Learning objectives.
By reading this article you should be able to:
-
•
Discuss the clinical significance of medication errors in anaesthesia.
-
•
List the causes of medication errors, including human factors.
-
•
Describe the role of medicine management systems in optimising medicines safety.
-
•
Outline the aspects of clinical practice that anaesthetists can use to reduce the likelihood of medication errors occurring.
Key points.
-
•
Medicines safety requires the administration of the correct medication to the correct patient, in the correct dose, by the correct route, at the correct time, with accurate documentation.
-
•
Human factors are a major source of medication errors and adverse drug reactions.
-
•
Unique risks for anaesthetists relate to working independently in a complex and distracting environment, with drugs that have potential for both harm and abuse.
-
•
Organisations should ensure that systems are in place to optimise medicines safety.
-
•
Anaesthetists should take steps to reduce the risk of human factors contributing to errors.
Anaesthetic practice involves the preparation and administration of medicines with a high potential for harm in a time-critical fashion. The working environment is distracting and medicines are often used without the support of colleagues or electronic clinical decision tools. There is a reliance on situational awareness, mental focus, and vigilance in order to reduce the chance of medication errors (MEs) occurring. However, human errors are an inevitable outcome of poor human reliability; so it is essential that steps are taken to minimise errors and harm. Robust medicines management is essential for the safe and effective use of drugs by anaesthetists. Processes must be in place to enable reliable and timely access to drugs for clinical efficiency and emergency treatment, whilst also restricting access to mitigate drug misdirection and abuse.1
This article reviews the medicine management systems and human factors relevant to medicines safety in anaesthetic practice.
Classification of MEs
Medication errors are ‘a failure in the treatment process that lead to, or have the potential to lead to, harm to the patient’.2 Classification of errors can be approached in a variety of ways, for example according to root cause or clinical outcome. In order to prioritise effectively those areas in need of risk mitigation strategies, critical incident reporting data can be used to identify trends in particular types of MEs. Some of the ways that errors can be categorised are listed in Table 1.
Table 1.
Categorisation of medication errors.
| Categorisation of medication error | Examples |
|---|---|
| Stage in the process | Medication selection; purchase; storage; dispensing or manipulation; distribution; prescribing; administration; documentation; or clinical monitoring |
| Causative factor | Supply failure; lack of clinician's knowledge; communication failures; or distracting work environment |
| Mechanism of error | Surge in demand for a drug with low stock levels or mis-selection of a syringe from a tray of drugs |
| Based on psychological theory | Errors when planning actions, errors in the execution of well-planned actions, or deliberate violations |
| Clinical impact (actual or potential) | From no harm to life-threatening complications |
Clinical significance of medicines safety
The reported incidence of MEs in anaesthetic practice varies widely between studies, partly because of varying definitions and study methodologies. In studies that rely on anaesthetists to recognise and self-report their errors the incidence is relatively low, with an error rate per case ranging from to 0.008 to 0.03.3, 4 Prospective trials with data collection by an observer report significantly higher error rates, especially errors in the documentation of medication administration. The reported incidence per anaesthetic case is as high as 0.96, and the rate per perioperative medication administration is as high as 11.6%.5
Adverse drug reactions (ADRs), an unexpected or dangerous reaction to a drug, have been observed to occur as a result of one third of MEs in anaesthesia.6 An observational study in intensive care found that the occurrence of two or more MEs resulting in ADRs independently increased the odds ratio of death by 3, after adjusting for confounding variables.7
Medication safety issues are an endemic source of preventable harm throughout healthcare; accordingly, they account for five out of the 15 ‘never events’ published by NHS Improvement in 2018. Those directly relevant to anaesthesia include: (i) mis-selection of high strength midazolam during conscious sedation; (ii) mis-selection of a strong potassium solution; (iii) administration of medication by the wrong route, including (a) intravenous chemotherapy by the intrathecal route and (b) intravenous administration of an epidural medication that was not intended to be administered by the iv route; and (iv) overdose of insulin caused by the use of abbreviations or an incorrect device.8
In 2017 the World Health Organization identified its third ‘Global Patient Safety Challenge’ as improving medication safety by strengthening the systems for reducing MEs and avoidable medication-related harm. The three key priorities for safety improvements are high-risk medication, polypharmacy, and transition of care.9
Medicines management systems
The UK Department of Health defines medicine management as ‘the clinical, cost-effective and safe use of medicines to ensure patients get the maximum benefit from the medicines they need, while at the same time minimising potential harm’.10 Medicines management looks at systems surrounding selection, purchase, storage, dispensing, prescribing and administration. To minimise the potential for MEs in the perioperative period, it is recommended in the Royal College of Anaesthetists' Guidelines for the Provision of Anaesthetic Services (GPAS) that ‘reliable medicine-management systems should be in place, and appropriate safety measures should be taken to minimise errors’.11 This includes ensuring appropriate training for all staff, access to appropriate and up-to-date resources, and following legislation for medications (in particular for controlled drugs [CDs]). It is recommended that all hospitals have standard operating procedures in place surrounding the supply and use of medications to ensure that medication safety is maintained.2 GPAS recommends access to a clinical pharmacy service for advice.11
Supply and storage of medications
GPAS recommends that there are ‘facilities for medication storage…that allow timely access when required for patient care, while maintaining integrity of the medicines and aiding organisations to comply with safe and secure storage requirements’.11 Besides appropriate storage, it is strongly recommended that robust systems are in place to ensure reliable medicines management, stock review, supply, expiry checks, and access to appropriately trained pharmacy staff to manage any drug shortages.11
A robust system needs to be in place to ensure that an appropriate range and quantity of in-date stock medications. Recent guidance highlights that ‘it is not possible to provide a definitive list of “emergency drugs”’ and that stock lists need to reflect the surgical procedures performed.13 To meet the Royal College of Anaesthetists (RCoA) Anaesthesia Clinical Services Accreditation (ACSA) standards, every site where anaesthesia is provided must have an in-date supply maintained of emergency drugs such as intralipid, sugammadex, and dantrolene.
Timeliness of access to medication is essential, as even short delays have the potential to lead to an adverse outcome for the patient. The Care Quality Commission recently asked for clarification regarding storage of non-CDs in anaesthetic rooms; the RCoA and Association of Anaesthetists convened a working party that concluded that patient safety is paramount and timely access to emergency medications is essential; however, all medicines should be stored in a locked medicine cupboard when an operating theatre is not in use.13 Legislation is more clearly defined for CDs, with operating theatre CD registers used for recording issue, receipt, form of administration (including administration via intravenous infusion or driven syringes), names of patients receiving the CDs, and ampoules/vials returned/disposed of.12
The reliability and resilience of medication supplies must be ensured. An increasing incidence of drug shortages (e.g. in recent years there have been shortages of preservative-free diamorphine and piperacillin–tazobactam) has necessitated recent recommendations that ‘hospitals should invest in sufficiently large buffer stock to be able to maintain continuity of supply’ during shortages of high-risk medications.14 If issues do arise with the supply of medications, it is important that any changes in drug presentation are advertised clearly ahead of time.15
Sources of error
In contrast to other areas of medicine, where medications are prescribed, checked, and then administered by different healthcare professionals, anaesthetists routinely prescribe, dispense, and then administer i.v. drugs in a short time period.15 Injectable medicines are known to be associated with a higher incidence of errors than other forms of medicines, thereby highlighting the need to ensure that systems are in place to minimise MEs.16 Injectable medicines account for 25% of all medication incidents, and 58% of those that result in death or serious harm to patients.17
Errors, caused by one drug being mistaken for another, can occur as a result of: (i) similar looking ampoules/packaging or ‘look alike’; (ii) similar sounding names or ‘sound alike’; (iii) falsely anticipated or confounded storage location; (iv) concentration of the preparation; and (v) intended route of administration of the preparation.18
A third of reported medication incidents may be caused by confusion over packaging and labelling.17 Pharmaceutical companies and NHS procurement should comply with national guidelines regarding packaging and labelling of injectable medicines. If an end user has any concerns regarding packaging and labelling, they should be highlighted via the pharmacy medicines management chain to the Medicines and Healthcare products Regulatory Agency (MHRA).18
It is recommended that the number of syringes that are prepared in the operating theatres is minimised to increase safety, especially of high-risk medications, for example heparin, morphine, and noradrenaline (norepinephrine). These should either be made in a pharmacy's aseptic unit or bought in as a prefilled product. Prefilled syringes are made in a system designed to ensure the quality of the product and with enhanced labelling as per National Patient Safety Agency Design (NPSA) guidance.17 Again, if there are any changes to the drug presentation these should be advertised ahead of time.15
If a prefilled product is not available then the anaesthetist will often have to prescribe, dispense, and manipulate the product for administration. It is essential that all syringes containing drugs are clearly labelled, and it is recommended that the label includes a colour as defined by international norm ISO 26825.17, 19 Clear labelling on both ampoule and syringe should be read carefully before the drug is drawn up or injected. Ideally drugs should be drawn up and labelled by the anaesthetist who administers them. When infusions may be continued outside of the operating theatre they must be labelled at the time of preparation with the drug name, concentration, diluent, and time of preparation, and be checked and countersigned by another trained member of staff.
Storage of medicines should also be considered, with each drug having a clear and distinct location, which is labelled and separated from its neighbour (e.g. through drawer dividers). As per a NPSA patient safety alert, it is recommended that drugs intended for regional anaesthesia are stored separately from those intended for intravenous use so as to minimise the risk of wrong route administration.20 Any drugs considered to be potentially hazardous should be stored separately and removed if possible. ‘Sound-alike’ drugs (e.g. adrenaline and atropine) should be physically separated where possible. The concentration of medications should be standardised to help prevent inadvertent mis-selection (e.g. heparin, morphine, and midazolam), and where possible only one concentration should be stocked.
Human factors in medicines safety
The human factors theory examines the relationship between human beings and the systems in which they interact and describes the interaction between individuals at work, the task at hand, and the workplace. Human errors are summarised in Fig. 1.2 They can occur as a consequence of mistakes in planning actions or skill-based errors during the execution of well-planned actions. Human errors contribute to over half of ADRs related to anaesthesia.6
Fig 1.
Classification of medication errors based on a psychological approach.2 Reproduced, with permission from Jeffrey Aronson.
Mistakes
Mistakes are unintentional failures in the planning of actions. Knowledge-based mistakes result from insufficient knowledge; rule-based mistakes result from either the application of a bad rule or the failure to apply a good rule.
High quality training, proactive supervision, robust competency assessment, and a team dynamic in which it is normal for colleagues to observe and challenge each other can reduce mistakes.
Training and continuing professional development in anaesthesia necessitates an up-to-date understanding of clinical pharmacology and medicines management. The General Medical Council states that ‘good doctors keep their knowledge and skills up to date and work within the limits of their competence’.21
Rule-based errors relating to local and national guidelines can be reduced with pre-emptive training and a workspace layout which facilitates safe access to the necessary drugs and equipment. Up-to-date versions of guidelines should be readily available—for example by including them on emergency treatment carts (e.g. dantrolene for malignant hyperpyrexia or intralipid for local anaesthetic toxicity).
Application of the Association of Anaesthetists’ guidelines ‘Checking anaesthetic equipment’ will mitigate the risk of vaporiser errors including use of an empty or incorrectly mounted vaporiser.22
Skill-based errors
Skill-based errors tend to affect experienced and capable individuals during repetitive or routine activities. They are subdivided into memory-based errors (lapses), which result from forgetting to correctly execute planned actions, and action-based errors (slips), which are unintentional actions. Well-recognised skill-based errors include selecting the incorrect vial when drawing up drugs, selecting the wrong label for a syringe, failure to draw up a drug in a diluent, and administering the incorrect syringe from a tray of prepared syringes.
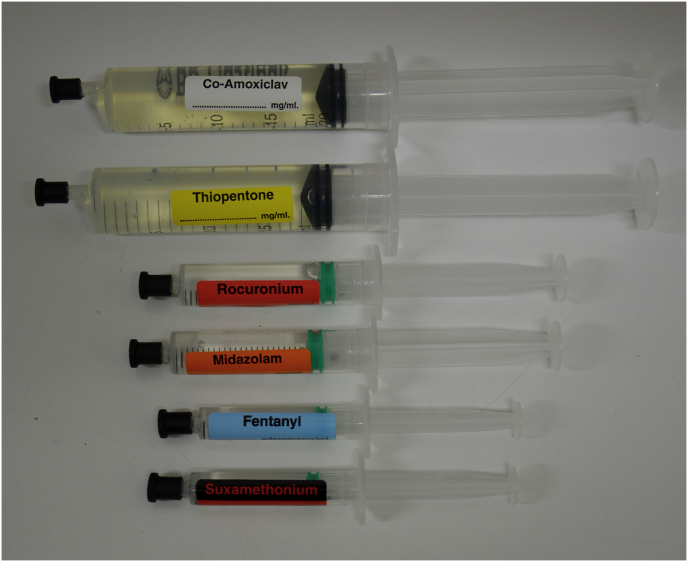
The 5th National Audit Project (NAP5), a year-long nationwide audit on accidental awareness during general anaesthesia, found that all reports of awareness caused by syringe swaps involved similar coloured fluids in similar sized syringes.23 Figure 2 demonstrates examples of syringe swaps that have resulted in accidental awareness. Suggested safety mechanisms within the report included reserving 5 ml syringes for neuromuscular block only, double-labelling of these syringes or even using coloured syringes or different syringe types.
Fig 2.
Accidental awareness during general anaesthesia has been caused by a syringe swap of 2 ml (e.g. succinylcholine rather than fentanyl), 5 ml (e.g. midazolam rather than non-depolarising drugs), or 20 ml (e.g. antibiotics rather than thiopental).
A well-designed workplace and effective medicine management system reduces opportunities for skill-based errors. Individual performance is enhanced by adequate rest, active supervision, avoiding distractions, and engagement with checklists and technology.
MEs in anaesthesia are often associated with distraction, fatigue, and organisational issues such as last minute changes in operating list order. Statistically significant associations have been found with medication critical incidents and very junior anaesthetists, when anaesthesia is provided on a non-elective basis and outside of normal working hours.23
An awareness of specific situational risks enables interventions to mitigate harm. For instance, the increased risk of dose miscalculations in paediatric patients can be managed by using dose cross-checking tools.
Although evidence supporting the clinical benefits of double-checking medicines is lacking, it has been widely adopted as a means of reducing human error outside of anaesthetic practice. A two-person technique is limited by human resources and the potential to compromise safety paradoxically because of ‘involuntary automaticity’ in which the conscious attention of both individuals is reduced.23 Computerised barcode-assisted double-checking before drug administration has been found to be more feasible than a two-person technique. It is effective at reducing errors in record keeping and improving legibility but is limited by unfavourable attitudes to double-checking and failures in the utilisation of the technology. This technique is enhanced by the routine use of prefilled syringes in order to prevent inadvertently scanning mislabelled syringes.6
Although technological innovations have radically improved patient safety in certain aspects of anaesthesia, such as with medical gas supplies and ventilators, the process of giving medication has changed little over recent decades. If intelligent computerised drug decision-support systems were integrated with electronic patient records, they could mitigate human errors by providing patient-specific assistance with dose calculations, drug administration reminders, and warnings about drug interactions or adverse reactions.
Depth of anaesthesia monitoring is a technological development that is widely available. NAP5 recommended that its use should be especially considered during the use of total intravenous anaesthesia (TIVA) when there are risk factors for accidental awareness such as with neuromuscular blocking drugs, during patient transfer, and when converting volatile anaesthesia to TIVA.23
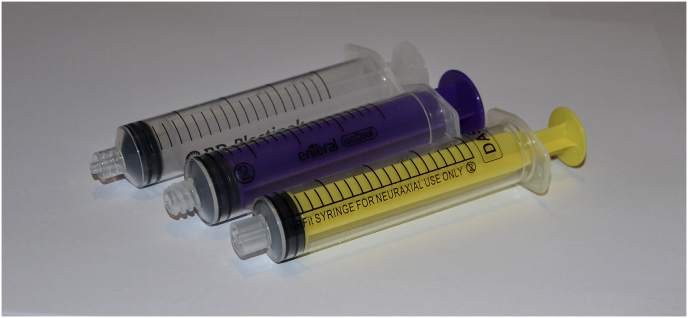
Product design can be used to minimise the likelihood of errors occurring. New worldwide standards for small-bore non-Luer connectors (ISO 80369), which are not cross-compatible with Luer connectors, have been developed to reduce the risk of wrong route errors. The ENFit ISO standard describes the system developed for administering medication via the oral or enteral route, whereas the NRFit ISO standard describes the system developed for the neuraxial route (or for major regional anaesthesia). Figure 3 demonstrates the different designs.24
Fig 3.
From left to right: Luer-lock syringe for intravenous use, ENFit standard syringe for enteral use, and NRFit standard syringe for central neuraxial and regional anaesthesia.
There are multiple international recommendations focusing on improving medicine safety by reducing MEs. These are summarised in Table 2 by the stage at which they at occur in the planning and administration of medication in a clinical encounter. The recommendations are mostly based on expert opinions.25 However, an RCT found that anaesthetists who complied with the principles of safe drug administration were less prone to errors than non-conformers.5
Table 2.
Mechanisms to reduce the likelihood of a medication error occurring during planning and administration of a medicine.
| Stage of administration | Recommendation |
|---|---|
| Preoperative |
|
| Preparation |
|
| Administration |
|
| After administration |
|
Promoting safety culture
The routine reporting of MEs, ADRs, and near misses should be easy to perform and actively encouraged. Root cause investigations should be fair and transparent, with compassionate support and feedback afforded to those involved. Investigations should focus on the various latent factors arising from the working environment in conjunction with the final ‘action error’. Analysis of individual reports and regular audit of collective reports enables the development of quality improvement interventions. Important policy changes should be implemented in a timely fashion and be well publicised to the relevant staff, and subsequently re-evaluated.
A ‘just culture’ model of shared accountability between management and staff has been advocated. Although a non-punitive approach is appropriate for most errors, individuals are accountable if they deliberately or repeatedly compromise patient safety.26
Conclusion
Failures of medicine safety in anaesthetic practice remain a prevalent source of avoidable iatrogenic harm. In order to improve medicine safety, there is a need for effective medicine management systems and for anaesthetists to embrace the principles of medicines safety culture.
Declaration of Interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Steve Webber FRCA in a consultant in anaesthesia and critical care at Sheffield Teaching Hospitals. His clinical interests are clinical governance and patient safety, critical care, and anaesthesia for urological surgery.
Jen Jennings MPharm MSc is a critical care pharmacist at Sheffield Teaching Hospitals. Her clinical interests are clinical governance and patient safety, in particular understanding the factors that lead to medication errors.
Euan Mackay FRCA FICM is a specialist registrar at Sheffield Teaching Hospitals who has recently been appointed as a consultant in anaesthesia and critical care at Chesterfield Royal Hospital.
Matrix codes: 1A02, 2A12, 3I00.
References
- 1.Australian and New Zealand College of Anesthetists PS51 Guidelines for the safe management and use of medications in anaesthesia. 2018. http://www.anzca.edu.au/documents/ps51-2009-guidelines-for-the-safe-administration-o.pdf Available from. [Google Scholar]
- 2.Aronson J.K. Medication errors: definitions and classification. Br J Clin Pharmacol. 2009;67:599–604. doi: 10.1111/j.1365-2125.2009.03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webster C.S., Merry A.F., Larsson L., McGrath K.A., Weller J. The frequency and nature of drug administration error during anaesthesia. Anaesth Intensive Care. 2001;29:494–500. doi: 10.1177/0310057X0102900508. [DOI] [PubMed] [Google Scholar]
- 4.Gariel C., Cogniat B., Desgranges F.P., Chassard D., Bouvet L. Incidence, characteristics, and predictive factors for medication errors in paediatric anaesthesia: a prospective incident monitoring study. Br J Anaesth. 2018;120:563–570. doi: 10.1016/j.bja.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Merry A.F., Webster C.S., Hannam J. Multimodal system designed to reduce errors in recording and administration of drugs in anaesthesia: prospective randomised clinical evaluation. BMJ. 2011;343:d5543. doi: 10.1136/bmj.d5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanji K.C., Patel A., Shaikh S., Seger D.L., Bates D.W. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124:25–34. doi: 10.1097/ALN.0000000000000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrouste-Orgeas M., Timsit J.F., Vesin A. Selected medical errors in the intensive care unit: results of the IATROREF study: parts I and II. Am J Respir Crit Care Med. 2010;181:134–142. doi: 10.1164/rccm.200812-1820OC. [DOI] [PubMed] [Google Scholar]
- 8.NHS Improvement . 2018. Never events list.https://improvement.nhs.uk/documents/2266/Never_Events_list_2018_FINAL_v5.pdf Available from. [Google Scholar]
- 9.World Health Organisation . 2017. Medication without harm. WHO global patient safety challenge.http://apps.who.int/iris/bitstream/handle/10665/255263/WHO-HIS-SDS-2017.6-eng.pdf;jsessionid=F77ACDA0FF696133A9992C2CEFCB70E7?sequence=1 Available from. [DOI] [PubMed] [Google Scholar]
- 10.Department of Health . 2004. Management of medicines, a resource to support implementation of the wider aspects of medicines management for the national service frameworks for diabetes, renal services and long-term conditions.http://webarchive.nationalarchives.gov.uk/20121101234412/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4088755.pdf Available from. [Google Scholar]
- 11.Royal College of Anaesthetists . 2018. Chapter 3, guidelines for the provision of anaesthesia services (GPAS)https://www.rcoa.ac.uk/system/files/GPAS-2018-03-INTRAOP.pdf Available from. [Google Scholar]
- 12.Royal Pharmaceutical Society of Great Britain . 2005. The safe and secure handling of medicines: a team approach.https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Publications/Safe%20and%20Secure%20Handling%20of%20Medicines%202005.pdf Available from. [Google Scholar]
- 13.Royal College of Anaesthetists and the Association of Anaesthetists of Great Britain and Ireland Storage of drugs in anaesthetic rooms: guidance on best practice from the RCoA and AAGBI 2016. https://www.rcoa.ac.uk/system/files/StorageDrugs2016.pdf Available from.
- 14.Whitaker D., Brattebø G., Trenkler S. The European Board of Anaesthesiology recommendations for safe medication practice: first update. Eur J Anaesthesiol. 2017;34:4–7. doi: 10.1097/EJA.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 15.National Patient Safety Agency . 2012. Safe Anaesthesia Liaison Group, patient safety update, including the summary of reported incidents relating to anaesthesia (Jan 2012–Mar-2012)https://www.aagbi.org/sites/default/files/images/PATIENT%20SAFETY%20UPDATE%20-%20Mar%202012.pdf Available from. [Google Scholar]
- 16.National Patient Safety Agency . 2007. Promoting safer use of injectable medicines. Multi-professional safer practice standards for: prescribing, preparing and administering injectable medicines in clinical areas.https://www.sps.nhs.uk/wp-content/uploads/2016/12/2474_Inject_Multi-prof-1.pdf Available from. [Google Scholar]
- 17.National Patient Safety Agency Design for patient safety—a guide to labelling and packaging of injectable medicines. https://webarchive.nationalarchives.gov.uk/20150402184112/http://www.nrls.nhs.uk/resources/healthcare-setting/hospital-pharmacy/?entryid45=59831&cord=ASC&p=2 Available from. Reference Number 0592.
- 18.Kaufmann J., Wolf A.R., Becke K., Laschat M., Wappler F., Engelhardt T. Drug safety in paediatric anaesthesia. Br J Anaesth. 2017;118:670–679. doi: 10.1093/bja/aex072. [DOI] [PubMed] [Google Scholar]
- 19.Association of Anaesthetists . 2016. Syringe labelling in critical care areas review 2014.https://www.aagbi.org/sites/default/files/SYRINGE_LABELLING_2014_updated_20November_2016_0.pdf (updated November 2016) Available from. [Google Scholar]
- 20.Royal College of Anaesthetists . 2015. Royal College of anaesthetists' accreditation standard 2.2.4.1.https://www.rcoa.ac.uk/system/files/ACSA-STANDARDS-2015_9.pdf Available from. [Google Scholar]
- 21.General Medical Council . 2014. Good medical practice.https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/good-medical-practice Available from. [Google Scholar]
- 22.Association of Anaesthetists of Great Britain and Ireland . 2012. Checking anaesthetic equipment.https://www.aagbi.org/sites/default/files/checking_anaesthetic_equipment_2012.pdf Available from. [DOI] [PubMed] [Google Scholar]
- 23.Pandit J.J., Andrade J., Bogod D.G. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: summary of main findings and risk factors. Br J Anaesth. 2014;113:549–559. doi: 10.1093/bja/aeu313. [DOI] [PubMed] [Google Scholar]
- 24.NHS Improvement Small bore connectors: an introduction to safe use 2017. https://improvement.nhs.uk/resources/small-bore-connectors-safety-introduction/ Available from.
- 25.Wahr J.A., Abernathy J.H., Lazarra E.H. Medication safety in the operating room: literature and expert-based recommendations. Br J Anaesth. 2017;118:32–43. doi: 10.1093/bja/aew379. [DOI] [PubMed] [Google Scholar]
- 26.Rogers E., Griffin E., Carnie W., Melucci J., Weber R.J. A just culture approach to managing medication errors. Hosp Pharm. 2017;52:308–315. doi: 10.1310/hpj5204-308. [DOI] [PMC free article] [PubMed] [Google Scholar]