Key points.
-
•
Gastric ultrasound is used when prandial status is uncertain or gastric emptying might be delayed.
-
•
Images are acquired with a curvilinear transducer in the epigastrium.
-
•
The gastric antrum may be empty or contain fluid or solid. If the antrum contains fluid, quantification of the volume of fluid will guide risk stratification.
-
•
A gastric antrum that is empty or contains <1.5 ml kg−1 of clear fluid is consistent with a state of fasting, while a volume of ≥1.5 ml kg−1 of clear fluid or solids is consistent with a ‘full stomach’.
-
•
Gastric ultrasound is feasible in obese, pregnant, and paediatric patients.
Learning objectives.
By reading this article you should be able to:
-
•
Explain the indications and limitations of point-of-care gastric ultrasound.
-
•
Describe the acquisition and interpretation of images for qualitative and quantitative analysis of gastric content for the purposes of pulmonary aspiration risk stratification.
-
•
Discuss the role of gastric ultrasound in the management of obese, pregnant, and paediatric patients.
Pulmonary aspiration of gastric contents occurs once every 2000–3000 elective general anaesthetics and is associated with a 20% incidence of in-hospital mortality.1 In patients undergoing surgery, the incidence of pulmonary aspiration is at least three times greater, up to one in 895 general anaesthetics. Indeed, pulmonary aspiration accounts for half of airway-related mortality associated with anaesthesia. In adults, pulmonary aspiration causes significant morbidity including respiratory failure, acute lung injury, and multi-organ failure. Several measures can be used to mitigate the risk and severity of pulmonary aspiration, including prokinetic and antacid drugs, rapid-sequence induction of anaesthesia, and tracheal intubation. However, the single most commonly used measure is appropriate fasting before anaesthesia. Currently accepted guidance in the UK and Europe is for surgical patients to abstain from consuming solid foods for ≥6 h before induction of anaesthesia, and to withhold clear fluids for ≥2 h. Recommendations in North America vary by specifying that a full meal (of fried/fatty food or meat) should be avoided for ≥8 h, while light meals, such as tea and toast, should be not be taken within ≥6 h. However, there is significant variability between individuals in gastric emptying time, and even when these prespecified time-points have been adhered to, the stomach may contain either solid or high-volume liquid in up to 4.5% of patients.2 Furthermore, patients with delayed gastric emptying present a challenge, as fasting guidelines are not directly relevant.
It is useful to qualify and quantify gastric content, volume, and transit time. Several invasive methods are available, including assessment of paracetamol absorption, electrical impedance tomography, radiolabelled diet, polyethylene glycol dilution studies, or suctioning of gastric content via gastric tubes. However, these are both invasive and time-consuming, and are not practical in perioperative practice.
Point-of-care gastric ultrasound
Gastric sonography has been previously investigated in the assessment of gastric motility and emptying, by visualising solid matter in the stomach and comparing it with ingestion times. It has also been used to detect the presence of liquid and solids, and to report the correlation between gastric cross-sectional area (CSA) and fasting times. However, the real-time utility of gastric sonography at the bedside is a novel application.
The increasing value of point-of-care ultrasonography, particularly after its early adoption in emergency care, has allowed focused assessment of the abdomen, lungs, and heart in a rapid, non-invasive, accurate and structured manner to guide clinical practice. Point-of-care gastric ultrasound is an emerging diagnostic tool that allows qualification and quantification of gastric content to aid perioperative clinical decision-making.3 By visualising the contents of the stomach (empty or containing fluid or solids) the risk of pulmonary aspiration can be determined more accurately compared with reliance solely on predefined fasting times. Along with an understanding of the anatomic principles, implementation of gastric ultrasound using the Indication; Acquisition; Interpretation; Medical management (I-AIM framework; Table 1) may be useful for both new and existing practitioners of point-of-care gastric ultrasound.
Table 1.
The I-AIM framework for the performance of point-of-care gastric ultrasound.
| Indication | Uncertain prandial status |
|
| Known or suspected delayed gastric emptying |
|
|
| Acquisition | Device selection |
|
| Patient position |
|
|
| Sonographic imaging |
|
|
| Interpretation | Empty |
|
| Clear fluid |
|
|
| Solid |
|
|
| Medical management | Clinical considerations |
|
| Image analysis |
|
|
| Decision-making |
|
Anatomical concepts
The stomach has five distinct sections: the cardia, fundus, body, antrum, and pylorus (Fig. 1). The gastric antrum is of particular interest as it is easily identifiable on ultrasound in the epigastric region. It is also the more dependent area of the stomach, meaning any gastric content will gravitate towards this region. The antral wall is composed of five distinct layers, which may or may not be clearly visible on ultrasound. These are, from luminal to extra-luminal: mucosa, muscularis mucosae, submucosa, muscularis propriae, and serosa (Fig. 1). The gastric antrum lies posterior and inferior to the medial margin of the left lobe of the liver and anterior to the tail of the pancreas and the aorta and its proximal branches, particularly the superior mesenteric artery.
Fig 1.
Graphical representation of the different gastric sections. A representative cross-section of the five layers of the gastric antrum that can be seen sonographically is demonstrated on the right.
Indications for gastric ultrasound
Point-of-care gastric ultrasound has yet to be incorporated into recommended standards of practice, but its indications are in the clinical scenarios in which prandial status is uncertain, or gastric emptying may be delayed. Uncertainty over prandial status may occur in patients with acute or chronic cognitive dysfunction, language barriers, or those presenting with an unclear history, such as in paediatric patients. Delayed gastric emptying occurs in those with systemic pathologies such as chronic kidney disease or diabetes, or in acute pain states. Parturients, the obese and patients who have been treated with systemic medications that may delay gastric emptying, such as opioids, may also benefit from having gastric ultrasound to guide management of anaesthesia.
Acquisition of images
Device selection
An ultrasound machine that can measure CSA is required. In adults, a curved-array, low-frequency (1–5 MHz) transducer is used, with standard abdominal settings selected. This transducer allows sufficient penetration of the abdominal compartment to produce sonographic images of the key landmarks required. In adults with low body weight or paediatric patients weighing <40 kg, a linear-array, high-frequency (5–12 MHz) transducer may be selected to provide greater resolution of the superficial antrum and surrounding structures. Ultrasound gel is placed on the transducer to act as an acoustic medium. The depth is adjusted according to patient body habitus, and gain adapted to optimise sonographic visualisation of the gastric antrum.
Positioning the patient
The upper abdomen needs to be fully exposed. Both supine and right lateral decubitus (RLD) positions may be used to locate the gastric antrum. In the supine position, large quantities of gastric content will be readily visualised in the gastric antrum, but smaller quantities may remain within the gastric fundus, which is more dependent in this position and difficult to visualise, resulting in under-appreciation of gastric content. In contrast, the RLD position encourages gravitational drainage of gastric content to the dependent antrum, and increases the sensitivity of ultrasound to detect smaller volumes.3, 4 The RLD position is therefore the ideal patient position used to confirm antral content. Although some advocate performing gastric sonography with a patient in the semi-recumbent position, this is less accurate than RLD positioning in quantifying gastric volumes.5 Nonetheless, RLD positioning is impractical in some patients, such as the critically ill, trauma, or pregnant patient, in which case the scanning in the semirecumbent position is a reasonable alternative.6
Sonographic imaging
The gastric antrum is initially imaged with the transducer in a sagittal plane in the epigastric region, immediately below the xiphisternum (Fig. 2).7 By convention, the transducer orientation is such that cephalad is on the left of the screen. The ultrasound transducer is placed perpendicular to the skin, sweeping from the left costal margin seeking the following key sonographic landmarks, from deep to superficial: vertebral bodies, long axis of the abdominal aorta, head or neck of the pancreas, inferior margin of the left lobe of the liver, and the gastric antrum in short axis (Fig. 3). The liver provides an acoustic window to the gastric antrum, which can be distinguished from other hollow viscera such as duodenum or bowel by its thick, hypoechoic muscularis layer along with the hyperechoic serosa and mucosal layers, which are typically 4 mm in thickness (Fig. 3), and superficial anatomical location. It is also the most amenable section of the stomach to sonography, as the fundus is difficult to fully observe, and the body of the stomach often contains air.3, 4, 5, 8 Obtaining the ideal sonographic window may require sliding the transducer from left to right, or right to left, to observe the antrum in short axis at the level of the aorta. Heel-to-toe manoeuvres or transducer rotation can be used to minimise obliquity in antral views.
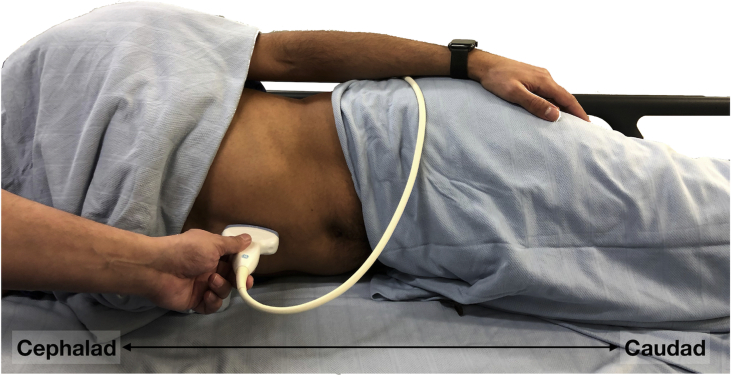
Fig 2.
Patient positioning for performing gastric ultrasound in the RLD position, with the ultrasound transducer placed in the epigastrium beneath the xiphoid process.
Fig 3.
Ultrasound images of an empty gastric antrum, with the key sonographic landmarks identified on the right. SMA, superior mesenteric artery.
Image interpretation
Once the optimal sonographic views are obtained, the practitioner's aim is first to qualify the contents of the gastric antrum. The antrum may be empty, contain variable volumes of fluid, or contain solids.
Empty
When the stomach is empty, it appears small, flat, and collapsed in both the supine and RLD positions. The walls of the stomach can appear relatively thick, and when round or ovoid in shape it has been described as having a ‘bulls-eye’ appearance (Fig. 3).8 It may be possible to observe the multiple hyper- and hypoechoic layers of the gastric antrum when empty. In particular, the muscularis mucosa layer may be prominent when the muscle is in a relaxed state. Occasionally, mucosal folds within the antral lumen can be seen, but the character and size of the antrum should make these folds clearly distinguishable from solids, as the antrum will be small in size and will not contain any moving content. Diagnosis of an empty gastric antrum can only be made in the RLD position after continued observation and is associated with a low risk of pulmonary aspiration.9
Clear fluids
Gastric secretions and clear fluids, such as water, clear juices, or black tea, are anechoic or hypoechoic in appearance.7 In contrast, thick fluids, such as milk or juice containing pulp, appear more echoic and homogenous in nature. When containing fluid, the antrum begins to distend and becomes thin-walled, unlike the thicker-walled appearance in the empty state. The hypoechoic muscularis propriae layer also becomes thinner as the antrum distends. The physical process of swallowing is inevitably associated with swallowing of air as well. Thus, recent consumption of clear fluids or carbonated drinks can produce air bubbles within the fluid that may appear as hyperechoic dots (Fig. 4A and B).10 This is sometimes referred to as a ‘starry night’ appearance.
Fig 4.
Ultrasound images of different antral qualitative appearances. (A) Gastric antrum (A) containing fluid with some air bubbles. (B) Gastric antrum containing fluid with a ‘starry night’ appearance. Antral CSA was calculated with a calliper trace tool (yellow dotted line), and a CSA of 27.34 cm2 was quantified. (C) Gastric antrum after recent ingestion of solids, with a ‘frosted glass’ appearance. The anterior antral wall is visible, but there are no clear structures seen deep to the anterior antral wall. (D) Gastric antrum containing solids, with heterogeneous echogenicity representing different consistency of solids consumed. Note the thin, hypoechoic muscularis propriae compared to Fig 3. SMA, superior mesenteric artery.
Solid
Chewing and swallowing of solid matter are invariably associated with the ingestion of air. In the early stages after solid consumption, this air prevents sonographic visualisation of deeper structures and creates a ‘frosted glass’ curtain-like image from the mucosal-air interface of the anterior antral wall (Fig. 4C). No structures deep to the anterior wall of the antrum are seen. After this initial stage, the air in the antrum becomes either absorbed or displaced, and solids appear hyperechoic with a heterogeneous consistency within a distended antrum (Fig. 4D). The full circumference of the antrum will be visible, peristalsis might be apparent, and the movement of particulate matter within the antrum can be observed.9 Thicker fluids such as milk or yoghurt appear more homogeneous and hyperechoic. On occasion, a biphasic appearance of a hyperechoic and a hypoechoic area may be seen as a result of curdling of dairy products when mixed with gastric acid.
Quantitative assessment
Two-thirds of fasted patients have an empty gastric antrum, but a low volume (<1.5 ml kg−1) of hypoechoic gastric secretions is also a normal finding in fasted patients. Therefore, quantifying the volume of clear fluid can help with risk stratification. The purpose of volume assessment is to determine whether the volume present is consistent with a baseline fasting state or is likely caused by the ingestion of fluids. This is achieved by determining the antral CSA in the RLD position, as this is the most sensitive position for observing changes in gastric volume.3 Careful observation of the antrum for peristaltic contractions is valuable, as measurements are most sensitive when taken between contractions when the antrum is at its largest. Freezing the image with the ideal sonographic end-points is followed by determining the antral CSA using the trace calliper function and tracing the entire circumference following the serosal layer (Fig. 4B). A mean of three readings is then calculated. There are several models that correlate antral CSA to the volume of fluids. The most statistically robust model that demonstrates high reliability within and between observers was developed by performing endoscopic suctioning of gastric fluid in the process of mathematical modelling.4, 5, 11 It is calculated as follows:
| Gastric volume (ml)=27+(14.6×RLD-CSA)–(1.28×age [yrs]). | (1) |
Risk stratification
If an empty, solid-containing or thick fluid-containing gastric antrum is seen in the RLD position, no further assessment is required. An empty antrum represents a low risk of pulmonary aspiration of gastric content, as there is no visible content. An antrum that contains solids, particulate matter, or thick fluid is inconsistent with a fasting state and is likely to represent a higher-than-baseline risk of pulmonary aspiration, regardless of the quantity of solid content. However, quantification of gastric content is important in the presence of clear fluid, as a low volume of clear fluid is appreciable with ultrasound in half of all fasted patients.12 Despite some controversy, the most accepted upper limit of normal for gastric secretion or clear fluid content is 1.5 ml kg−1 of actual body weight, or approximately 100–130 ml in the average adult. This volume correlates with the 95th centile for fasted elective surgical (obstetric or non-obstetric) patients.4, 5, 12, 13 Therefore, in the presence of antral fluid, a volume <1.5 ml kg−1 may be consistent with baseline gastric secretions and likely carries a low risk of pulmonary aspiration. However, a volume of clear fluid ≥1.5 ml kg−1 is rarely seen in the fasting state and likely suggests recent ingestion of fluids or delayed emptying, possibly carrying a higher than baseline risk. Quantification of gastric fluid is therefore imperative for risk stratification, particularly in the emergency patient in whom prandial status is often questionable.14
An alternative qualitative approach to risk stratification may also be used. Observing an empty gastric antrum in both the supine and RLD positions suggests minimal volume and low risk of aspiration; it is therefore classified as a Grade 0 antrum and is present in 45–50% of fasting elective surgical subjects.4 An antrum that appears empty in the supine position, but contains clear fluids in the RLD position, correlates with an antral volume of <1.5 ml kg−1, and is classed as a low-risk Grade 1 antrum. About 45–50% of fasting elective surgical subjects have a Grade 1 antrum.4 Finally, the presence of fluid in both the supine and RLD positions likely correlates with a volume of ≥1.5 ml kg−1 and is categorised as a Grade 2 antrum (Fig. 4A and B). A Grade 2 antrum is uncommon but possible in fasting individuals. About 3–5% of fasting elective surgical subjects have a Grade 2 antrum.4
Implications for clinical practice
With an appropriate medical history, physical examination, and gastric ultrasound-assisted risk assessment, anaesthetic management can be appropriately tailored for individual patients. Gastric ultrasound should be viewed as an adjunctive tool to increase the margin of safety when performing anaesthetic interventions, with high sensitivity (1.0), specificity (0.975), positive predictive value (0.976), and negative predictive value (1.0) in the presence of clinical equipoise.15 Inexperienced point-of-care gastric sonographers have been found to require 33 supervised scans to achieve a 95% accuracy in qualitative assessment of gastric content.16 In conjunction with considering the urgency of surgery, medical factors, and alternative options for anaesthesia, gastric ultrasound has been shown to lead to changes in anaesthetic management.17 Armed with accurate sonographic findings, anaesthetists may decide to delay or cancel surgery, or proceed with surgery but modify the anaesthetic technique, such as using rapid-sequence induction, tracheal intubation, or regional anaesthesia.
Specific patient cohorts
Obesity
Patients who are obese feature highly in cases of airway complications, with aspiration of gastric contents being the main complication. Obesity is also associated with multiple comorbidities, some of which might delay gastric emptying.18, 19 Obese patients may benefit from a tailored approach to anaesthesia using gastric sonography to reduce the risk of pulmonary aspiration. Despite the perceived increased technical challenge, visualisation of the antrum is feasible in the RLD position, and can be achieved in >90% of patients with a BMI of >40 kg m−2 despite a depth of >7 cm.17 Fasted obese patients tend to have a higher antral CSA than non-obese subjects, but quantification of gastric fluid volume per unit of body weight is similar. Moreover, the mathematical model used in non-obese subjects to quantify fluid volume performs well in subjects with a BMI of >35 kg m−2.20 The key differences in gastric sonography of obese patients is the increased depth at which the antrum is found, with a greater quantity of tissue superficial to the muscles of the anterior abdominal wall.
Pregnancy
Performing a gastric ultrasound on the parturient poses technical challenges. The gravid uterus displaces visceral organs cephalad and to the right, which makes antral identification more difficult. Tachypnoea of pregnancy and hyperdynamic circulatory changes might make obtaining adequate sonographic windows more challenging. Finally, the position and placement of the transducer can be more testing because of the limited space between the xiphisternum and the gravid abdomen.7 Despite these challenges, consistency has been shown with gastric ultrasound assessment in third trimester parturients with good feasibility and reproducibility for the detection of gastric contents.21 The antrum remains at a similar depth, or may be found deeper than that of the non-pregnant patient. Gastric sonography may be aided in pregnant patients by sitting in a semirecumbent position, manually displacing the uterus, and asking the patient to hold their breath in end-expiration for optimal sonographic windows. Similar to the non-pregnant adult, an empty antrum in both the sitting and RLD position is classified as a low-risk antrum (Grade 0), and the presence of solids in either position is a high-risk antrum. However, the mathematical modelling and quantification of fluid volumes remain debated. It is thought that distinguishing a Grade 1 and Grade 2 gastric antrum may be achieved by observing an antral CSA of ≥9.6 cm2 in the semi-recumbent RLD position, which has been demonstrated as the discriminating correlator of a volume ≥1.5 ml kg−1.6
Paediatrics
Acquisition of sonographic images for qualitative and quantitative assessment of antral contents in paediatric patients is easily achieved. With high-frequency linear transducers and the superficial location of the stomach (antral depth is usually fewer than a few centimetres), the five layers of the gastric antrum are readily seen, and content recognisable. Moreover, the linearity of antral volumes with CSA measured in the RLD position is comparable in paediatric patients as it is in adults, and may similarly influence anaesthetic management in a variety of settings.22, 23, 24 The following mathematical model, however, has only been validated for low fasting volumes of up to 1.5 ml kg−1 in patients between 1 and 18 years of age:25
| Gastric volume (ml)=−7.8+(3.5×RLD-CSA)+(0.127)×age (months). | (2) |
Limitations
Gastric ultrasound is associated with few limitations, but these must be considered when modifying medical management accordingly. Sonography can be inconclusive in 2–3% of subjects, even with adequate technique and experienced sonographers. This can be caused by anatomical variation, misinterpretation of other hollow viscus structures, or the presence of air in nearby structures such as the bowel, obscuring antral views. Moreover, sonographic findings may not be accurate or reliable in patients who have had previous gastric surgery, and those with a large hiatus hernia. The risks of inaccurate sonography are especially high in patients with a large hiatus hernia, because ingested food or fluid may be localised in the herniated portion of the stomach that is not accessible to ultrasound examination, leading to an underappreciation of gastric content and risk. Finally, gastric ultrasound has traditionally only been reported before induction of anaesthesia, but its role before tracheal extubation has not been examined fully.
Conclusions
Point-of-care gastric ultrasound is an emerging tool that is simple, painless, non-invasive and quick. It can be used to examine gastric contents in adult, obese, pregnant, and paediatric patients. It is reliable, replicable, and can be used to influence anaesthetic management. Gastric ultrasound is an invaluable technique to complement the use of fasting guidelines, particularly when these guidelines have not been followed, or may not be relevant. Further research is required to determine the influence of point-of-care gastric sonography on perioperative outcomes.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Kariem El-Boghdadly BSc MSc FRCA EDRA is a consultant anaesthetist at Guy's and St Thomas' NHS Foundation Trust London. Dr El-Boghdadly is a leading figure in teaching gastric ultrasound, is on the editorial board of Anaesthesia, and is the editor of Anaesthesia Reports.
Thomas Wojcikiewicz BSc MRCP FRCA is a specialty registrar in anaesthesia at Guy's and St Thomas' NHS Foundation Trust, London. He has undertaken fellowships in regional, thoracic, and bariatric anaesthesia and is a committee member of the Association of Anaesthetists Trainees.
Anahi Perlas FRCPC is professor of anaesthesia at the University of Toronto and a faculty member at Toronto Western Hospital, who has been prominent in the development of gastric ultrasound. Professor Perlas is on the editorial board of Regional Anesthesia & Pain Medicine and is a board member of the American Society of Regional Anesthesia.
Matrix codes: 1A03, 2A03, 3A09
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2019.03.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Engelhardt T., Webster N.R. Pulmonary aspiration of gastric contents in anaesthesia. Br J Anaesth. 1999;83:453–460. doi: 10.1093/bja/83.3.453. [DOI] [PubMed] [Google Scholar]
- 2.Van De Putte P., Vernieuwe L., Jerjir A., Verschueren L., Tacken M., Perlas A. When fasted is not empty: a retrospective cohort study of gastric content in fasted surgical patients. Br J Anaesth. 2017;118:363–371. doi: 10.1093/bja/aew435. [DOI] [PubMed] [Google Scholar]
- 3.Perlas A., Chan V.W.S., Lupu C.M., Mitsakakis N., Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111:82–89. doi: 10.1097/ALN.0b013e3181a97250. [DOI] [PubMed] [Google Scholar]
- 4.Perlas A., Mitsakakis N., Liu L. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357–363. doi: 10.1213/ANE.0b013e318274fc19. [DOI] [PubMed] [Google Scholar]
- 5.Van De Putte P., Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth. 2014;113:12–22. doi: 10.1093/bja/aeu151. [DOI] [PubMed] [Google Scholar]
- 6.Arzola C., Perlas A., Siddiqui N.T., Downey K., Ye X.Y., Carvalho J.C.A. Gastric ultrasound in the third trimester of pregnancy: a randomised controlled trial to develop a predictive model of volume assessment. Anaesthesia. 2018;73:295–303. doi: 10.1111/anae.14131. [DOI] [PubMed] [Google Scholar]
- 7.Perlas A., Arzola C., Van de Putte P. Point-of-care gastric ultrasound and aspiration risk assessment: a narrative review. Can J Anesth. 2017;65:437–448. doi: 10.1007/s12630-017-1031-9. [DOI] [PubMed] [Google Scholar]
- 8.Cubillos J., Tse C., Chan V.W.S., Perlas A. Bedside ultrasound assessment of gastric content: an observational study. Can J Anesth. 2012;59:416–423. doi: 10.1007/s12630-011-9661-9. [DOI] [PubMed] [Google Scholar]
- 9.El-Boghdadly K., Kruisselbrink R., Chan V.W.S., Perlas A. Images in anesthesiology: gastric ultrasound. Anesthesiology. 2016;125:595. doi: 10.1097/ALN.0000000000001043. [DOI] [PubMed] [Google Scholar]
- 10.Haskins S.C., Tanaka C.Y., Boublik J., Wu C.L., Sloth E. Gastric ultrasound for the regional anesthesiologist and pain specialist. Reg Anesth Pain Med. 2017;42:632–644. doi: 10.1097/AAP.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 11.Kruisselbrink R., Arzola C., Endersby R., Tse C., Chan V., Perlas A. Intra- and interrater reliability of ultrasound assessment of gastric volume. Anesthesiology. 2014;121:46–51. doi: 10.1097/ALN.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 12.Perlas A., Davis L., Khan M., Mitsakakis N., Chan V.W.S. Gastric sonography in the fasted surgical patient: a prospective descriptive study. Anesth Analg. 2011;113:93–97. doi: 10.1213/ANE.0b013e31821b98c0. [DOI] [PubMed] [Google Scholar]
- 13.Perlas A., Van De Putte P., Van Houwe P., Chan V.W.S. I-AIM framework for point-of-care gastric ultrasound. Br J Anaesth. 2016;116:7–11. doi: 10.1093/bja/aev113. [DOI] [PubMed] [Google Scholar]
- 14.Dupont G., Gavory J., Lambert P. Ultrasonographic gastric volume before unplanned surgery. Anaesthesia. 2017;72:1112–1116. doi: 10.1111/anae.13963. [DOI] [PubMed] [Google Scholar]
- 15.Kruisselbrink R., Gharapetian A., Chaparro L.E. Diagnostic accuracy of point-of-care gastric ultrasound. Anesth Analg. 2019;128:89–95. doi: 10.1213/ANE.0000000000003372. [DOI] [PubMed] [Google Scholar]
- 16.Arzola C., Carvalho J.C., Cubillos J., Ye X.Y., Perlas A. Anesthesiologists’ learning curves for bedside qualitative ultrasound assessment of gastric content: a cohort study. Can J Anesth. 2013;60:771–779. doi: 10.1007/s12630-013-9974-y. [DOI] [PubMed] [Google Scholar]
- 17.Alakkad H., Kruisselbrink R., Chin K.J. Point-of-care ultrasound defines gastric content and changes the anesthetic management of elective surgical patients who have not followed fasting instructions: a prospective case series. Can J Anesth. 2015;62:1188–1195. doi: 10.1007/s12630-015-0449-1. [DOI] [PubMed] [Google Scholar]
- 18.Cook T.M., Woodall N., Frerk C. Fourth national audit project. Major complications of airway management in the UK: results of the fourth national audit project of the royal college of anaesthetists and the difficult airway society. Part 1: anaesthesia. Br J Anaesth. 2011;106:617–631. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 19.Nightingale C.E., Margarson M.P. Peri-operative management of the obese surgical patient 2015: Association of Anaesthetists of Great Britain and Ireland society for obesity and bariatric anaesthesia. Anaesthesia. 2015;70:859–876. doi: 10.1111/anae.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruisselbrink R., Arzola C., Jackson T., Okrainec A., Chan V., Perlas A. Ultrasound assessment of gastric volume in severely obese individuals: a validation study. Br J Anaesth. 2017;118:77–82. doi: 10.1093/bja/aew400. [DOI] [PubMed] [Google Scholar]
- 21.Arzola C., Cubillos J., Perlas A., Downey K., Carvalho J.C. Interrater reliability of qualitative ultrasound assessment of gastric content in the third trimester of pregnancy. Br J Anaesth. 2014;113:1018–1023. doi: 10.1093/bja/aeu257. [DOI] [PubMed] [Google Scholar]
- 22.Gagey A.C., de Queiroz Siqueira M., Monard C. The effect of pre-operative gastric ultrasound examination on the choice of general anaesthetic induction technique for non-elective paediatric surgery. A prospective cohort study. Anaesthesia. 2018;73:304–312. doi: 10.1111/anae.14179. [DOI] [PubMed] [Google Scholar]
- 23.Gagey A.C., de Queiroz Siqueira M., Desgranges F.P. Ultrasound assessment of the gastric contents for the guidance of the anaesthetic strategy in infants with hypertrophic pyloric stenosis: a prospective cohort study. Br J Anaesth. 2016;116:649–654. doi: 10.1093/bja/aew070. [DOI] [PubMed] [Google Scholar]
- 24.Desgranges F.-P., Gagey Riegel A.-C., Aubergy C., de Queiroz Siqueira M., Chassard D., Bouvet L. Ultrasound assessment of gastric contents in children undergoing elective ear, nose and throat surgery: a prospective cohort study. Anaesthesia. 2017;72:1351–1356. doi: 10.1111/anae.14010. [DOI] [PubMed] [Google Scholar]
- 25.Spencer A.O., Walker A.M., Yeung A.K. Ultrasound assessment of gastric volume in the fasted pediatric patient undergoing upper gastrointestinal endoscopy: development of a predictive model using endoscopically suctioned volumes. Paediatr Anaesth. 2015;25:301–308. doi: 10.1111/pan.12581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.