Learning objectives.
By reading this article, you should be able to:
-
•
Outline the underlying pathology causing coccydynia and sacroiliac joint pain.
-
•
Discuss the diagnosis and management of coccydynia.
-
•
Summarise the diagnosis and management of sacroiliac joint pain.
Key points.
-
•
Coccydynia is a clinical diagnosis mainly seen in those with abnormal coccygeal mobility as a result of trauma.
-
•
Conservative non-surgical treatments are the ‘gold standard’ for coccydynia.
-
•
Sacroiliac joint pain accounts for 16–30% of incidences of chronic mechanical lower back pain.
-
•
Sacroiliac joint pain has a bimodal distribution, mainly affecting young athletic adults and the elderly.
-
•
Fluoroscopy-guided diagnostic blocks are the standard for diagnosis and treatment of sacroiliac joint pain.
Introduction
Coccydynia and sacroiliac joint (SIJ) pain account for a significant proportion of the cases of musculoskeletal lower back pain; they include a collection of pathologies that affect 60–85% of individuals in their lifetime, of which 95% are caused by musculoskeletal pathologies.1 Lower back pain is responsible for significant morbidity in the UK, resulting in a significant economic burden with the loss of 50 million working days each year at a cost of more than £5 billion. Coccydynia and SIJ pain are two groups of conditions that have different prevalences, aetiologies and management; therefore, they will be discussed separately in this article.
Coccydynia
Symptoms of pain localised to the region of the coccyx are referred to as coccydynia or coccygodynia. This pain disorder is typically exacerbated by pressure over the coccyx and is mainly seen in those with abnormal coccygeal mobility, although the specific origin of the pain can often be difficult to isolate. Although coccydynia can affect individuals of both sexes and all ages, it has a five times greater prevalence in women than in men, and the mean age of onset is 40 yrs.2
Anatomy
The coccyx forms the terminal aspect of the vertebral column. Its name originated from the Greek word for ‘cuckoo’, as its triangular shape is thought to resemble that of a bird's beak.2,3 The dorsal convex aspect of the coccyx has multiple paired coccygeal articular processes, and the most superior of these are termed the coccygeal cornu, which articulate with the sacral cornu of the terminal sacral vertebra, S5. The anterior sacrococcygeal ligaments and the levator ani muscle attach to the ventral concaved aspect. The lateral aspects of the coccyx provide attachments to the sacrosciatic ligaments. Posterior to these ligaments attach the fibres of the gluteus maximus and anterior to the ligaments attaches the coccygeus muscle. Attached to the tip of the coccyx is the tendon of the iliococcygeus muscle which helps protect and support the rectum. These attachments contribute to voluntary bowel control and pelvic floor support.4 There is large variability in the structure of the coccyx with respect to which particular joints fuse and to what degree discs fibrosis. This results in different configurations of the coccyx, with respect to its angulation in the vertical axis, with a predisposition to coccydynia with certain morphologies.5
Prevalence and aetiology
It is proposed that coccydynia is caused by chronic inflammation and contractions of the attachments to the coccyx, which results from pathological instability, and is associated in the majority of cases with a subluxed or hypermobile coccyx.2 The actual prevalence of coccydynia has not been reported. Factors associated with increased prevalence are BMI and female sex. Obesity is three times more common in those suffering from coccydynia, and the incidence is five times greater in females.4
Aetiology
Coccydynia is commonly classified according to its aetiology. The most common cause of coccydynia is trauma, specifically single direct axial trauma such as a fall, or cumulative trauma, which could result from sitting in a suboptimal position on a hard or uncomfortable surface for a prolonged period. Aside from external trauma, the coccyx is susceptible to internal trauma such as during a difficult childbirth. Non-traumatic causes include sacrococcygeal joint hyper- or hypomobility, degenerative disc or joint disease, infections, referred or radicular pain, and less commonly neoplasia. The predominance of coccydynia in females is in part associated with pregnancy. During pregnancy there is relaxation and increased mobility within the pelvic joints. This results from an increase in elasticity and softening of connective tissues and collagen as a consequence of increased concentrations of steroid sex hormones, specifically oestrogen, and the ovarian hormone relaxin. Non-organic causes such as somatisation and psychiatric disorders have also been associated with coccydynia.
Presentation and diagnosis
Coccydynia is a clinical diagnosis, with the classical presentation being pain localised over the coccyx. The pain is worse with prolonged sitting or standing and rising from the seated position. Pain can also be present during defecation and sexual intercourse, and there may be a history of the frequent need to defecate.4 There is often a relief from the pain when the patient sits on their feet or on one buttock. The history may reveal a recent axial load trauma or repeated prolonged episodes of sitting on hard uncomfortable surfaces.2
Physical examination will elicit tenderness, swelling or a causative mass on palpation of the coccyx. Rectal examination and bimanual manipulation of the coccyx will elicit pain and aid evaluation of any hypo- or hypermobility of the sacrococcygeal joint. The examination should also rule out other causes of pain in the region such as a pilonidal cyst. A faecal occult blood test should be conducted to assess gastrointestinal pathology.3
Imaging modalities are valuable in filtering the differential diagnoses but also assessing and evaluating coccydynia. Static plain radiographs are useful in eliminating other differential diagnoses but are limited in aiding the diagnosis of coccydynia. Dynamic radiographs are more useful, that is obtained in the sitting and standing positions.8,9 This allows the coccygeal angle of incidence to be measured (the angle at which the coccyx meets the seat surface).2 The coccyx usually pivots between 5° and 25° between the sitting and standing positions. Coccydynia is commonly associated with coccygeal displacement, immobility (<5° of movement), or hypermobility (>25° of movement).11,17 Advanced imaging modalities and provocative tests will aid diagnosis but may not be as useful as dynamic radiographs. MRI and technetium Tc-99m will demonstrate inflammation associated with hypermobility and exclude other underlying pathologies.12 Fluoroscopic or ultrasound-guided injection of local anaesthetic and steroid techniques can both aid diagnosis and be therapeutic.17
Treatment
A broad spectrum of treatments have been proposed for coccydynia. These range from conservative non-surgical to surgical interventions. Conservative non-surgical options remain the gold standard of treatment.2 In 90% of cases pain is resolved successfully, and many patients require relatively simple measures.10,13
Non-surgical interventions include NSAIDS, stool softeners, circular (‘doughnut’) cushions or wedge-shaped (coccygeal) cushions, ergonomic adaptations, physical therapies, intrarectal massage and manipulation, sacrococcygeal steroid and local anaesthetic injections, radiofrequency (RF) thermocoagulation and a ganglion impar block.3,6,7 When non-organic causes are suspected, psychotherapy is indicated.
NSAIDs are the main analgesics prescribed. Opioids are not commonly used and reserved for management of acute severe episodes refractory to other therapies. Topical NSAIDs have no specific evidence in the treatment of coccydynia, but their usefulness in other pain condition such as osteoarthritis would suggest they would be of some benefit, while avoiding the systemic adverse effects of an oral route.13 Ergonomic adaptation includes postural training and pelvic floor rehabilitation; these can be helpful in addressing the pelvic floor muscle spasm component of coccydynia. Massage and stretching of the levator ani muscle along with manipulation of the sacrococcygeal joint have been shown by Maigne and colleagues8 to have medium to moderate symptomatic improvement depending on the pathophysiology.15,16 Those with normal coccygeal mobility benefit the most, with a successful outcome in 43%; those with an immobile coccyx have the least benefit with only a 16% success rate; those with a hypermobile coccyx have moderate symptomatic improvement with a 25% success rate and those with subluxation of the coccyx have a similar moderate improvement with a 22.2% success rate.
Interventional procedures are becoming an increasingly popular treatment modality. They are being used as both a diagnostic and a therapeutic intervention despite a relative lack of supporting literature, especially in chronic coccydynia. There is no clear consensus with regard to the best site to inject. However, it is generally recommended to use image guidance to inject around the coccyx.4 The majority of practitioners uses fluoroscopy or ultrasound to inject local anaesthetic and steroid around the sacrococcygeal joint or ligament.4 Injection therapies also assist in identifying those with persistent debilitating pain despite all other conservative therapies, who would benefit from surgical intervention.3
Another interventional procedure is to target the sympathetic ganglion known as the ganglion impar, also known as the ganglion of Walther. The ganglion is located anterior to the sacrococcygeal junction in the midline, and is the pelvic component of the sympathetic trunk.4 This block is useful in cases of coccydynia that are refractory to other therapies and have an associated pelvic pain component to their symptoms, such as with pelvic cancers.
Blockade of the nociceptor and sympathetic fibres of the ganglion impar has been used for years to help treat coccydynia. It has been shown to improve symptoms with prolonged pain free periods especially when used in conjunction with conservative rehabilitation.5,7,8 This sympathetic ganglion blockade can be assisted using ultrasound, fluoroscopy, or CT.6 Diagnostic blocks with local anaesthetics are usually conducted first to confirm the efficacy of the block. Complications of ganglion impar blockade include rectal puncture, neuritis, and cauda equina syndrome. Radiofrequency ganglion ablation has been used to treat cases of severe oncological pain.4,7,9
Surgical interventions are reserved as a last course of action for patients with coccygeal instability, with subluxation and hypermobility of the coccyx and causing advanced degeneration, and in whom all other treatments have failed. It is in this group that the greatest improvement in symptoms and quality of life is seen, with success rates as high as 91%.2 This is higher than that for patients with normal coccygeal mobility where the surgical outcome is less favourable. Coccygectomy or resection of the mobile segment of the coccyx is the surgical option most commonly undertaken. There are significant associated complications including rectal injury, incontinence and the most frequent complication being wound infection, which occurs in up to 22% of cases.17 This has been attributed to the close proximity with the abundant perineal flora, the difficult access to the surgical site for postoperative care and also the high wound tension created in the sitting position. The significant complication rate and lack of data to support this type of surgery mean that it is still not generally recommended.4 Other surgical approaches described include coccygeoplasty, in which polymethylmethacrylate cement is injected to treat coccygeal fractures, and placing tension sutures to maintain the integrity of a potentially dislocated coccyx. More research is needed to explore the effectiveness of these techniques.4
Sacroiliac joint pain
Sacroiliac joint pain is defined as pain localised to the area of SIJ. It can be reproduced by stressing the joint with provocation testing and can be relieved reliably with local anaesthetic infiltration of the SIJ.16 It is a non-radicular cause of lower back pain, occasionally referred to as posterior pelvic pain.17 It is usually felt unilaterally within the gluteal region, but, pain can be referred into the lower limbs, groin, abdomen, and lumbar regions.
Different diagnostic criteria are used to diagnose SIJ pain including clinical history, examination, imaging and periarticular test blocks. Depending on the diagnostic criteria used, SIJ pain accounts for 16–30% of cases of chronic mechanical lower back pain.13
SIJ pain is one of the most common causes of mechanical lower back pain. It has a bimodal distribution, mainly affecting young athletic adults and also the elderly.16,17 An understanding of the anatomy and structure of the SIJ and how it changes with age, sex, hormonal influence and adaptation to external forces, along with an understanding of the biomechanics, function and the association of the SIJ within the biokinetic chain, help guide diagnosis and management.
Anatomy and function
The SIJ is a diarthrodial joint within the pelvic ring with an average surface area of 17.5 cm2.17 It consists of two surfaces, sacral and ilial, connected with a fibrous capsule containing synovial fluid. The sacral side is lined with thick hyaline cartilage and the ilial side with fibrocartilage. Associated muscular, ligamentous and fascial structures help limit the joint's mobility along its various axes, provide strength and stability to the joint, and allow it to fulfil its function. The associated structures include latissimus dorsi, gluteus maximus, piriformis and thoracolumbar fascia. The pelvic functions as a central base supporting the upper body and reducing the impact of ambulation. The SIJ functions to allow the transference of weight between the upper and lower body in a coordinated, stable and functional manner. This results in force transmission both directly and indirectly through or across the SIJ along different axes or degrees of freedom. It is proposed that the SIJ has 6 df with a rotational range of between 1° and 12°, meaning a translational range of between 3 and 16 mm.17
The SIJ has a complex innervation. The posterior capsule is of more relevance with regard to treatment and is generally better understood. It is mainly innervated from the dorsal rami of S1–S3 with contribution from L5 and S4 in some individuals.17
Aetiology
The aetiology of SIJ pain is wide-ranging, from intra-articular (IA) degeneration and inflammation to extra-articular (EA) causes, secondary to dysfunction of the periarticular structures (muscle, ligaments and fascia). IA causes include arthritis, infection, spondyloarthropathies and malignancy; EA causes include fractures, and myofascial and ligamentous injuries. In many cases, a specific cause may not be identified. Risk factors include spinal surgery, more commonly lumbar fusion, scoliosis and sacral fixation; true and functional leg length discrepancy, abnormal gait patterns, scoliosis, pregnancy, strenuous physical exertion and trauma. These all cause altered biomechanics resulting in an imbalance in the load and forces transmitted across the SIJ. These repetitive torsional and unidirectional sheer forces cause recurrent episodes of inflammation and hence result in SIJ pain. It has been demonstrated that in 40–50% of patients with injection confirmed SIJ pain can recall a precipitating event.16,17 The most frequent precipitants are a motor vehicle collision, falls, repetitive stress such as from jogging, and pregnancy.17
Presentation and diagnosis
The diagnosis of SIJ pain is underappreciated as a cause of chronic mechanical lower back pain and is extremely challenging to diagnose; this in part because of its size and complex anatomical structure, attachments and function. Diagnosis is made with a combination of diagnostic criteria as individually their predictive values are weak.13,14,15 This includes history, physical examination, provocative testing, imaging modalities and diagnostic blocks.
Patients may often present with a history of one of the risk factors mentioned above, or a history of an event with ensuing biomechanical dysfunction or a failure to restore biomechanical function secondary to adaptative changes resulting from another pathology that has either resolved or is ongoing. These can include a history of conditions such as lumbar discogenic pain, radiculopathy, hip pathology or facet syndrome.12 The characteristics and distribution of the pain vary from patient to patient. The majority present with lateral rather than central buttock pain, with the worst pain originating within 10 cm of the posterior superior iliac spine, which tends to extend into the posterolateral thigh, but can also present with extension in the groin or lower extremities.14,15 Patients may experience pain or a clicking/popping sensation when getting up from sitting or other transitional activities. Activities involving asymmetrical loading across the SIJ such as stepping, golf, bowling and skating can also provoke pain.12
A detailed physical examination must include static and non-static assessment of the kinetic chain: the spine, pelvis, hips and lower limbs. Detailed physical examination is usually undertaken by health professionals with in-depth musculoskeletal knowledge, as its diagnostic value and ability to guide management depends on the examiner's experience.
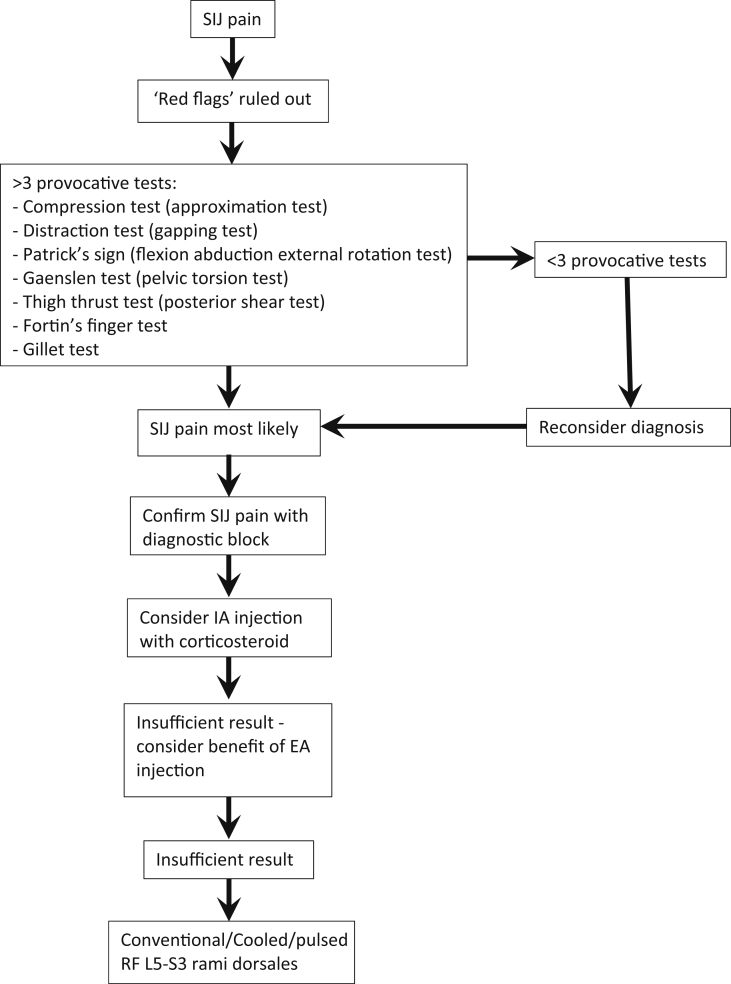
Historically it has been difficult to achieve accepted diagnostic criteria for SIJ pain, with the interpretation of diagnostic blocks by an experienced practitioner being the gold standard of diagnosis.13 The consensus from a multitude of studies and reviews is that the presence of three or more positive provocative tests (Fig. 1) has reasonable specificity and sensitivity in identifying those who would respond positively to a diagnostic SIJ injection.17 The International Association for the Study of Pain (IASP) states that pain should be alleviated after fluoroscopy-guided IA SIJ injection with local anaesthetic, and thus confirm the diagnosis of SIJ pain.16 Some suggest a double confirmatory block, using different local anaesthetics of differing durations. 13 Others suggest that a periarticular injection should be the diagnostic block as the location of nociceptors is extracapsular, and if this fails to relieve the pain then an IA injection should be done to confirm or reject the diagnosis of SIJ pain.16,17
Fig 1.
Algorithm for treating SIJ pain. Adapted from Vanelderen and colleagues.13
Imaging modalities vary in their ability to aid the diagnosis of SIJ pain depending on the underlying pathology. Imaging is useful in identifying the inciting pathology such as inflammation as a potential cause of SIJ pain and adding to the evidence for the diagnosis. Importantly, imaging allows clinicians to identify ‘red flags’ and differentiate other potential non-SIJ pathologies (Table 1). X-ray has very low sensitivity, and so is a poor screening modality. CT is a rapid imaging modality that identifies bony pathology and early joint narrowing, but also has low specificity and sensitivity for the diagnosis of SIJ pain. Radionucleotide imaging has very low sensitivity but high specificity; hence, it is a poor screening modality but may have use in confirming SIJ pathologies such as inflammation. Meanwhile, MRI has very high sensitivity in identifying inflammatory SIJ pathologies but is poor at identifying non-inflammatory pathologies.13,17
Table 1.
Differential diagnosis for SIJ pain.10
| Facetogenic pain |
|---|
| Lumbar nerve root compression |
| Spondyloarthropathy (e.g. reactive arthritis, ankylosing spondylitis or psoriatic arthritis) |
| Hip pain |
| Myofascial pain |
| Piriformis syndrome |
| Endometriosis |
Management
Conservative management
Conservative management is under-represented in the medical literature. It is an early management pathway that can provide long-lasting resolution of pain with significantly fewer risks than are associated with other management options. However, other interventions as mentioned later are often needed alongside conservative management. The focus is on control of symptoms, identification of imbalance and any functional biomechanical deficit, and correcting them. This can be a discrepancy in muscle length and strength, asymmetrical muscle triggering and recruitment patterns causing poor quality of motion creating excessive and detrimental sheer forced across the SIJ. Conservative management encompasses a staged multimodal approach (Fig. 1) and includes the use of physical therapies, analgesics, education, pelvic belts, activity modification, weight management and use of orthotics to correct any differences in leg length.9,10,15
Interventions
Patients in whom a course of conservative management fails or those unable to comply with therapy because of pain are offered interventional therapy with articular injection, ganglion impar blockade, RF treatment, or both. These interventions have varying levels of evidence supporting them, with IA injection with local anaesthetic and corticosteroids being the intervention with the highest level of evidence of 1B+ to support its use.17 Prolotherapy—the process of injecting non-active irritant solutions such as dextrose and platelet-rich plasma—has been suggested as a therapy. This is hypothesised to cause inflammation and improve blood flow to improve tissue healing, but, there is insufficient evidence of its efficacy to support its use in treating SIJ pain.
Injections
Injection therapy involves IA injection, EA injection, or their combination. IA injection of local anaesthetic and corticosteroid may provide good relief from pain for up to 1 yr. Successful therapy is generally regarded as >70% reduction in pain.13 The choice of IA or EA injection should be guided by what has been diagnosed to be the cause of the pain. Elderly patients tend to present with bilateral IA pathology such as arthritis, whereas the younger athletic patient tends to present with unilateral EA pathology originating from periarticular structures such as muscles and ligaments. Advanced imaging such as radionucleotide scanning or MRI may help to evaluate those patients with an inflammatory IA pathology who would benefit primarily from IA injections. However, even with a primarily IA pathology, there may be capsular distension causing pain that may not be wholly addressed by an IA injection; these patients may benefit from both IA and EA injections.15
RF treatment
Radiofrequency lesioning or denervation for SIJ pain has been used for many years. It usually targets the dorsal rami of the lateral branches of the nerves that innervate the SIJ, mainly the posterior capsule. Those who gain the most benefit from RF lesioning tend to be those who have had significant relief from an SIJ block, especially an EA block or a double block. Higher success rates are seen in the young patients who tend to present with EA pathology causing their SIJ pain as the nerves that innovate this area are the specific target for RF lesioning.
The use of RF varies with different temperatures, durations, and anatomical location being applied. Conventional RF creates a relatively small lesion with an approximate horizontal diameter of 4 mm.17 This is a potential limitation with a higher risk of missing nociceptive inputs. Therefore, multiple lesions need to be made around each foramen to ensure all nociceptive inputs from the SIJ are interrupted. Another technique is to increase the lesion size and therefore success, by injecting fluid, for example 2% lignocaine with or without steroid before lesioning; this technique may also reduce the incidence of neuritis.17 Bipolar RF uses a second electrode in close proximity passing a continuous current between the electrodes to help increase the lesion size. Cooled RF is a relatively new technique that allows larger lesion expansion, by preventing charring of surrounding tissue through the use of cooled electrode tips to allow slower heating to ablative temperatures. Pulsed RF is a non-neuroablative technique that aims to prevent nerve injury whilst providing pain relief. Pulsed RF creates an electric field to interrupt pain transmission from Aδ and C fibres but also enhancing descending modulatory pathways. There is no conclusive volume of evidence supporting the use of pulsed RF over other techniques to treat SIJ pain.
RF techniques rarely cause serious complications. Procedural complications of infection and bleeding are inherent to percutaneous procedures. Patients may experience worse pain for up to 10 days after the procedure, which is thought to be related to temporary neuritis and procedure-related tissue trauma.13 Use of peri-procedural injection of local anaesthetic and steroid at the lesion site can reduce this pain severity and duration. Twenty percent of patients complain of numbness or tingling caused by effects on the cutaneous sensory branches, but, this is rarely troublesome to the patient especially with successful relief from pain. Misplacement of needles that affect the sacral nerve can cause incontinence and weakness in the lower limbs. This is generally avoided through the use of seeker needles to confirm the position of the electrode before ablation.17
Surgical interventions
Surgical intervention relates to fixation or fusion of the SIJ. These procedures are usually reserved for patients requiring surgical fixation for fracture dislocation of the SIJ with good effect. Surgery can also be offered to those in whom conservative and other interventional management fails. However, the evidence is not clear whether surgical fixation benefits those in pain from a degenerative pathology when other therapies have failed.
Conclusions
Sacrococcygeal pain causes significant morbidity and loss of function, and affects the individual's ability to work and interact socially. The incidence of SIJ pain has a bimodal distribution affecting the young athletic adult and the elderly, whereas coccydynia more commonly predominates in middle-aged females. Trauma is a common underlying pathology in both, either from direct high impact or repetitive low impact trauma. Diagnosis is difficult; history and examination are essential with the aid of imaging to rule out differential diagnosis including ‘red flags’ and confirm a likely diagnosis of SIJ or coccydynia. Articular local anaesthetic and steroid blocks are the gold standard for the diagnosis of SIJ pathologies and play a significant part in management. Interventions are becoming increasingly popular in the management of coccydynia despite a paucity of evidence. Conservative management is underrepresented in the medical literature and underappreciated in practice; it is associated with fewer risks and adverse effects and should be the first part of the management of both SIJ pain and coccydynia.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Nishanth Sandrasegaram BSc MSc FRCA is a specialty registrar in anaesthesia on the South East Thames training scheme.
Rajesh Gupta MD FRCA FFPMRCA EDRA FIPP EDPM is a locum consultant in anaesthesia and pain management at Frimley Park Hospital. His interests include the use of ultrasound in pain medicine and regional anaesthesia.
Mohjir Baloch BSc MRCP FRCA FFPMRCA is a consultant in pain management at Frimley Park Hospital NHS Trust. He is an examiner for the FFPMRCA examination and is a course director for the annual Society of Ultrasound in Anaesthesia Pain Interventions workshop at the Royal College of Physicians.
Matrix codes: 1D01, 2E03, 3E00
References
- 1.Jackson M.A., Simpson K.H. Chronic back pain. BJA Educ. 2006;6:152–155. [Google Scholar]
- 2.Patel R., Appannagari A., Whang P.G. Coccydynia. Curr Rev Musculoskelet Med. 2008;1:223–226. doi: 10.1007/s12178-008-9028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan S.T., Fisher B.E., Roberts C.S. Coccydynia: a review of pathoanatomy, aetiology, treatment and outcome. J Bone Jt Surg [Br] 2010;92-B:1622–1627. doi: 10.1302/0301-620X.92B12.25486. [DOI] [PubMed] [Google Scholar]
- 4.Lirette L.S., Chaiban G., Tolba R., Eissa H. Coccydynia: an overview of the anatomy, etiology, and treatment of coccyx pain. The Ochsener J. 2014;14:84–87. [PMC free article] [PubMed] [Google Scholar]
- 5.Gonnade N., Mehta N., Khera P.S. Ganglion impar block in patients with chronic coccydynia. Indian J Radiol Imaging. 2017;27:324–328. doi: 10.4103/ijri.IJRI_294_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Huang-Lionnet J.H.Y., Cohen S.P. Radiofrequency ablation in coccydynia: a case series and comprehensive, evidence-based review. Pain Med. 2017;18:1111–1130. doi: 10.1093/pm/pnw268. [DOI] [PubMed] [Google Scholar]
- 7.Adas C., Ozdemir U., Toman H. Transsacrococcygeal approach to ganglion impar: radiofrequency application for the treatment of chronic intractable coccydynia. J Pain Res. 2016;9:117307. doi: 10.2147/JPR.S105506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maigne J.Y., Guedj S., Straus C. Idiopathic coccygodynia: lateral roentgenograms in the sitting position and coccygeal discography. Spine. 1994;19:930–934. doi: 10.1097/00007632-199404150-00011. [DOI] [PubMed] [Google Scholar]
- 9.Fogel G., Cunningham P., Esses S. Coccygodynia: evaluation and management. J Am Acad Orthop Surg. 2004;12:49–54. doi: 10.5435/00124635-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Trollegaard A.M., Aarby N.S., Hellberg S. Coccygectomy: and effective treatment option for chronic coccydynia: retrospective results in 41 consecutive patients. J Bone Jt Surg [Br] 2010;92-B:242–245. doi: 10.1302/0301-620X.92B2.23030. [DOI] [PubMed] [Google Scholar]
- 11.Merskey H., Bogduk N. 2nd Edn. IASP Press; Seattle: 1994. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. [Google Scholar]
- 12.Prather H., Hunt D. Sacroiliac joint pain. Dis Mon. 2004;50:670–683. doi: 10.1016/j.disamonth.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Vanelderen P., Szadek K., Cohen S.P. Sacroiliac joint pain. Pain Pract. 2010;5:470–478. doi: 10.1111/j.1533-2500.2010.00394.x. [DOI] [PubMed] [Google Scholar]
- 14.Tonosu J., Oka H., Watanabe K. Validation study of a diagnostic scoring system for sacroiliac joint-related pain. J Pain Re. 2018;11:1659–1663. doi: 10.2147/JPR.S167033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen S., Chen Y., Neufeld N.J. Sacroiliac joint pain: a comprehensive review of epidemiology, diagnosis and treatment. Expert Rev Neurother. 2013;13:99–116. doi: 10.1586/ern.12.148. [DOI] [PubMed] [Google Scholar]
- 16.Chou L.H., Slipman C.W., Bhagia S.M. Inciting events initiating injection-proven sacroiliac joint syndrome. Pain Med. 2004;5:26–32. doi: 10.1111/j.1526-4637.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- 17.Kurosawa D., Murakami E., Ozawa H. A diagnostic scoring system for sacroiliac joint pain originating from the posterior ligament. Pain Med. 2017;18:228–238. doi: 10.1093/pm/pnw117. [DOI] [PubMed] [Google Scholar]