Key points.
-
•
Inadvertent perioperative hypothermia (IPH) (core body temperature <36°C) is a common consequence of general and regional anaesthesia.
-
•
Strategies should be used before, during, and after surgery to maintain normothermia.
-
•
Risk factors for IPH include: high ASA grade, combined regional and general surgery, emergency major surgery, and low BMI.
-
•
Consequences of IPH include surgical site infection, coagulopathy and increased transfusion requirements, pain, altered drug metabolism, and adverse cardiac events.
-
•
The device used to measure temperature should be considered carefully; choose the most appropriate device for the situation and understand its limitations.
Learning objectives.
By reading this article, you should be able to:
-
•
Explain the physiology of thermoregulation in non-anaesthetised adults and children.
-
•
Describe the causes and consequences of perioperative hypothermia.
-
•
Summarise the steps the anaesthetist should take to prevent and treat inadvertent hypothermia at each perioperative stage.
Inadvertent perioperative hypothermia (IPH) is defined as a core body temperature <36.0°C. It is a common consequence of anaesthesia, which increases morbidity and potentially increases mortality. IPH has been the subject of the recently updated National Institute of Health and Care Excellence (NICE) Guideline 65 which summarises best practice for the prevention and treatment of hypothermia in adults undergoing surgery.1 In this article, we describe the physiology of temperature homeostasis and the biophysical causes of IPH; we discuss thermometry and the recommended devices used to measure a patient's temperature and provide a summary of the NICE guidelines and the supporting evidence. It is rare that one aspect of anaesthetic practice can so directly impact on a patient's perioperative health and therefore is relevant to all grades and all specialties of anaesthesia.
Note that deliberate induction of hypothermia, as used for example in cardiac surgery, is outside the scope of this article.
Thermoregulation physiology
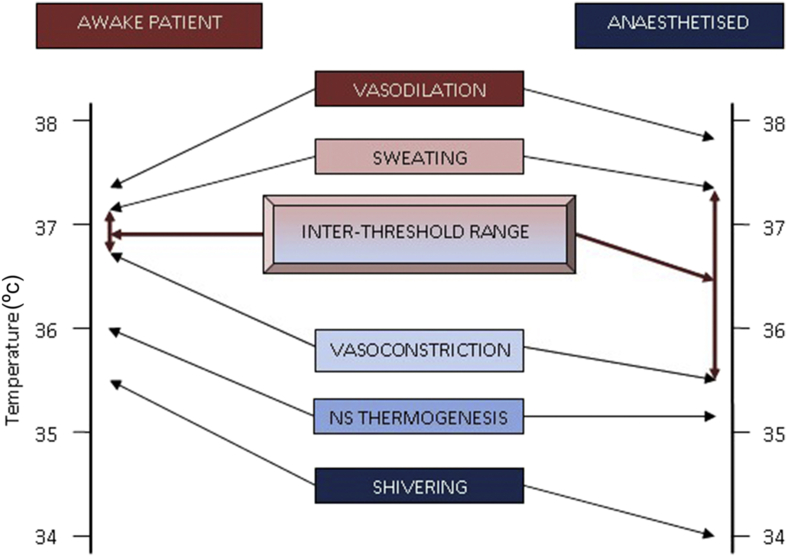
The core temperature of the body is tightly controlled within strict parameters for the effective function of many enzyme and transport mechanisms. Thermoregulation begins with the input from anatomically distinct heat and cold sensors found both peripherally, in the skin and deep tissues, and centrally, in the spinal cord, brain stem, and hypothalamus. Cold receptors are innervated by A-δ fibres; warm receptors by C fibres. Both receptors communicate with the pre-optic nucleus of the anterior hypothalamus via the lateral spinothalamic tract, or trigeminal nerve for the head and neck. In health, the posterior hypothalamus maintains a temperature set point between 36.7 and 37.1°C. Outside this inter-threshold range, the hypothalamus initiates homeostatic and behavioural mechanisms to return the body to normothermia (Fig. 1). Threshold is the temperature at which an effector is activated. Gain is the rate of response to a change in temperature.
Fig 1.
Thermoregulatory thresholds in awake and anaesthetised patients, showing the widened inter-threshold range after anaesthesia. The inter-threshold range describes the lower and upper limits of core body temperature between which no autonomic thermoregulatory effects are triggered. At a core temperature above this range, the body will initiate cooling measures such as sweating and vasodilatation; at a core temperature below this, vasoconstriction and shivering will commence. Anaesthesia widens the inter-threshold range so the core body temperature needs to be much lower than in the awake patient before homeostatic warming methods are triggered.
When cold, the effector mechanisms are firstly vasoconstriction, mediated by α1 adrenergic receptors, along with behavioural changes such as using clothing and shelter to keep warm. These are followed by non-shivering (NS) thermogenesis and then shivering. NS thermogenesis is mediated by β3 adrenergic receptors found in brown adipose tissue, which is more abundant in infants and can therefore double heat production in neonates, but has little significance in adults. Brown adipose tissue contains an increased number of mitochondria that increase lipid oxidation to generate both ATP and heat; NS thermogenesis is thus the production of metabolic heat without muscular activity. In contrast, shivering uses increased mechanical work to generate heat as a by-product; this can increase metabolic rate up to six-fold more than the basal metabolic rate.
When the core body temperature is higher than the inter-threshold range, the hypothalamus triggers methods of cooling such as sweating and vasodilatation along with appropriate behavioural changes such as shedding layers of clothing and seeking shade.
Heat balance
Heat balance describes the process of thermoregulation; heat is produced by metabolism and basal metabolic rate is independent of thermoregulatory feedback. Three quarters of the body's heat is lost by convection, radiation, and conduction and the remaining quarter by evaporation, predominantly in the respiratory tract. To maintain normothermia, any lost heat must be regenerated internally with exercise and shivering increasing metabolic rate significantly to achieve this.
Effect of anaesthesia on heat balance
Under general anaesthesia, this control system is severely challenged. Firstly, behavioural responses are completely abolished. Secondly, the inter-threshold range is widened from ∼0.4 to 4.0°C and homeostasis is compromised (Fig. 1). Vasoconstriction and shivering thresholds can be reduced even further in elderly patients. The effect of this is an inability of the body to respond effectively to the multiple causes of heat loss during surgery and anaesthesia.
Causes of heat loss under anaesthesia
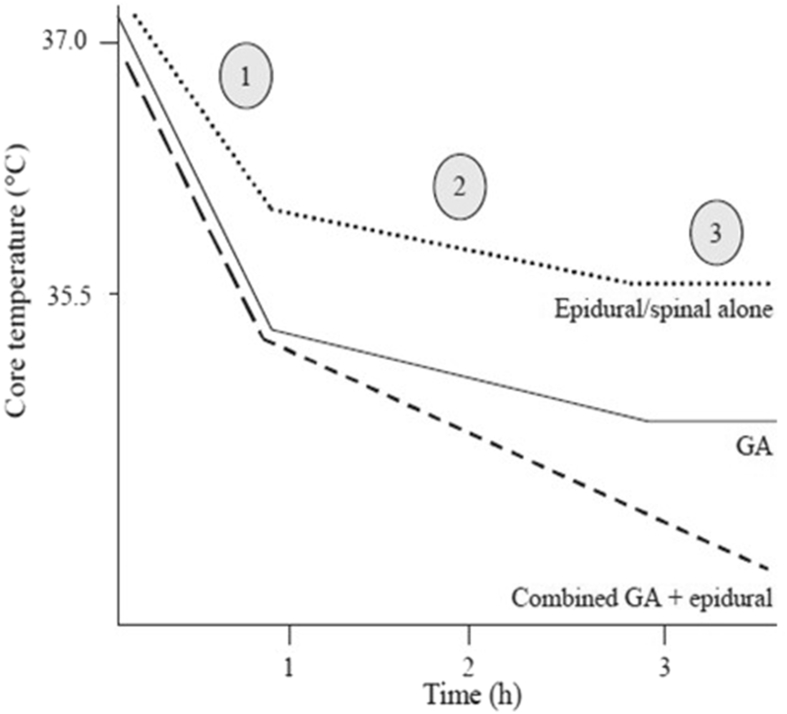
Heat loss under anaesthesia leads typically to a triphasic pattern of hypothermia (Fig. 2).
Fig 2.
Characteristic triphasic patterns of hypothermia under regional, general, or combined regional and general anaesthesia.2 The high risk of a combined regional and general technique is clearly seen. GA, general anaesthesia.
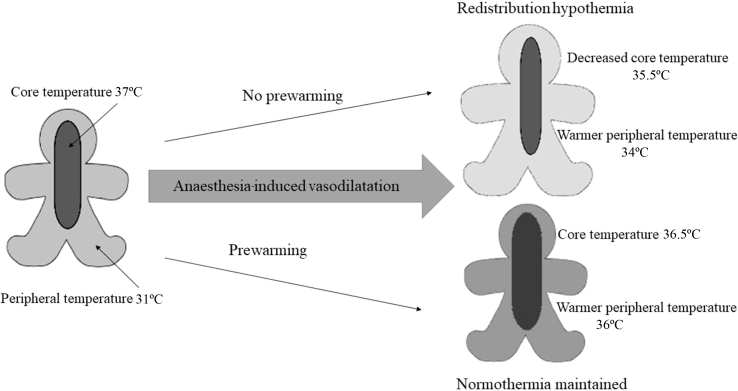
Redistribution causes the first rapid decline in temperature (Phase 1) when vasodilatation leads to warm blood reaching the peripheries and cool blood from the peripheries enters the core circulation (Fig. 3—redistribution hypothermia). Vasodilatation is caused by both the direct effect of anaesthetic agents and the indirect consequences of a lowered vasoconstriction threshold.
Fig 3.
Effect of anaesthesia induced vasodilation on core and peripheral temperature with and without prewarming.
The linear Phase 2 occurs because heat loss exceeds heat produced from metabolism. Metabolic rate is reduced by 15–40% during general anaesthesia.3 The reduced heat generated coupled with the increased heat lost during anaesthesia leads to a negative heat balance, and consequent hypothermia. Heat loss occurs by the following mechanisms:
Radiation (40%)—thermal radiation, which behaves like the electromagnetic spectrum; it travels in straight lines and can be reflected. Heat loss is proportional to the temperature difference to the power 4.
Convection (30%)—caused by a movement away from a heat source by molecules that have increased velocity because of transfer of heat energy. Heat loss is proportional to the velocity of air currents around the body and is exacerbated in the operating theatre by air currents and laminar flow.
Evaporation (25%)—heat is lost as a result of the latent heat of vaporisation, when heat from the body is used to turn a liquid into a gas. Examples include sweating or evaporation from exposed body cavities or mucosal surfaces, skin preparation fluids, and the humidification of any dry respiratory gases.
Conduction (5%)—caused by a transfer of heat from a warm object in direct contact with a cooler object, for example, the use of non-warmed i.v. fluids or contact with a cold theatre table or mattress.
Finally, the linear phase ends and the plateau begins (Phase 3), largely because of maximal vasoconstriction, when any ongoing heat loss is balanced by that produced metabolically.
Regional anaesthesia
Regional anaesthesia decreases the shivering and vasoconstriction threshold below the level of the block, probably because of decreased afferent input from the peripheral thermal centres. Initial hypothermia occurs by redistribution of cooler peripheral blood to the core, because of the vasodilatation induced by regional anaesthesia, but vasoconstriction above the level of the block can compensate to some degree. The greatest risk of heat loss is during combined regional and general anaesthesia (Fig. 2).
Paediatrics
Children (especially neonates) are at particular risk of IPH; children lose more heat than adults via conduction and radiation because they have a higher surface area to volume ratio and less insulating subcutaneous adipose tissue. Children have an increased basal metabolic rate compared with adults. Infants have an immature hypothalamic thermoregulatory capacity and a high resting vagal tone so are less able to vasoconstrict. Furthermore, general anaesthesia lowers the threshold for vasoconstriction and other compensatory mechanisms including NS thermogenesis.
Risk factors for IPH in paediatrics are young age, length of surgery >30 min, major surgery and temperature <36.5°C before induction of anaesthesia.
Other risk factors for incidence of IPH
Table 1 shows the patients who should be considered to be at high risk of IPH.1 Other risk factors include:
Table 1.
Risk factors for inadvertent perioperative hypothermia. Patients should be managed as high risk if two or more of the following apply
| ASA grade 2–5 (the higher the grade, the greater the risk) |
| Preoperative temperature <36.0°C (and preoperative warming is not possible because of clinical urgency) |
| Undergoing combined general and regional anaesthesia |
| Undergoing major or intermediate surgery |
| At risk of cardiovascular complications |
| Low BMI |
Age: The evidence is inconclusive, although elderly patients are at more risk of any of the complications of IPH.
Duration of surgery: NICE recommends warming all surgical patients for more than 30 min duration. Long cases tend to be easier to warm to normothermia by the end of surgery; those lasting about an hour are most at risk, as this is the period that corresponds with the maximal physiological disturbances.4
Environmental temperature: Patients in a cold environment before surgery will have a greater core–periphery gradient and are at increased risk. Likewise, a cold operating theatre environment increases the likelihood of IPH in anaesthetised patients until active warming is applied.
Consequences of IPH
The consequences of IPH impact on morbidity, mortality, and length of hospital stay (Table 2). Furthermore, patients describe being cold in the PACU as one of the most distressing aspects of their surgery.
Table 2.
Consequences of IPH
| SSI | IPH leads to decreased blood flow and decreased oxygen flux to the tissues; oxygen tension is directly related to oxidative neutrophil destruction of bacteria for 4 h after exposure Hypothermia reduces superoxide radical production at any given oxygen tension |
| Drug metabolism | MAC for isoflurane decreases by 5% per 1°C decrease in core temperature Tissue solubility of volatile anaesthetics increases with hypothermia, causing delayed recovery Hepatic metabolism is reduced, leading to the prolonged action of propofol and opiates Longer action of neuromuscular block is caused by reduced hepatic metabolism and decreased rate of Hoffman degradation |
| Increased bleeding and transfusion requirements | Impaired platelet function Impaired coagulation cascade Temperatures at 35.5°C have been shown to increase the relative risk of transfusion by 22%4 |
| Increased rate of cardiac events | Mediated by increased postoperative catecholamine concentrations leading to increased arterial BP which increases myocardial workload Ischaemic cardiac events may also be compounded by the increased skeletal muscle oxygen demand of any concomitant shivering5 |
| Shivering | Increases postoperative pain and makes monitoring unreliable Independently increases carbon dioxide production, catecholamine release, and cardiac output |
The increased risk of surgical site infection (SSI) caused by IPH is well established and is independent of the increased risk of SSI associated with perioperative blood transfusion.6 Maintenance of normothermia is a method recommended by NICE for preventing SSI.7 Normothermic patients have increased collagen at the wound site and faster wound healing.6
There is less evidence of the effect of IPH in paediatric patients, but many of the consequences described above can be extrapolated to children. In addition, noradrenaline (norepinephrine) release in response to hypothermia is increased in neonates, with increased oxygen and glucose uptake. This can cause a metabolic acidosis with increased pulmonary vascular resistance, right-to-left shunting, and decreased tissue perfusion and oxygen flux. Hypothermic children have delayed recovery from anaesthesia and postoperative respiratory depression can occur.
Risks and avoidance of overheating
Intraoperative hyperthermia is rare; the body's warm defences are relatively well-preserved during general anaesthesia. Infants and children are most at risk of overheating. Pathological causes of active hyperthermia such as malignant hyperthermia (MH), sepsis, blood in the cerebral ventricles, or adverse reactions to a drug or blood transfusion should always be considered.
The risks of overheating include increased peripheral blood flow, increased capillary permeability, and oedema. Patients sweat in an attempt to lose heat. Hyperthermia increases the minimum alveolar concentration (MAC) of inhaled anaesthetic agents and reduces the duration of action of neuromuscular blockers.
Frequent or continuous core temperature monitoring will detect hyperthermia. Treatment of active hyperthermia depends on the aetiology, but may include antipyretic medications or active cooling, as well as treating the cause. Passive overheating is easily preventable by vigilance and treated by removing the warming device or insulation.
Thermometry
Temperature should ideally be recorded every 30 min in patients undergoing anaesthesia. This includes patients having regional techniques. There are various devices available and several anatomical sites that can be used to measure temperature; specific consideration of these ensures core temperature is accurately estimated. It has long been known that certain anatomical sites are unreliable for core temperature measurement, with axillary, forehead, and toe temperature being significantly lower than core.8 There is a balance between accuracy, reliability, and acceptability. Under general anaesthesia, invasive techniques that measure core temperature measurements to within 0.5°C of accuracy should be used. These include distal oesophageal (inserted to approximately 40 cm), intravesical, or nasopharyngeal (inserted to approximately 10 cm). In the awake patient, however, many of the sites available give only an indirect estimate of core temperature. These include infra-red tympanic and temporal artery thermometers that use a correction factor before displaying the final temperature. These devices are not accurate to within 0.5°C and are also prone to user error. Aural readings are affected by ear debris and measurement of the ear canal temperature instead of that of the tympanum. Low readings can be checked in the opposite ear. If a reading is questionable, then alternative technology should be used to confirm hypothermia. Digital oral (sublingual) temperatures or zero heat flux technology are more accurate. Rectal temperatures are unreliable and their slow response to changes render them of less use, especially in early detection of MH.
NICE guideline 65: hypothermia—prevention and management in adults having surgery
The first NICE guideline was published in 2008 and was updated in December 2016.1 It provides a useful summary of the prevention and treatment of IPH at each perioperative step (Table 3).
Table 3.
Strategies for the prevention of IPH
| Recommendation | Comments | |
|---|---|---|
| Before surgery | Identify patients at high risk of IPH (Table 1) Measure patient's core temperature |
Active warming should be started before operation in hypothermic or high-risk patients |
| Patient should not be transferred to theatre unless their core temperature is >36°C | ||
| Patient should be encouraged to walk to theatre where possible | This increases heat generated by metabolism | |
| During anaesthesia and surgery | Induction of anaesthesia should not be started until the patient's core temperature is >36°C unless clinically urgent | |
| Active warming is recommended for all high-risk patients regardless of the length of the procedure, and for all patients with total anaesthesia time >30 min | Forced air warmer is the recommended device The temperature setting should be set at maximum and then adjusted to maintain a patient temperature of at least 36.5°C |
|
| Ambient temperature should be >21°C while the patient is exposed to reduce heat loss by convection and radiation | Thereafter, ambient temperature can be reduced for staff comfort Equipment to cool the surgical team should also be considered |
|
| Warm i.v. fluids | Use a continuous fluid warmer incorporated into the giving set Prewarmed fluids are as effective if given within 30 min of removal from warming cabinet9 |
|
| Humidification of respiratory gases | Although only a small amount of metabolic heat loss occurs through the respiratory tract, the use of a heat moisture exchanger filter or alternative humidification device is recommended | |
| The patient's temperature should be measured at least every 30 min and active warming titrated to effect | This is useful to monitor for both hypothermia and hyperthermia This includes patients having regional techniques |
|
| After surgery | The core temperature should be measured with the observations in PACU on admission and then every 15 min | Forced air warming should be continued if the patient is hypothermic (warm blankets offer comfort, but do not actively warm the patient) The patient should stay in PACU until the core temperature is >36°C |
| Patients should be kept comfortably warm for 24 h after surgery with a duvet and blankets |
Perioperative temperature management
Communication and patient empowerment is emphasised; patients should be educated about the potential to feel cold in hospital and advised to bring something to keep themselves comfortably warm and to communicate thermal discomfort to staff.
The 2016 guideline has added a recommendation to pay particular attention to the perioperative thermal comfort of patients with communication difficulties.
Before surgery
Prewarming reduces the temperature difference from periphery to core, thus decreasing the amount of heat lost by redistribution after induction (Fig. 3). Unless patients are hypothermic, a short period of prewarming should have no effect on their measured core temperature, as it is the peripheries that are warmed. The updated NICE guidelines now recommend 30 min prewarming as a way of minimising the incidence of IPH in all patients. There is evidence that even 10 min warming before induction of anaesthesia decreases the incidence of IPH.10 This can easily be instigated in the anaesthetic room whilst preparing the patient for anaesthesia.
During surgery
A forced air warming device is the recommended method of active warming during surgery; it has been shown to be superior to resistive devices in preventing IPH.11 It is worth noting the controversy surrounding the use of forced air warmers and the potential for disruption of laminar flow. There is mixed evidence for this perceived problem, but if a forced air warmer is unsuitable, a resistive heating mattress is recommended.12, 13 Ideally, both these warming technologies should be used together in patient groups at high risk of IPH such as those having combined regional and general techniques.
Cool i.v. fluids can have a clinically significant effect on core temperature: 1 L of fluid at room temperature or one unit of blood at 4°C can decrease core body temperature by 0.25°C.14 All i.v. fluids should therefore be warmed before administration. Clear fluid stored in a warming cabinet at 39°C can be used for low volume infusions.
Prevention of IPH in children
Many of the methods described above and recommended by NICE are applicable to paediatric patients; children should be kept warm before anaesthesia with blankets and encouraged to walk to theatre where possible. Premature infants should be nursed in an incubator. The ambient temperature of the operating theatre is often kept high, especially for neonates, and the appropriate use of active warming with a forced air warmer and fluid warmer can be extrapolated from the guidelines for adults. Continuous temperature monitoring should be used to prevent hyperthermia and burns.
Obstetrics
Obstetric anaesthesia is not specifically covered in the NICE guidelines, but IPH during Caesarean section is uncommon, probably because the mother is in a vasodilated state before anaesthesia.15 Women should be encouraged to walk to the operating theatre in the elective setting and temperature measurement should be routine; a resistive heating mattress and fluid warming is often used in obstetrics and should be mandatory if regional anaesthesia and surgery is likely to last longer than 30 min.
Treatment of postanaesthetic shivering
Shivering is a recognised consequence of both general and regional anaesthesia; it can occur independently of temperature, but should never be treated independently of temperature. Pharmacological treatment of shivering works by lowering the shivering threshold and lowers metabolic heat production, therefore, it would compound hypothermia if not addressed in parallel. As above, the most efficient way of warming a hypothermic patient is with a forced air warmer.
Drugs used commonly to treat shivering include pethidine 25 mg, clonidine 150 μg, and doxapram 100 mg.
Summary
In summary, it is possible to avoid perioperative hypothermia if all types of potential heat loss are addressed. Short surgical cases are at risk of hypothermia; prewarming may be needed for high-risk patients if redistribution hypothermia is to be avoided. Local audit will dictate if certain patients (e.g. combined regional/general anaesthesia) require two methods of intraoperative warming such as an induction mattress and forced air warming.
Declaration of interest
Dr Andrzejowski has received payment for advice and lecturing from various companies involved in marketing active warming devices.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Catherine Riley MA, MRCP, FRCA is a specialty trainee in anaesthesia at Sheffield Teaching Hospitals NHS Trust.
John Andrzejowski FRCA, FFICM is a consultant in neuroanaesthesia & neurocritical care at the Royal Hallamshire Hospital, Sheffield. He is past President of the Neuro Anaesthesia & Critical Care Society of Great Britain and Ireland. He was an advisor for the NICE guidance on depth of anaesthesia monitoring in 2012 and the updated CG65 IPH guidelines in 2016.
Matrix codes: 1A01, 1A03, 2A03, 3A03, 3A09, 3D00
References
- 1.NICE guideline 65 Hypothermia: prevention and management in adults having surgery. Available from: www.nice.org.uk/guidance/cg65 (Accessed 17 May 2018).
- 2.Kirkbride D.A., Buggy D.G. Thermoregulation and mild peri-operative hypothermia. Br J Anaesth CEPD Rev. 2003;3:24–28. [Google Scholar]
- 3.Sessler D.I. Perioperative heat balance. Anesthesiology. 2000;92:578–596. doi: 10.1097/00000542-200002000-00042. [DOI] [PubMed] [Google Scholar]
- 4.Sun Z., Honar H., Sessler D.I. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anaesthesiology. 2015;122:276–285. doi: 10.1097/ALN.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank S.M., Fleisher L.A., Breslow M.J. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277:1127–1134. [PubMed] [Google Scholar]
- 6.Mauermann W.J., Nemergut E.C. The anesthesiologist's role in the prevention of surgical site infections. Anesthesiology. 2006;105:413–421. doi: 10.1097/00000542-200608000-00025. [DOI] [PubMed] [Google Scholar]
- 7.NICE guideline 74 Surgical site infections: prevention and treatment. Available from: www.nice.org.uk/guidance/cg74 (Accessed 17 May 2018).
- 8.Cork R.C., Vaughan R.W., Humphrey L.S. Precision and accuracy of intraoperative temperature monitoring. Anesth Analg. 1983;62:211–214. [PubMed] [Google Scholar]
- 9.Andrzejowski J., Turnbull D., Nandakumar A. A randomised single blinded study of the administration of pre-warmed fluid vs active fluid warming on the incidence of peri-operative hypothermia in short surgical procedures. Anaesthesia. 2010;65:942–945. doi: 10.1111/j.1365-2044.2010.06473.x. [DOI] [PubMed] [Google Scholar]
- 10.Horn E.P., Bein B., Böhm R. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia. 2012;67:612–617. doi: 10.1111/j.1365-2044.2012.07073.x. [DOI] [PubMed] [Google Scholar]
- 11.John M., Crook D., Dasari K. Comparison of resistive heating and forced air warming to prevent inadvertent perioperative hypothermia. Br J Anaesth. 2016;116:249–254. doi: 10.1093/bja/aev412. [DOI] [PubMed] [Google Scholar]
- 12.Moretti B., Larocca A.M., Napoli C. Active warming systems to maintain perioperative normothermia in hip replacement surgery: a therapeutic aid or a vector of infection? J Hosp Infect. 2009;73:58–63. doi: 10.1016/j.jhin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Wood A.M., Moss C., Keenan A. Infection control hazards associated with the use of forced-air warming in operating theatres. J Hosp Infect. 2014;88:132–140. doi: 10.1016/j.jhin.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Sessler D.I. Consequences and treatment of perioperative hypothermia. Anesthiol Clin N A. 1994;12:425–456. [Google Scholar]
- 15.Cheebout R., Newton R.S., Walters M. Does the addition of active body warming to in-line intravenous fluid warming prevent maternal hypothermia during elective Caesarean section? A randomised controlled trial. Int J Obstet Anesth. 2017;31:37–44. doi: 10.1016/j.ijoa.2017.04.008. [DOI] [PubMed] [Google Scholar]