Learning objectives.
By reading this article, you should be able to:
-
•
Assess the place of neurological prognostication in the overall critical care management of a patient after cardiac arrest.
-
•
Describe the outcome scores in common usage for post-cardiac arrest patients.
-
•
Explain the value and limitation of clinical, electrophysiological, biochemical, and imaging signs in neurological prognostication.
Key points.
-
•
Return of spontaneous circulation after cardiopulmonary arrest is a common presentation to critical care.
-
•
Neurological prognostication should not occur within 72 h of the return of spontaneous circulation.
-
•
Additional investigations are becoming routinely available to aid neurological prognostication.
-
•
Neurological prognostication should be multimodal.
-
•
Patients who survive critical care require coordinated neurorehabilitation.
A recent study in England found that the Emergency Medical Services treat approximately 30 000 patients with an out-of-hospital cardiac arrest annually, with a crude survival rate to hospital discharge of 7.9%.1 Other European countries report survival rates of up to 25%. The presenting rhythm and location of the arrest are significant and associated with different survival rates. The incidence of in-hospital cardiac arrest in the UK is 1.6 per 1000 hospital admissions. Overall survival to hospital discharge for this cohort is 18.4%; this is sub-divided to 49% and 11% for patients who present with shockable and non-shockable rhythms, respectively.2 Survivors of cardiac arrest can make a full recovery without disability; however, many experience significant disability. The wide range of survival rates quoted in the literature is partly because of non-standardised definitions and data collection practices; therefore, many international statistics are not directly comparable with UK practice.
The UK Resuscitation Council describe a chain of survival for patients who have had a cardiac arrest.3 This chain starts with: (i) early recognition, (ii) early cardiopulmonary resuscitation, (iii) early defibrillation, and (iv) post-resuscitation care. There are ongoing national initiatives to strengthen each link, both in hospital and the community. This article focuses on the final link for patients who remain comatose after return of spontaneous circulation (ROSC) and require critical care management. These patients develop post-resuscitation syndrome,4 a multiorgan phenomenon with four key components: (i) brain injury, (ii) myocardial dysfunction, (iii) systemic ischaemia and reperfusion response, and (iv) precipitating pathology. These components provide a useful conceptual framework for management and prognostication.
Early critical care management
The Resuscitation Council guidance describes three phases of treatment after ROSC: (i) immediate treatment, (ii) diagnosis, and (iii) optimising recovery. Immediate treatment follows an Airway-Breathing-Circulation (ABC) approach with specific focus on maintaining a temperature between 32 and 36°C. A full clinical examination should take place including collateral history and assessment of comorbidities at the earliest opportunity. The precipitating cause of the arrest must be elucidated and treated. On admission to critical care a treatment bundle to optimise recovery is initiated, as summarised in Table 1.
Table1.
Critical Care treatment bundle to optimise recovery following Return of Spontaneous Circulation.
| Goals: Optimise recovery for survivors; dignity in death for non-survivors |
| Full physical examination and review history and comorbidities |
| Identify and manage precipitating cause of cardiac arrest |
| Maintain normoxia, normocapnia; lung protective ventilation |
| Optimise haemodynamics using cardiac output monitoring or serial bedside echocardiogram |
| Maintain normoglycemia |
| Diagnose and treat seizures |
| Temperature control between 32 and 36°C for >24 h; prevent fever for >72 h |
| Continuous renal replacement therapy |
| Delay neurological prognostication until at least 72 h |
| Key investigations to consider: Echocardiogram, coronary angiography, computed tomography pulmonary angiography, CT of the head |
Resuscitation and post-resuscitation care are frequently delivered where there is limited information about the patient. When it becomes apparent that treatment is futile, this should be discussed with relatives and life-sustaining treatment should be withdrawn. During the first 72 h of intensive care unit (ICU) treatment, consideration of futility should focus on clinical details other than the patients neurological state. The severity of the insult, pre-existing cardiopulmonary reserve, and reversibility of the pathology within the context of the patient's comorbidities should be considered. There are multiple case series that demonstrate that neurological prognostication becomes more accurate further into the ICU stay; current guidance is to wait at least 72 h after the arrest.
A period of cardiac stunning is frequently seen; this usually has some degree of reversibility; focusing on initial post-arrest investigations will lead to an overly pessimistic view. Systemic reperfusion initiates an inflammatory response, which can lead to multiorgan failure and the requirement of several organ support modalities; this will have some degree of reversibility. There will be a subset of patients where survival is unlikely either because of a pre-existing terminal illness, poor cardiopulmonary reserve or irreversibility of the cause of the cardiac arrest. In these patients, it is inappropriate to extend care to a point where neurological prognostication can take place. Where life-sustaining treatment is withdrawn, consideration should be given to offering the opportunity for the patient to become an organ donor as part of end-of-life care.
Neurological prognostication
Hypoxic–ischaemic brain injury is a common sequel to cardiac arrest in patients who gain ROSC. Neurological injury is the cause of death in two-thirds of patients who suffer an out-of-hospital cardiac arrest. These deaths largely occur because life-sustaining treatment is withdrawn, based on prediction of a poor neurological outcome.
Prognostication is the art and science of predicting the clinical course of a medical condition. Studies of neurological prognostication after a cardiac arrest have focussed on predicting poor outcomes; few studies have been conducted into predicting good outcomes. Early identification of patients with a predicted poor neurological outcome avoids futile treatment and allows for a dignified death. As new investigations are adopted into routine clinical practice identification of patients with a poor predicted neurological outcome is becoming increasing possible.
Pyrexia is detrimental to patients with a hypoxic–ischaemic brain injury; various lines of enquiry have suggested that cooling may confer neurological protection. Over recent years, large, well-constructed trials have demonstrated that therapeutic hypothermia delivers significant clinical benefit to this patient group. More recent work has shown that targeting temperature of 36°C to be not inferior to a lower temperature of 33°C.5 Many units have elected to target the higher temperature as this is technically easier to achieve. This new target is often interpreted as ‘do nothing’ and subsequently there is evidence that this target is missed and patient outcomes are affected.6 Temperature management interferes with prognostication based on clinical examination; for this reason, prognostication is deferred until a period of rewarming has occurred.
Measuring and predicting outcomes
Outcome after cardiopulmonary resuscitation is reported both in terms of absolute survival and neurological functioning. A basic understanding of the outcome measures commonly used is helpful when appraising the literature. Many studies have methodological flaws including low patient numbers and lack of appropriate blinding. The most common mode of death is this patient group is withdrawal of life-sustaining treatment because of predicted poor neurological prognosis. Therefore, in the study setting, mortality is best thought of as a marker of clinical prognostication.
For survivors, cerebral performance and disability are most commonly measured using Cerebral Performance Categories scale (CPC) and the modified Rankin Scale (mRS), respectively (Table 2, Table 3). While the scores are similar, CPC couches outcome within purely neurological terms, and mRS focuses upon disability and considers pre-existing neurological function. Studies then group the various scores into ‘good’ (CPC grades 1 and 2) and ‘poor’ (grades 4 and 5); grade 3 was considered good in older studies but now poor in more recent ones. By grouping similar scores, it is possible to perform higher powered studies with fewer patients. However, on an individual level, what a particular patient might deem a ‘good’ outcome will not necessarily tally with the group they have been assigned in the context of a study. Although about 70–80% of survivors will be defined as CPC 1 or 2, half of all survivors will have cognitive impairment detectable by more sophisticated tests than those commonly used in resuscitation trials. Many patients experience an improvement of their CPC between discharge and 6 months.
Table 2.
Modified Rankin Scale.
| Modified Rankin Scale |
|---|
| 0 – No symptoms |
| 1 – No significant disability |
| Able to look after own affairs without assistance but unable to carry out all previous activities. |
| 2 – Slight disability |
| Able to look after own affairs without assistance, but unable to carry out all previous activities. |
| 3 – Moderate disability |
| Requires some help, but unable to walk unassisted. |
| 4 – Moderately severe disability |
| Unable to attend to own bodily needs without assistance and unable to walk without assistance |
| 5 – Severe disability |
| Requires constant nursing care and attention, bedridden, incontinent. |
| 6 - Deceased |
Table 3.
Cerebral Performance Categories scale (CPC).
| CPC |
|---|
|
CPC 1 – Good cerebral performance Conscious, alert, able to work, might have mild neurological or psychological deficit. |
|
CPC 2 – Moderate cerebral disability Conscious, sufficient cerebral function for independent activities of daily life. Able to work in a sheltered environment. |
|
CPC 3 – Severe cerebral disability Conscious, dependent on others for daily support because of impaired brain function. Ranges from ambulatory state to severe dementia or paralysis. |
|
CPC 4 – Coma or vegetative state Any degree of coma without the presence of all brain death criteria. Unawareness, even if appears awake (vegetative state) without interaction with environment; may have spontaneous eye opening and sleep/wake cycles. Cerebral unresponsiveness. |
|
CPC 5 – Brain death Apnoea, areflexia, EEG silence, etc. |
Integrating prognostic data
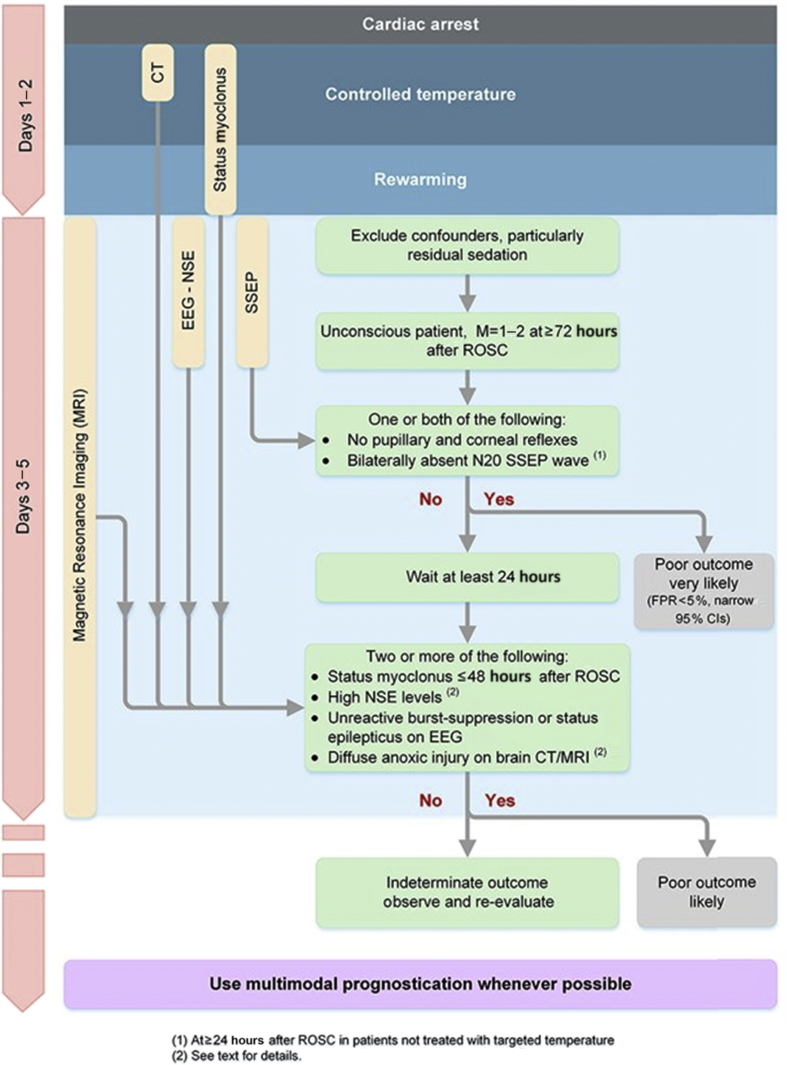
In the recent resuscitation guidelines, an algorithm for prognostication is provided to aid integration of a multimodal investigations (Fig. 1). This pathway demonstrates the utility of clinical examination, neurophysiological studies, biochemical markers and radiological investigations. It provides a timeline to demonstrate the point at which various investigations should be considered. The purpose of the algorithm is to aid the clinician to combine the various findings to aid decision making. The algorithm uses a low false positive rate narrow confidence interval as a cut off for a poor neurological outcome. Here we outline each of the investigative modalities discussed.
Fig 1.
CT, computed tomography; EEG, electroencephalogram; NSE, Neuron-specific enolase; SSEP, Somatosensory Evoked Potential; ROSC, return of spontaneous circulation; FPR, False positive rate; CI, Confidence interval.
Clinical assessment
Clinical examination is inexpensive and easy to perform, but no single clinical finding reliably predicts a poor outcome independently. The results of clinical assessment are influenced by timing of the observation from initial ROSC, core body temperature, and metabolic and pharmacological status, in addition to the experience of the clinician assessing the patient. Before neurological assessment, residual sedation, neuromuscular blocking agents, hypothermia, and metabolic disturbances should be corrected.
The combination of bilateral loss of the pupillary reflex to light and corneal reflexes, when assessed at 72 h reliably predicts a poor outcome.7 The use of corneal reflexes alone are less useful because of the potential influence of residual neuromuscular blockade. An extensor or absent motor response to pain is more sensitive than eye signs, but less specific; for this reason, it is used as a screening tool during assessment. Where central reflexes are absent, the motor score is 1 and there is apnoea, consideration should be given to brainstem death testing.
Single seizures are not helpful for predicting long-term outcome. Prolonged seizures or repeated seizures (status epilepticus) are associated with a poor clinical outcome, although cases of neurological recovery have been described. Myoclonus, brief involuntary jerking caused by muscular contraction, is associated with poor outcome, but has a false positive rate of 9%. Lance–Adams syndrome is an important but rare differential, characterised by generalised or repetitive myoclonus elicited by voluntary action or sensory stimulus; it persists with return consciousness and does not predict poor outcome.
Computed tomography
Most patients should have a computed tomography (CT) brain scan after ROSC before admission to ICU. Scanning is useful where there is suspicion of acute ischaemic infarction or haemorrhage; although an acute ischaemic infarction may not be initially apparent. Anoxic brain damage is primarily seen as a loss of grey–white differentiation. Although there is a standardised approach to the measurement of grey–white matter changes; common UK practice is to make a subjective assessment.8 Because of the subjective nature of this finding, it should be used in combination with other findings to make predictions about neurological outcome.
Magnetic resonance imaging
The use of magnetic resonance imaging (MRI) from day 3 after cardiac arrest is more sensitive than CT imaging at detecting global anoxic–ischaemic injury. It should not be interpreted alone but in combination with other assessments of neurological function. MRI scanning in a critically unwell patient may pose practical difficulties; smaller hospitals may not have access to this modality.
Electroencephalogram
Electroencephalogram (EEG) is a non-invasive investigation, used to record electrical brain activity. Electrical reactivity to a noxious stimulus, presence of status epileptics, and presence of burst suppression have each been used to predict neurological outcome. Absence of EEG reactivity predicts poor outcome with a low false positive rate, but a high confidence interval. This test lacks a standardised noxious stimulus; interpretation has a degree of subjectivity. Status epilepticus can be confirmed on EEG and is associated with a poor outcome; it has a false positive rate of 0–6%. Burst suppression refers to the features of an EEG where >50% of the trace consists of EEG low voltage complexes with alternating bursts. The finding of burst suppression at >72 h post ROSC is associated with poor outcome.
Short-latency somatosensory evoked potentials
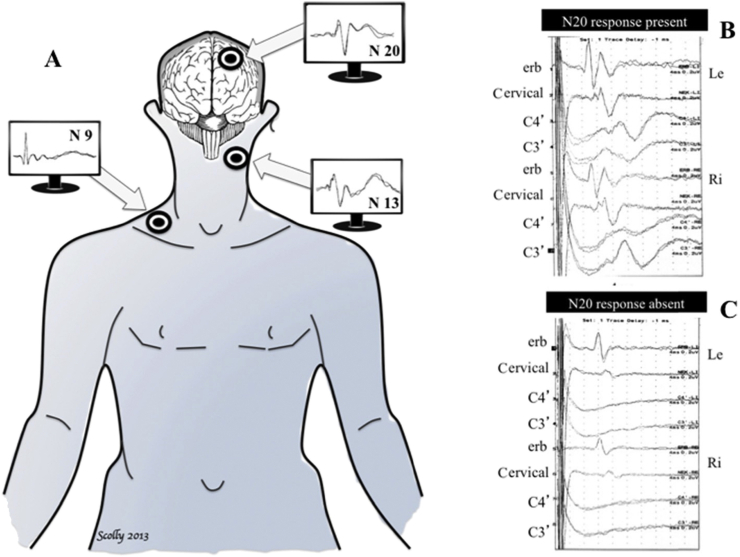
Short-latency somatosensory evoked potentials (SSEP) is probably the most reliable single electrophysiological test of intact neurological function. At a basic level, SSEP testing examines the integrity of neurological pathways that all transit through the brain stem and records the response of the primary somatosensory cortex (PSC) to stimulation. Median nerve stimulation will lead to a N20 signal at the receiving electrode at the PSC when all pathways are intact, as demonstrated in Figure 2. N20 refers to the 20 ms it takes from signal induction at the nerve to its reception at the PSC. Bilateral loss of this signal has a 0% false positive rate9 after 24 h of ROSC. The loss of signal can only be reliably confirmed when testing of nerve function at the higher part of the cervical spinal cord (N13) and brachial plexus (N9/10) are undertaken. This is because a lesion or damage at these levels will not reflect neurological functioning at the level of the cortex or brain stem. SSEPs can safely be interpreted with sedative and opioid medications and are unaffected by neuromuscular blocking agents. The use of SSEPs is limited by its availability and requirement for expert interpretation. Caution must be taken to reduce the effect of electrical or muscle movement artefact on interpretation.
Fig 2.
Somatosensory evoked potentials. (A) In comatose survivors after cardiac arrest, somatosensory evoked potentials are elicited by transcutaneous electrical stimulation applied to the median nerve and then recorded at Erb's point (N9), the cervical medulla (N13) and the controlateral cortex (N20). (B) Example of present N20 cortical response (C3′) in two comatose patients after cardiac arrest. (C) Example of absent N20 cortical response (C3′) in two comatose patients after cardiac arrest.
Biomarkers
Protein biomarkers are released in response to neuronal tissue damage occurring at the point of and after cardiac arrest. The greater the anoxic–ischaemic insult, the greater the levels of protein biomarkers present in the serum. Neuron-specific enolase (NSE) and S-100B are released from neurons and glial cells, respectively. NSE is increasingly used in clinical practice. There have been initial concerns regarding the threshold concentration at which certainty about poor prognosis can made. Other issues include the variability of measured levels between the use of different analysers, extraneuronal production of biomarkers, kinetics and timing of measurement.
Recently NSE has been evaluated in two large patient cohorts. Serum NSE concentrations were assessed in a nested group within the TTM trial at 24, 48, and 72 h after ROSC.10 High NSE cut-off values with low false positive rates and tight confidence intervals reliably predicted outcome.10 A similar European study prospectively analysed NSE serum concentrations in >1000 patients who were cooled to 33°C after cardiac arrest.11 They found that high concentrations reliably predicted poor outcome at critical care discharge.11 While biomarker use is uncommon in current UK practice, interpretation should be in combination with other forms of testing.
Case study
The case below is used to illustrate the use of the various prognostic tests and their integration to assist clinical decision making.
Case
A 61-yr-old man was found slumped by the side of his car by a bystander. Cardiopulmonary resuscitation was initiated and an ambulance called. On the arrival of the paramedics, he was found to be in ventricular fibrillation; one shock was delivered and he gained ROSC. He was not breathing, so a supraglottic airway was placed and mandatory breaths were delivered. On arrival at hospital, he was making spontaneous breaths. He was in sinus rhythm with ST-segment elevation in leads V1-4; electrocardiograph changes resolved within 20 min. He had a mean arterial pressure of 80 mm Hg, was Glasgow Coma Scale 4 (E1, V1, M2) and had pupils reactive to light. He had a normal CT scan of his brain; there were no injures identified on secondary survey. Further investigation revealed a history of hypertension; he was independent, walking more than a mile each day. He was discussed with an interventional cardiologist who advised treatment with antiplatelet drugs, heparin, and a statin. He was stabilised in critical care and a bundle of care was offered to him (Table 1).
During his stay in critical care he developed no organ failure. He had a significantly raised troponin. After 48 h after ROSC sedation was stopped. At 72 h, he had a motor score of 2, took occasional spontaneous breaths and coughed on suctioning. He had no pupillary reflex to light, but had preserved corneal reflexes. A repeat CT scan and EEG were arranged for the next day. The CT demonstrated loss of grey–white differentiation; the EEG demonstrated unreactive burst suppression. There were no changes on neurological examination at 96 h after ROSC. His family were informed that he was unlikely to make a neurological recovery and that life-sustaining treatment should be withdrawn. A second interview with the specialist nurse for organ donation was arranged, where the family declined organ donation after death. An end-of-life care pathway was started, and active treatment stopped; the patient died 30 min after terminal extubation. The patient was discussed with the coroner, who allowed a medical certificate of cause of death (MCCD) to be issued: 1a Hypoxic ischaemic brain injury, 1b Myocardial infarction, 2 Hypertension.
Discussion
In this case, the patient had ROSC, but failed to regain consciousness. Initial investigations suggested the cause of the arrest was myocardial ischaemia and a cardiological opinion was sought. The patient was taken to critical care. The patients' medical and functional history suggested he had the reserve to survive organ support. After a period of targeted temperature management his sedation was stopped; time was given to allow the sedative drugs to be eliminated. The ability to take occasional spontaneous breaths and cough on tracheal suctioning demonstrated that patient was not brain dead. However, the poor motor score and absent pupillary reflexes indicated a likely poor neurological outcome. The CT scan, EEG report and unchanged neurological examination confirmed a likely poor neurological outcome. As with all suspected deaths in critical care, this patient was referred to the organ donation team—had the family agreed, the patient could have donated organs after death by the donation after cardiac death pathway. The patient was discussed with the coroner given that his collapse was unwitnessed, but, given that on the balance of probabilities, the hypoxic–ischaemic brain injury was a result of a myocardial infarction, the coroner allowed the attending doctor to issue an MCCD.
Conclusion
The journey from prevention of to rehabilitation from cardiac arrest is described as a chain of survival. Prognostication after cardiac arrest forms part of the comprehensive critical care offered to patients who remain comatose after ROSC. After 72 h of critical care, it is possible to start identifying patients who are predicted to have a poor neurological outcome by combining the results from various tests. Knowledge of how these tests are performed, and the implication of their results in this patient group is important for clinicians involved in their care.
There is a proposal for centralisation of care for patients who arrest in the community at cardiac arrest centres to strengthen the chain of survival for patients who suffer cardiac arrest. These hubs will have immediate access to the time-critical treatments and investigations required in the immediate post-arrest period. Additional services that facilitate prognostication, such as MRI of intubated patients, biomarkers, and SSEPs should reasonably be available at such centres. Integrated pathways from pre-hospital care to return to the community will facilitate the best outcomes possible. Advances in both clinical science and organisation of services might be expected to offer better outcomes than have been seen historically.
Declaration of interest
None declared.
MCQs
The associated MCQs (to support CME/CPD activity) can be accessed at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Matthew Jackson BSc FFICM FRCA is a consultant in anaesthesia and intensive care at Stockport NHS Foundation Trust.
Andrew Mockridge is a specialty registrar in Anaesthesia and Intensive Care Medicine in the Northwest of England.
Matrix codes: 1B04, 2C06, 3C00
References
- 1.Hawkes C., Booth S., Ji C. Epidemiology and outcomes from out-of-hospital cardiac arrests in England. Resuscitation. 2017;110:133–140. doi: 10.1016/j.resuscitation.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Nolan J., Soar J., Smith G. Incidence and outcome of in-hospital cardiac arrest in the United Kingdom national cardiac arrest audit. Resuscitation. 2014;85:987–992. doi: 10.1016/j.resuscitation.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Nolan J. 7th ed. Resuscitation Council UK; London: 2016. Advanced life support. [Google Scholar]
- 4.Nolan J., Neumar R., Adrie C. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen N., Wetterslev J., Cronberg T. Targeted temperature management at 33°C versus 36°C after cardiac arrest. New Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 6.Bray J., Stub D., Bloom J. Changing target temperature from 33 °C to 36 °C in the ICU management of out-of-hospital cardiac arrest: a before and after study. Resuscitation. 2017;113:39–43. doi: 10.1016/j.resuscitation.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Sandroni C., Cavallaro F., Callaway C.W. Predictors of poor neurological outcome in adult comatose survivors of cardiac arrest: a systematic review and meta-analysis. Part 2: patients treated with therapeutic hypothermia. Resuscitation. 2013;84:1324–1338. doi: 10.1016/j.resuscitation.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Torbey M.T., Selim M., Knorr J., Bigelow C., Recht L. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke. 2000;31:2163–2167. doi: 10.1161/01.str.31.9.2163. [DOI] [PubMed] [Google Scholar]
- 9.Horn J., Tjepkema-Cloostermans M.C. Somatosensory evoked potentials in patients with hypoxic–ischemic brain injury. Semin Neurol. 2017;37:60–65. doi: 10.1055/s-0036-1594252. [DOI] [PubMed] [Google Scholar]
- 10.Stammet P., Collignon O., Hassager C. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33°C and 36°C. J Am Coll Cardiol. 2015;65:2104–2114. doi: 10.1016/j.jacc.2015.03.538. [DOI] [PubMed] [Google Scholar]
- 11.Streitberger K., Leithner C., Wattenberg M. Neuron-specific enolase predicts poor outcome after cardiac arrest and targeted temperature management. Crit Care Med. 2017;45:1145–1151. doi: 10.1097/CCM.0000000000002335. [DOI] [PubMed] [Google Scholar]