minimize the potential for survivorship bias, only patients with incident PDAC were included in the analysis. Incident PDAC was defined as gBRCA1-2pv screening within 3 months of diagnosis.
Information on patient demographics, family history, and disease characteristics (including age at diagnosis, gender, birthplace, site of residence, cancer pathology, stage of disease, previous cancer history, family history of cancer, and genetic test results) were retrieved from medical records by the participants and sent to the coordinating institution.
Molecular analysis of BRCA1 and BRCA2 included sequencing of the whole coding regions and intronic junctions, as well as multiplex ligation-dependent probe amplification analysis for detection of large intragenic deletions/duplications.
Classification of variants was carried out in agreement with the American College of Medical Genetics and Genomics and the Association for Molecular Pathology28 and Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) criteria (www.enigmaconsortium.org).
Statistical analysis
Patients or disease characteristics were examined using the χ2 test or Fisher's exact test for qualitative variables, and the Student t-test or Mann–Whitney test for continuous variables, as appropriate. All analyses were carried out using Statistica 12.0 statistical package for Windows (Statsoft Inc, Tulsa, OK). All tests were two-sided and P values <0.05 were considered statistically significant.
For the purposes of our analysis, a diagnosis of breast, ovarian, pancreas, or prostate cancer in a patient's family history was considered as potentially BRCA-associated.
Results
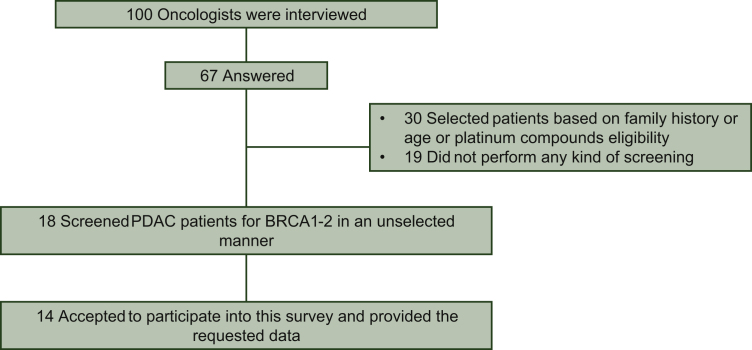
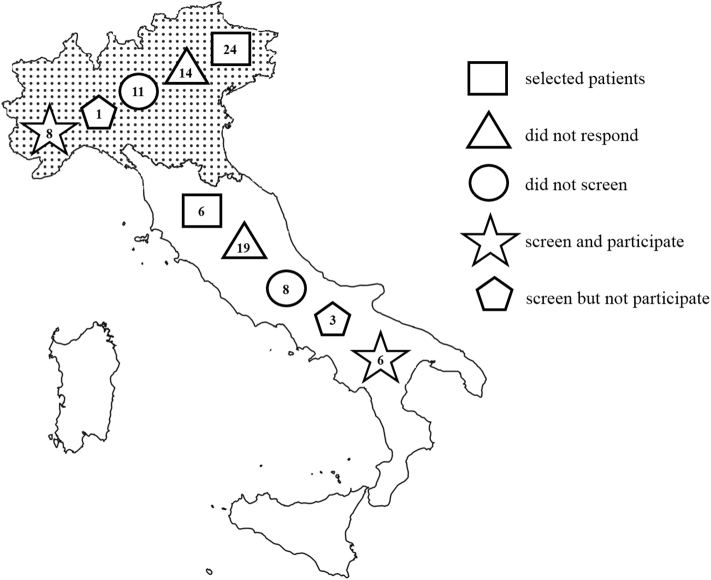
One hundred gastrointestinal oncologists were interviewed and 67 answered: 18 (26.9%) screened PDAC patients for BRCA1-2pv in an unselected manner, apart, for some centers, focusing only on metastatic patients, starting from different time points since June 2015; 30 (44.8%) selected patients based on family history or age or platinum compounds eligibility; and 19 (28.4%) did not perform any kind of screening (Figures 1 and 2). Among 18 screeners, 14 (77.8%) agreed to participate in this survey and provided the requested data. Overall, 939 patients were screened between June 2015 and May 2020. A range of 4 to 290 patients were registered by each institution. The two largest centers included more than half of the cases (54%) with a screening rate of about seven patients per month. Patients' characteristics are reported in Table 1.
Figure 1.
Description of gastrointestinal oncologists response to e-mail interview on BRCA testing.
Figure 2.
Northern Italy is highlighted in dotted area.
Cyphers in the geometric shapes refer to the number of oncologists who gave that specific response to e-mail interview.
Table 1.
Baseline characteristics
| TOTAL | Without a known BRCA1-2pv | BRCA1-2pv | BRCA1-2vus | |
|---|---|---|---|---|
| Number | 939 | 820 | 76 | 43 |
| Gender | ||||
| Male, n (%) | 492 (52) | 431 (53) | 40 (53) | 21 (49) |
| Female, n (%) | 447 (48) | 389 (47) | 36 (47) | 22 (51) |
| Age, n (%) | ||||
| Median | 62 | 62 | 59 | 64 |
| Range | 28-87 | 28-87 | 33-73 | 45-78 |
| <41 | 35 (4) | 29 (4) | 6 (8) | 0 |
| 41-50 | 115 (12) | 100 (12) | 12 (16) | 3 (7) |
| 51-60 | 282 (30) | 243 (30) | 26 (34) | 13 (30) |
| 61-70 | 327 (35) | 287 (35) | 22 (29) | 18 (42) |
| >70 | 178 (19) | 159 (19) | 10 (13) | 9 (21) |
| Missing | 2 | 2 | ||
| Stage | ||||
| IV, n (%) | 569 (61) | 483 (61) | 52 (68) | 23 (55) |
| III, n (%) | 168 (18) | 139 (17) | 10 (13) | 10 (26) |
| I-II, n (%) | 176 (19) | 152 (19) | 12 (16) | 8 (19) |
| Missing, n (%) | 26 (3) | 23 (3) | 2 (3) | 1 (2) |
| Familiar history for PDAC, n (%) | ||||
| Yes | 119 (13) | 100 (12) | 15 (20) | 4 (9) |
| No | 616 (65) | 533 (65) | 51 (67) | 32 (74) |
| Unknown | 204 (22) | 187 (23) | 10 (13) | 7 (16) |
| Familiar history for BRCAness, n (%) | ||||
| Yes | 282 (30) | 232 (28)∗ | 42 (55)∗ | 8 (19)∗ |
| No | 456 (49) | 403 (49) | 24 (32) | 29 (67) |
| Unknown | 201 (21) | 185 (23) | 10 (13) | 6 (14) |
PDAC, pancreatic ductal adenocarcinoma; pv, pathogenic variant; VUS, variant of uncertain significance.
P < 0.005.
gBRCA1-2pv were found in 76 patients (8.1%), gBRCA2pv in 64, and gBRCA1pv in 12. Variants of uncertain significance were found in 43 patients (4.6%). Patients with a pathogenic variant were younger compared with wild-type (59 versus 62 years, P = 0.01; 56 years in gBRCA1pv; 59 years in gBRCA2pv). The likelihood of finding a pathogenic variant was 17.1% among patients <40 years old, 10.4% among patients 41-50 years old, 9.2% among patients 51-60 years old, 6.7% for patients 61-70 years old, and 5.6% among those who were >70 years (0/94 >73 years old). The pathogenic variant frequency in 845 patients <74 years old was 9.0%. Patients with a family history of potentially BRCA-associated tumors had 14.9% with pathogenic variants as opposed to 5.3% for those without and 4.9% for those with an unknown family history (P < 0.0001). No significant difference was found based on gender (8.1% in females versus 8.1% in males) or stage (9.1% in all stage IV patients and 10.1% in stage IV patients <74 years old versus 6.4% in all stage I-III patients and 7.2% in patients <74 years old). All but three (two of whom had a CA19.9 baseline value >1400 UI/ml) gBRCA1pv were found in metastatic patients. The 2 largest institutions had overlapping prevalence rates of gBRCA1-2pv between them (8.3% and 8.2%) and with the other 12 institutions (7.9%). No significant difference in the prevalence of gBRCA1-2pv across different Italian regions based on birthplace was detected (data not shown). However, the pooled frequency in 588 patients living in the northern regions was 9.5%, numerically higher compared with 5.7% in 348 patients living in the central and southern regions (P = 0.050).
Discussion
In this large and unselected series of Italian patients affected by any stage of incident PDAC, the prevalence of newly diagnosed gBRCA1-2pv was 8.1%. When focusing on 569 patients with metastatic disease, the observed 9.0% rate is consistently greater than expected in this geographic area from a previous smaller series (6.0% of 249 patients)11 and is in the range of that observed in those countries with a higher prevalence of gBRCA1-2pv, such as the USA (9.5% of 275 patients), France (7.6% of 289 patients), and Israel (7.4% of 242 patients).11 Few centers have participated in this survey. However, these centers are mainly large tertiary referral institutions for pancreatic adenocarcinoma treatment that are located in the eight most densely populated Italian regions (Lombardy, Lazio, Campania, Veneto, Emilia Romagna, Piedmont, Apulia, Abruzzi) where approximately 68% of the Italian population is distributed. Accordingly, the population selected for this study may be considered a reliable representation of the Italian population. The current epidemiological data might have important implications in clinical practice. In fact, based on the higher prevalence of gBRCA1-2pv in the US population, National Comprehensive Cancer Network guidelines recommend that all individuals with a diagnosis of pancreatic cancer meet the criteria for genetic testing, irrespective of family history or age at diagnosis.29 Noteworthy, the prevalence in the USA is widely variable, ranging from 1.8% in 274 patients from Utah17; 2.5% in 283 patients with resected PDAC at Memorial Sloan Kettering Cancer Center (MSKCC)30; 1.8%-3.0% in two large series of patients who underwent pancreatic resection at Johns Hopkins31,32; 2.8% in 3078 patients from Mayo Clinic19; and 6.3% in 63 consecutive non Ashkenazi Jewish patients at MSKCC.7 A remarkable geographic variability has been reported and sparse data are available mostly from small retrospective series from single institutions without information on population characteristics, heterogeneous distribution of age and stage, and often undeclared eligibility criteria. Also, treatment-related selection biases are often embedded in eligibility criteria of analyzed series. Overall, prevalence was lower outside the USA or Israel (5.1%),11 in Spain (1.7%),33 and in Canada (1.0%-4.6%).8,13,18,34 Notwithstanding the limitation of being retrospective, the higher prevalence observed in our large, multi-institutional, real-life series of patients who were not enrolled based on treatment recommendations, was unlikely related to a more stringent patients' selection. In fact, centers were involved in the study only if they declared that no limitation was implemented in recommending genetic screening. Consistently, 568/939 patients (60%) had an unknown or negative oncologic familial history and were >50 years old, thus suggesting that in most cases these variables were not used as selection criteria. Age was also superimposable across the present series and others.7,11,20,32 Furthermore, the enrolment pace was very fast and the gBRCA1-2pv rate was superimposable across centers and geographical regions adding reliability to our findings. The opposite may be true because the POLO trial selected patients who were candidates to receive FOLinic Acid, Fluorouracil, IRINotecan, and OXaliplatin (FOLFIRINOX), and testing was sometimes carried out during treatment compared with incident cases in our series. Accordingly, the possibility that a subgroup of patients with gBRCA1-2pv was excluded from POLO screening procedures because they were affected by a more aggressive or platinum-resistant disease, or because they presented with an inadequate performance status to be recommended for FOLFIRINOX leading to an underestimation of the true gBRCA1-2pv rate, cannot be ruled out. Platinum resistance is not unusual among patients with gBRCA1-2pv and was reported in 43/247 (17.4%) of patients with Eastern Cooperative Oncology Group performance status 0-1 who were screened for the POLO trial.13 Also, gBRCA1-2pv was associated with earlier onset and more aggressive/higher-grade prostate cancers with poorer outcomes.16 The prognostic value of gBRCA1-2pv in PDAC is poorly explored and confounded by the predictive value and we cannot exclude that a similar effect is also true in PDAC as suggested by observing more metastatic (52/76 or 68% versus 517/863 or 60%) patients among gBRCA1-2pv compared with the wild-type population in our series. Of note, similar differences were also previously reported in a Canadian series (50% versus 42% metastatic in pathologic variants and wild-type, respectively) and in Mayo clinic series (46.2% versus 35.6%).8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Furthermore, in a large series of patients undergoing resection for PDAC, mostly treated without platinum-containing chemotherapy or without any chemotherapy at Johns Hopkins, disease-free survival and OS were significantly worse among gBRCA1-2pv patients compared with the wild-type matched population.32
With regard to age, none of 94 patients >73 years old had a gBRCA1-2pv suggesting that screening may be avoided in this population, taking also into account that the administration of platinum-based regimens may be challenging in this setting.
A large number of non-metastatic patients were also assessed in this current study. Despite a lower numerical prevalence compared with the metastatic population, a non-negligible 6.4% (or 7.2% if only those <74 years old are considered) of gBRCA1-2pv was observed. Routinely also testing non-metastatic patients should therefore be recommended because therapeutic choices may be influenced and driven by screening results.
Surprisingly, we found a gBRCA2pv/gBRCA1pv ratio (5.3 : 1 in the whole population; 4.8 : 1 in metastatic patients) that was consistently different from the 2-3 : 1 ratio observed in other series.8,11,23,35,36 Whether this finding is related to a geographical peculiarity or to the less stringent patients' selection in our series may be a topic for further investigation.
Of note, 9 of 12 gBRCA1pv were detected in patients with metastatic disease and 2 of 3 non-metastatic patients had a CA19.9 baseline value suggesting the presence of undetected systemic disease.
Another topic of extreme interest that may be a subject for speculation is the difference in gBRCA1pv prevalence between northern and central-southern regions. One possible explanation is the presence of different environmental carcinogenic factors favoring the development of (pancreatic) cancer in genetically predisposed subjects.
Finally, our survey strongly suggests that genetic testing in patients with PDAC is not carried out on a regular basis by more than 75% of Italian gastrointestinal oncologists. This figure is probably underestimated if we consider that about one-third of interviewed colleagues did not respond to the questionnaire. Although BRCA testing is technically available universally in Italy, Regional Health System refunding is heterogeneous and may account for minimal screening in some geographical areas. Nevertheless, the choice to perform this test appears mainly related to cultural reasons. Accordingly, awareness of the crucial relevance of genetic screening in PDAC patients must be urgently fostered in the oncological community. While it is not the purpose of this retrospective study to properly evaluate whether the universal gBRCA1-2 testing in PDAC is cost-effective, our observation may provide useful information to health authorities to estimate and optimize the cost-effectiveness of this procedure. In effect, such a policy may help to recommend more suitable treatment strategies with a possible survival impact on this disease with a dismal prognosis and, additionally, may spare lives by means of screening programs in patients' relatives.
Pancreatic cancer may also be associated with pathogenic variants in other genes such as ATM, MLH1, MSH2, MSH6, TP53, PALB2, CDKN2A, and others. Accordingly, epidemiological data about the prevalence of pathogenic variants on a geographical basis are eagerly needed and national database collections should be encouraged.
In conclusion, based on our findings, we recommend testing for gBRCA1-2pv in all PDAC patients <74 years old, regardless of family history, stage, and candidature to receive platinum-based chemotherapy.
Acknowledgments
Funding
This study was partially supported by MyEverest ONLUS (no grant number).
Disclosure
MR reports travel expenses and personal honoraria for Advisory Boards from Celgene, Merck, Astra-Zeneca, Baxalta (2016), Baxter, Sanofi (2017), Servier, Shire, Eli Lilly, Pfizer (2016), Novocure (2016), and Novartis (2016), personal honoraria for steering committee work for AstraZeneca, and non-remunerated steering committee activities for Boston Pharmaceuticals. MF reports travel expenses and personal honoraria for Advisory Boards from Celgene, Novartis, Advanced Accelerators Applications, and Ipsen. SC reports travel expenses and personal honoraria for the following companies. Speaker: Amgen, Bayer, Eli Lilly, Servier. Advisory Boards: Amgen, Eli Lilly, Bayer, Baxter, Merck Sharp & Dohme (MSD), Servier. Consultant: Amgen, Baxter, Eli Lilly, Celgene, Novartis, MSD. Research grant: Celgene, Eisai. GT: Travel expenses and personal honoraria for Advisory Boards from Celgene, Merck, Astra-Zeneca, Servier. LS reports travel expenses and personal honoraria for Advisory Boards from Merck-Serono, Celgene, Roche, Sanofi, Servier, Bayer, Astra-Zeneca, Amgen. DM reports research grants and personal fees for consulting from Incyte Corporation; research grants and personal fees for consulting from Shire, Evotec, and iOnctura; research grants from Celgene, and personal fees for consulting from Eli Lilly and Baxter. FDV reports travel expenses and personal honoraria for Advisory Boards: Roche, Amgen, Celgene, Eli Lilly, Servier. MDM reports travel expenses and personal honoraria for Advisory Boards from Celgene. GG reports travel expenses and personal honoraria for Advisory Boards from Celgene (2016–2017–2018), Sanofi (2016–2018), Servier (2019). FDB reports personal honoraria for: Consultant Advisory Board: Ignyta, Bristol-Myers Squibb (BMS), Daiichi Sankyo, Pfizer, Octimet Oncology, Incyte, Teofarma, Pierre Fabre, Roche, EMD Serono, Sanofi, NMS Nerviano Medical Science, Pharm Research Associated (UK) Ltd; Speaker: BMS, Roche, MSD, Ignyta, Bayer, ACCMED, Dephaforum S.r.l., Nadirex, Merck, Biotechspert Ltd, PriME Oncology, Pfizer, Servier, Celgene, Tesaro, Loxo Oncology Inc., Sanofi, Healthcare Research & Pharmacoepidemiology. MN reports travel expenses from Celgene and personal honoraria for Advisory Board from EMD Serono. All the other authors have declared no conflicts of interest.
Patient consent
Before testing, all patients signed an informed consensus statement that was revised and approved by a local ethics committee and allowed, for genetic testing, and data collection, and analysis and elaboration. Data were irreversibly anonymized before entering into the database.
Data availability statement
Data are available upon reasonable request.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Waddell N., Pajic M., Patch A.-M. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey P., Chang D.K., Nones K. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor M.J. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Petersen G.M. Familial pancreatic cancer. Semin Oncol. 2016;43:548–553. doi: 10.1053/j.seminoncol.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): genetic/familial high-risk assessment: breast and ovarian (version 2.2019) https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf Available at:
- 7.Salo-Mullen E.E., O'Reilly E.M., Kelsen D.P. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer. 2015;121:4382–4388. doi: 10.1002/cncr.29664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holter S., Borgida A., Dodd A. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol. 2015;33(28):3124–3129. doi: 10.1200/JCO.2014.59.7401. [DOI] [PubMed] [Google Scholar]
- 9.Couch F.J., Johnson M.R., Rabe K.G. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(2):342–346. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 10.Ferrone C.R., Levine D.A., Tang L.H. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27(3):433–438. doi: 10.1200/JCO.2008.18.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golan T., Kindler H.L., Park J.O. Geographic and ethnic heterogeneity of germline BRCA1 or BRCA2 mutation prevalence among patients with metastatic pancreatic cancer screened for entry into the POLO trial. J Clin Oncol. 2020;38:1442–1454. doi: 10.1200/JCO.19.01890. [DOI] [PubMed] [Google Scholar]
- 12.Pihlak R., Valle J.W., McNamara M.G. Germline mutations in pancreatic cancer and potential new therapeutic options. Oncotarget. 2017;8(42):73240–73257. doi: 10.18632/oncotarget.17291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golan T., Hammel P., Reni M. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tutt A., Tovey H., Cheang M.C.U. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med. 2018;24(5):628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebelatto T.F., Falavigna M., Pozzari M. Should platinum-based chemotherapy be preferred for germline BReast CAncer genes (BRCA) 1 and 2-mutated pancreatic ductal adenocarcinoma (PDAC) patients? A systematic review and meta-analysis. Cancer Treat Rev. 2019;80:101895. doi: 10.1016/j.ctrv.2019.101895. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher D.J., Gaudet M.M., Pal P. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16:2115–2121. doi: 10.1158/1078-0432.CCR-09-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young E.L., Thompson B.A., Neklason D.W. Pancreatic cancer as a sentinel for hereditary cancer predisposition. BMC Cancer. 2018;18:697. doi: 10.1186/s12885-018-4573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant R.C., Selander I., Connor A.A. Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Gastroenterology. 2015;148:556–564. doi: 10.1053/j.gastro.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav S., Kasi P.M., Bamlet W.R. Effect of germline mutations in homologous recombination repair genes on overall survival of patients with pancreatic adenocarcinoma. Clin Cancer Res. 2020;26(24):6505–6512. doi: 10.1158/1078-0432.CCR-20-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelker D., Zhang L., Kemel Y. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA. 2017;318:825–835. doi: 10.1001/jama.2017.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner N., Tutt A., Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4(10):814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 22.Kote-Jarai Z., Leongamornlert D., Saunders E. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105:1230–1234. doi: 10.1038/bjc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkitaraman A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 24.Thompson D., Easton D., Breast Cancer Linkage Consortium Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410–419. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canto M.I., Harinck F., Hruban R.H. International Cancer of the Pancreas Screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–347. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toss A., Venturelli M., Molinaro E. Hereditary pancreatic cancer: a retrospective single-center study of 5143 Italian families with history of BRCA-related malignancies. Cancers (Basel) 2019;11(2):193. doi: 10.3390/cancers11020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy-Lahad E., Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2007;96:11–15. doi: 10.1038/sj.bjc.6603535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): genetic/familial high-risk assessment: colorectal (version 1.2018) https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf Available at: [DOI] [PubMed]
- 30.McIntyre C.A., Lawrence S.A., Richards A.L. Alterations in driver genes are predictive of survival in patients with resected pancreatic ductal adenocarcinoma. Cancer. 2020;126:3939–3949. doi: 10.1002/cncr.33038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shindo K., Yu J., Suenaga M. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol. 2017;35(30):3382–3390. doi: 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blair A.B., Groot V.P., Gemenetzis G. BRCA1/BRCA2 germline mutation carriers and sporadic pancreatic ductal adenocarcinoma. J Am Coll Surg. 2018;226(4):630–637. doi: 10.1016/j.jamcollsurg.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Real F.X., Malats N., Lesca G. Family history of cancer and germline BRCA2 mutations in sporadic exocrine pancreatic cancer. Gut. 2002;50:653–657. doi: 10.1136/gut.50.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lal G., Liu G., Schmocker B. Inherited preposition to pancreatic adenocarcinoma: role of family history and germ-line p16, BRCA1, and BRCA2 mutations. Cancer Res. 2000;60:409–416. [PubMed] [Google Scholar]
- 35.Zhen D.B., Rabe K.G., Gallinger S. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med. 2015;17:569–577. doi: 10.1038/gim.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi S., Doi M., Ikari N., Yamamoto M., Furukawa T. Mutations in BRCA1, BRCA2, and PALB2, and a panel of 50 cancer-associated genes in pancreatic ductal adenocarcinoma. Sci Rep. 2018;8:8105. doi: 10.1038/s41598-018-26526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.