Learning objectives.
By reading this article you should be able to:
-
•
Explain the difference between reconstruction and windowing of CT images.
-
•
Recall common indications for CT head and specific indications where post-contrast imaging, CT angiogram (CTA) or CT venography (CTV) might be required.
-
•
Remember the basic anatomy and the suggested order for reviewing a CT head scan.
-
•
Describe the radiological appearance of common pathologies and distinguish the generic signs of mass effect from those of volume loss.
Key points.
-
•
Full review of a CT head scan requires use of brain, bone, and soft tissue windows.
-
•
Post-contrast imaging may add value in intracranial infection and malignant disease.
-
•
Review scans systematically from outside to in.
-
•
Acute haemorrhage is denser than brain tissue and appears relatively bright but the density reduces over time.
-
•
The effect of pathology on the surrounding tissues (mass effect or volume loss) is often important in detection, diagnosis, and management.
CT of the head revolutionised diagnosis of intracranial pathology in the 1970s and subsequently replaced plain radiographs, air encephalography, and even angiography for many indications. CT is often pivotal in the diagnosis of acute intracranial pathology, and scanners are now ubiquitous in developed countries with 24 h access the norm. As such, a good basic understanding of the modality, its applications, and interpretation is extremely valuable to anaesthetists and intensivists.
Basic principles
The basic physics and the risks of CT are explained in a previous article in this journal.1 In addition a few relevant factors are highlighted here.
Most modern scanners have detectors and X-ray tubes that spin or surround the patient as the bed moves through the scanner, thereby imaging the desired volume of tissue (i.e. the whole head) in a single spiral acquisition. The information acquired may then be reconstructed to produce 2D greyscale images of slices through the patient, which are effectively maps of tissue density: high-density tissue such as bone appearing white and low densities appearing darker. It is also possible to produce 3D surface rendered images.
Reconstruction is performed by the radiographer who will vary the technique depending on the indication. Brain and/or soft tissue algorithms are used when producing images for assessment of the brain and soft tissues and should always be available on the picture archiving and communication system (PACS), at least as axial slices. Bone algorithms use edge enhancement to better depict bone and are usually produced and sent to PACS for trauma patients or where bone pathology is suspected. Slice thickness can also be varied with thick slices (e.g. 5 mm) providing better tissue contrast, useful for example in assessing grey/white differentiation, and thin slices (e.g. 0.5–1.25 mm) providing greater spatial resolution and therefore detail.
‘Windowing’ is performed by the clinician reviewing the scan from PACS and involves altering the way the greyscale is applied across the range of densities in the image, in order to allow assessment of the different tissues. Brain, bone, and soft tissue windows should all be used when reviewing a CT head scan.
Cumulative radiation to the lens of the eye over time produces cataracts and is an important consideration when scanning the head. The scan plane is usually parallel to the skull base and should commence above the level of the lens. Where assessment of the orbit is required or images are to be used for neurosurgical navigation, the scanning protocol is altered.
Indications
The indications for head CT are numerous, but a number of the more common are given in Table 1, which is based on the American College of Radiology guidance.2 The National Institute for Health and Care Excellence (NICE) head injury guideline provides a detailed list of indications for adults and children in the context of trauma.3
Table 1.
Common indications for CT scanning of the head, based on guidance from the American College of Radiology2 and NICE.3
| Indications |
|---|
| Acute head injury (as per NICE guidance3) |
| Acute neurological deficit |
| Acute severe headache |
| Suspected ischaemic stroke |
| Suspected intracranial haemorrhage |
| Suspected intracranial infection∗ |
| Suspected hydrocephalus |
| Suspected shunt malfunction |
| Suspected raised intracranial pressure |
| Suspected intracranial mass∗ |
| Assessment of bony lesions of the skull |
| CTA for suspected vascular abnormalities, e.g. aneurysms or AVMs |
| CTV for suspected venous sinus thrombosis |
AVM, arteriovenous malformation; CTA, CT angiogram; CTV, CT venogram; NICE, National Institute for Health and Care Excellence.
Indications where standard post-contrast imaging may be useful.
Normal anatomy and review methodology
By convention, axial images are displayed as if looking from the patient's feet towards the head; hence, the right cerebral hemisphere is on the left of the image.
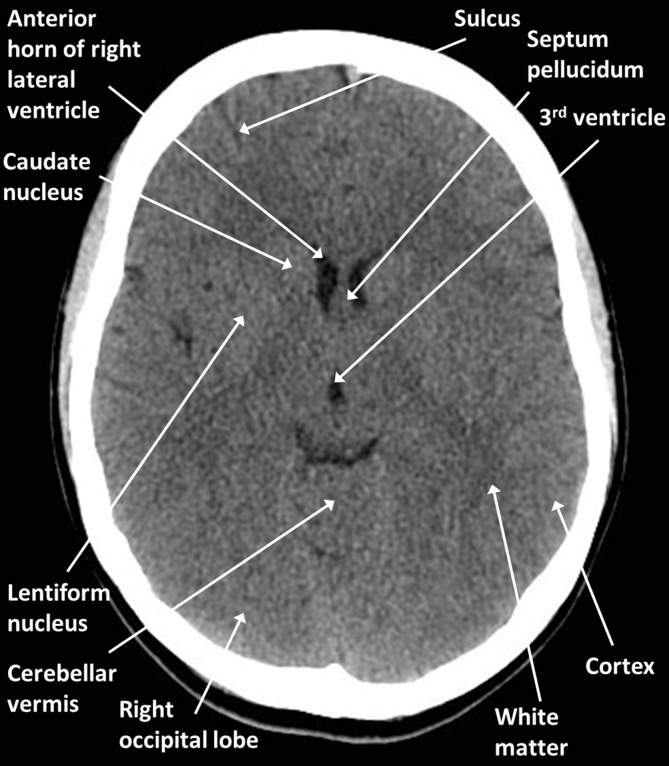
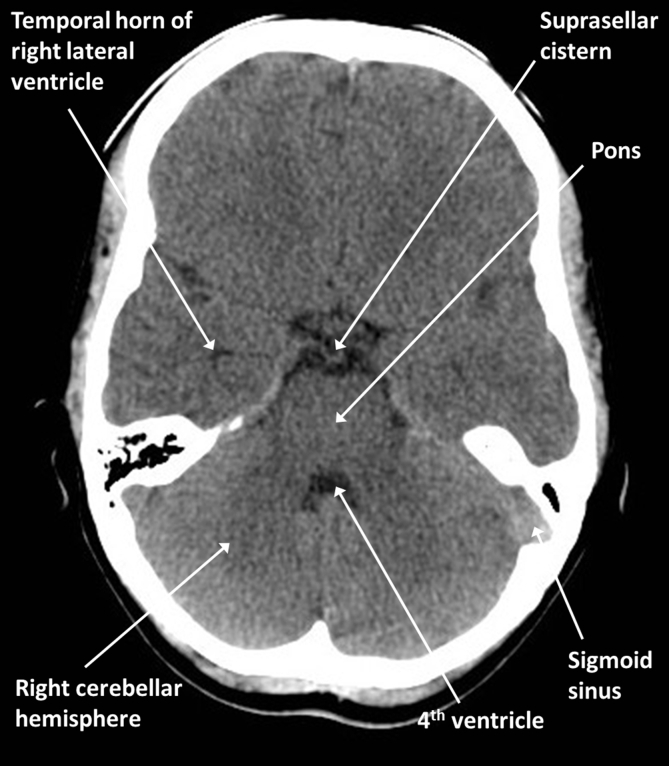
Fig 1, Fig 2 are brain optimised slices through a normal young adult brain. Familiarise yourself with the positions of the labelled structures taking particular note of the midline structures and CSF spaces. Note that normal scans should be relatively symmetrical and that the cortex and deep grey nuclei can be distinguished from the white matter because of their slightly higher density.
Fig 1.
Brain anatomy: axial CT at the level of the third ventricle.
Fig 2.
Brain anatomy: axial CT at the level of the basal cisterns.
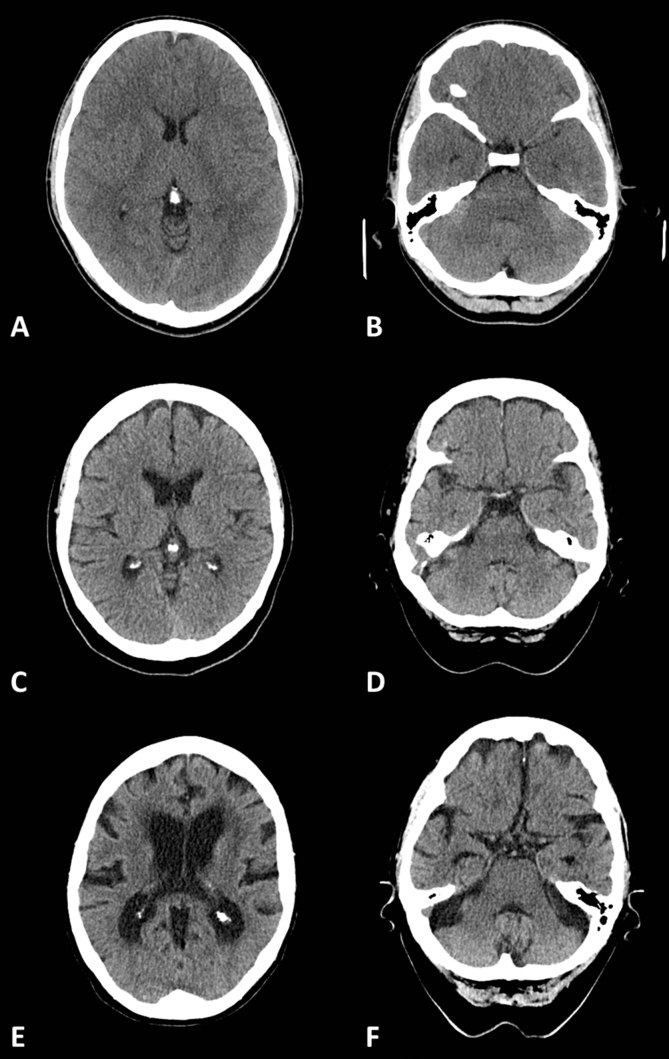
When an abnormality is found it is important to assess the effect it has on the surrounding tissue: with volume loss, normal structures will move towards the abnormality and sulci enlarge, whereas mass effect will push structures away and sulci and ventricles will become effaced. The reader should bear in mind that the brain atrophies with normal aging and the sulci and ventricles will therefore be larger in older patients (Fig. 3). In normal younger patients, few sulci may be visible; however, the third ventricle should be seen, at least as a thin slit, and a few sulci are generally visible towards the vertex.
Fig 3.
Brain atrophy with normal ageing: (A, B) 20 yr old; (C, D) 60 yr old; and (E, F) 90 yr old.
When starting out, a systematic approach is key and the authors advise working your way from outside to in, regardless of the indication (Table 2). However, the clinical context is vital and you should pay extra attention to specific areas as appropriate.
Table 2.
Suggested order of review for CT scans of the head
| Order | Window setting | Structures to examine |
|---|---|---|
| 1 | Soft tissue | Scalp, upper neck and orbital soft tissues (if included) |
| 2 | Bone | Calvarium, skull base, paranasal sinuses, mastoids, external/middle/inner ears |
| 3 | Brain | Extra-axial CSF spaces (sulci/basal cisterns) and potential spaces (subdural/extradural) |
| 4 | Brain | Cerebral hemispheres, deep grey nuclei, cerebellum, brainstem |
| 5 | Brain | Ventricles (lateral/third/fourth) |
Pathology
A note on mass effect, secondary herniation, and intracranial pressure
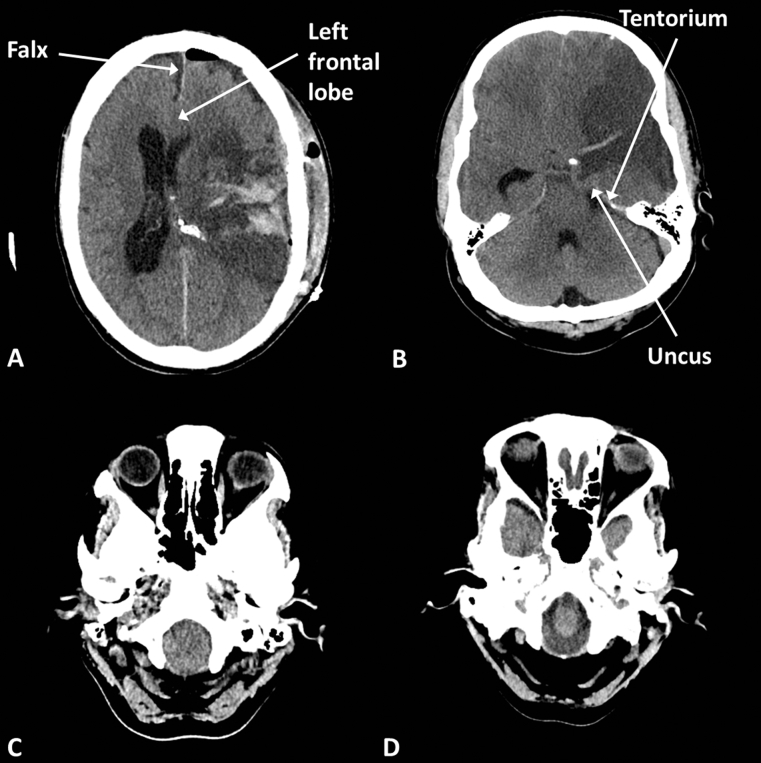
Most acute pathology will result in mass effect as described above. The sulci, ventricles, and basal cisterns may efface because of brain oedema or displacement by a lesion (Fig. 4). With significant mass effect from any cause brain structures may herniate between compartments, for example subfalcine herniation where the medial aspect of a cerebral hemisphere herniates beneath the falx from one side to the other or uncal herniation where the medial aspect of the temporal lobe herniates over the edge of the tentorium into the infratentorial compartment. Herniation potentially endangers specific neurovascular structures and brain tissue, depending on the particular site.
Fig 4.
Brain herniations. (A) Postoperative haematoma causing subfalcine herniation, the left frontal lobe bulges beneath the falx; (B) left middle cerebral artery (MCA) infarct (notice the dense vessel and loss of grey/white differentiation) causing uncal herniation, the left uncus bulges over the tentorium; (C) tonsillar herniation, the cerebellar tonsils bulge into the foramen magnum; and (D) normal foramen magnum with CSF visible around the medulla.
Important signs of subtle swelling or mass effect are loss (effacement) of the third ventricle and loss (effacement) of the sulci towards the vertex. Importantly, although raised intracranial pressure may be inferred from evidence of mass effect on CT imaging, a normal scan does not mean that the pressure is normal and raised pressure cannot be ruled out on imaging.
Trauma
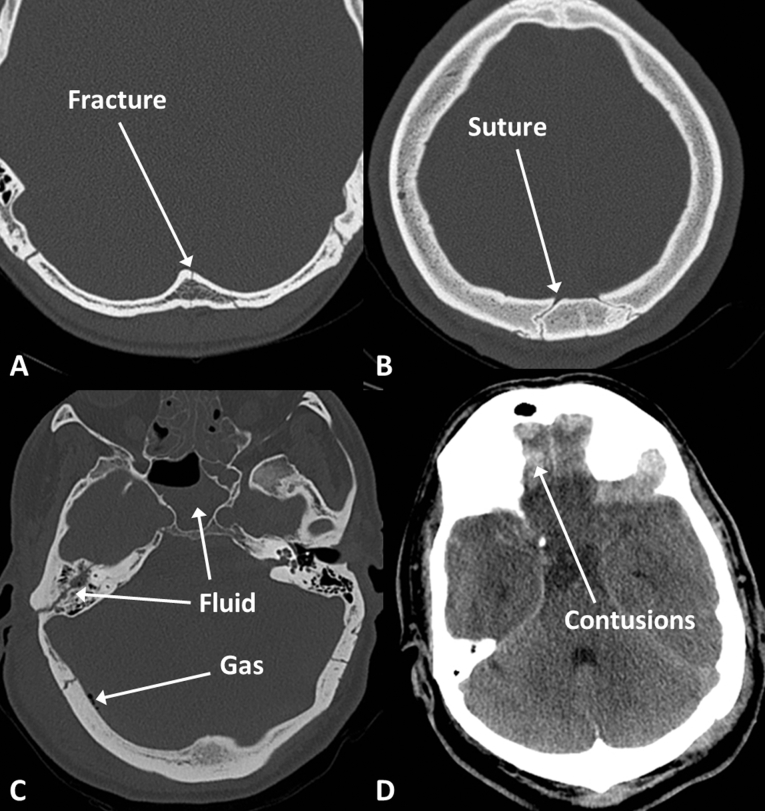
Scalp haematomas are relatively dense and alter the contour of the skin. Fractures are often straight lucent lines through the inner and outer tables of the skull with exposed marrow on either side, as opposed to sutures, which are usually wavy lines with sclerotic margins (Fig. 5). Skull base fractures may involve the paranasal sinuses, mastoids, or middle ear resulting in anatomically predictable haemorrhage, fluid levels, and pneumocephalus.
Fig 5.
Fractures and contusions. (A) Linear fracture; (B) suture line; (C) fluid in the mastoid air cells and sphenoid sinus suggesting skull base fracture, pneumocephalus indicated by gas density within the cranium; and (D) frontal contusions indicated by high density within the brain parenchyma.
Injuries may be found at the site of initial impact (coup) and on the opposite side of the head where the brain may impact the skull after rebounding from the initial impact (contrecoup).
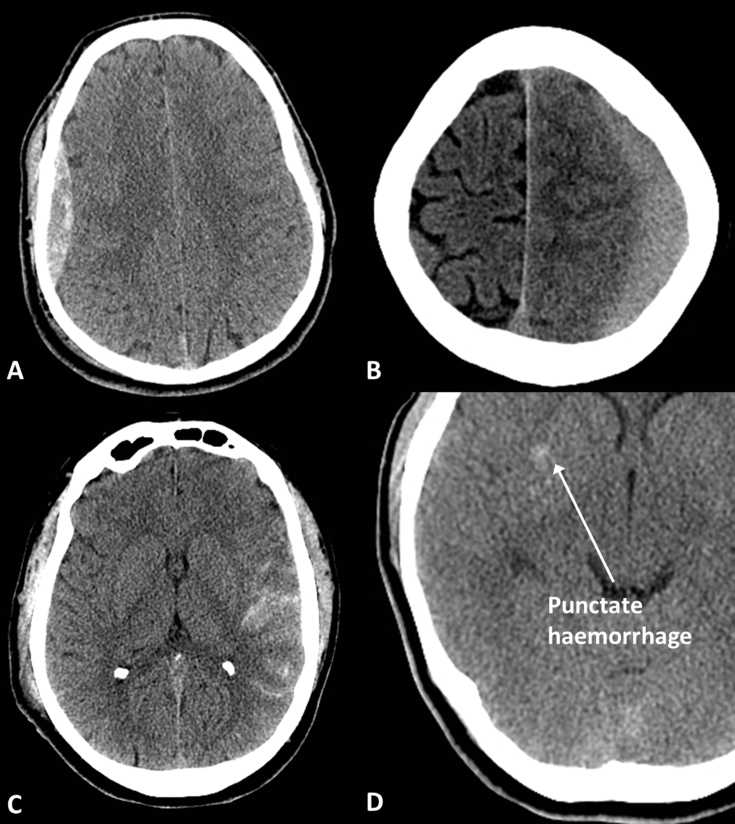
Acute extra-axial haematomas are dense areas outside the brain parenchyma which may be (Fig. 6):
-
(i)
Extradural—often associated with a fracture, lens shaped, will not cross the coronal or lambdoid sutures but can cross the midline
-
(ii)
Subdural—crescent shaped, can cross sutures but will not cross the midline and may extend along the falx or tentorium
-
(iii)
Subarachnoid—extends into the sulci, basal cisterns and may reflux into the ventricles.
Fig 6.
Traumatic haemorrhage. (A) Extradural haematoma; (B) subdural haematoma; (C) subarachnoid haemorrhage; and (D) subtle punctate haemorrhage indicating diffuse axonal injury.
Trauma-associated intra-axial haemorrhage:
-
(i)
Contusion—haemorrhage within gyri at sites of impact with the overlying bone or dura. Often found in the inferior frontal lobes, anterolateral temporal lobes and parasagittal frontoparietal lobes (Fig. 5).
-
(ii)
Diffuse axonal injury—often subtle, punctate haemorrhage at the grey/white matter junction, within the corpus callosum or brainstem. Related to significant acceleration/deceleration injury and axon damage caused by shear forces. The extent of brain injury is often underestimated by CT but the patient will often have a low Glasgow Coma Scale (GCS) and proceed to MRI, which depicts the far more extensive injury.
Note that acute haemorrhage, regardless of anatomical site, is relatively dense compared with brain tissue, but with time the density decreases such that it becomes isodense (usually within 2–3 weeks, depending on size) to brain in subacute haematomas and less dense than brain in chronic haematomas (>4 weeks old, depending on size). Other causes of hyperdensity include calcification and metallic implants or foreign bodies.
Non-traumatic haemorrhage
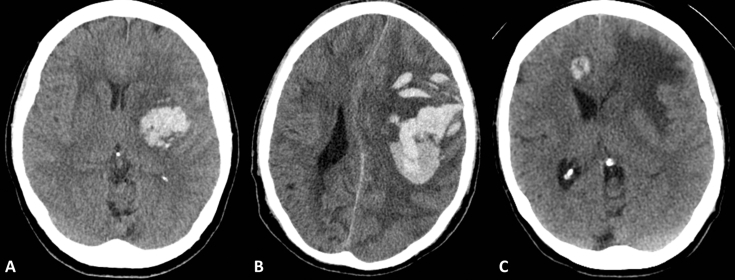
Common causes of intra-axial haemorrhage in older patients include hypertension, amyloid angiopathy, and metastatic disease (Fig. 7). Other causes include vascular malformations and illicit drug use which are more common in younger patients and primary brain tumours.
Fig 7.
Non-traumatic haemorrhage. (A) Hypertensive basal ganglia haemorrhage; (B) lobar haemorrhage in amyloid angiopathy; and (C) well-defined haemorrhagic metastasis in right frontal lobe; notice the low density (oedema) in the left frontal lobe which was related to another lesion on a lower slice.
Hypertensive haemorrhages commonly affect the basal ganglia, thalami, pons, and cerebellum, whereas haemorrhage related to amyloid angiopathy is more commonly peripheral or lobar and may be associated with sulcal subarachnoid haemorrhage.
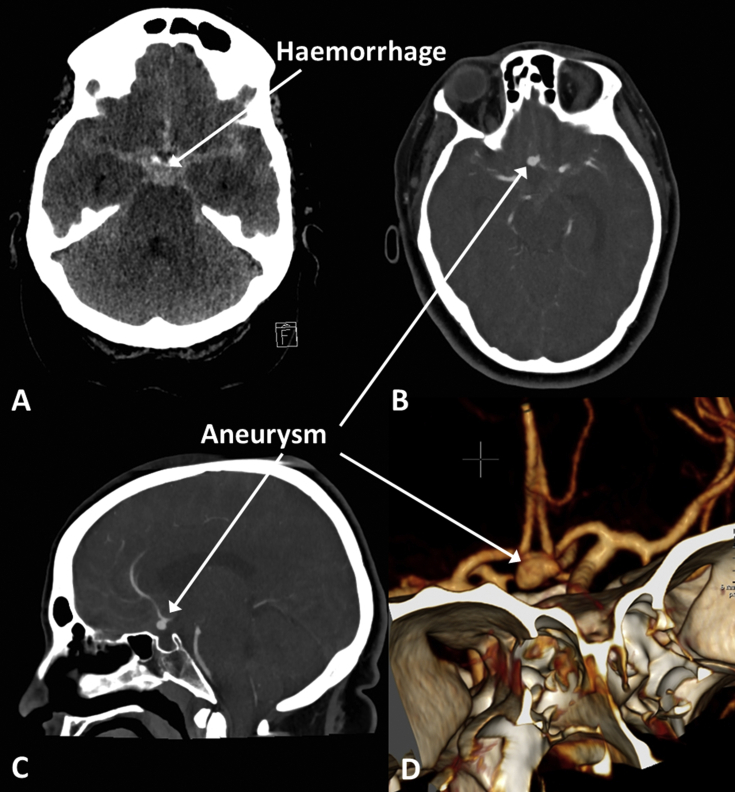
Spontaneous subarachnoid haemorrhage, particularly when extensive, is often the result of a ruptured aneurysm (Fig. 8). High-density material fills the basal cisterns and sulcal spaces which usually contain low-density CSF, and hydrocephalus ensues. A CT angiogram (CTA) requires intravenous injection of a contrast agent and is timed such that the intracranial arteries are opacified during the scan. Aneurysms are usually detected with CTA, but catheter angiography may be required for further evaluation and an endovascular approach is often used in treatment.
Fig 8.
Aneurysmal subarachnoid haemorrhage. (A) High-density acute blood fills the basal cisterns; (B) axial CT angiogram (CTA) showing anterior communicating artery (Acom) aneurysm; (C) sagittal CTA showing Acom aneurysm; and (D) 3D surface rendered reconstruction of CTA showing Acom aneurysm.
Other causes of spontaneous subarachnoid haemorrhage generally produce small volumes of sulcal haemorrhage and include amyloid angiopathy, cortical vein thrombosis, reversible cerebral vasoconstriction syndrome, and vasculitis.
Infarction
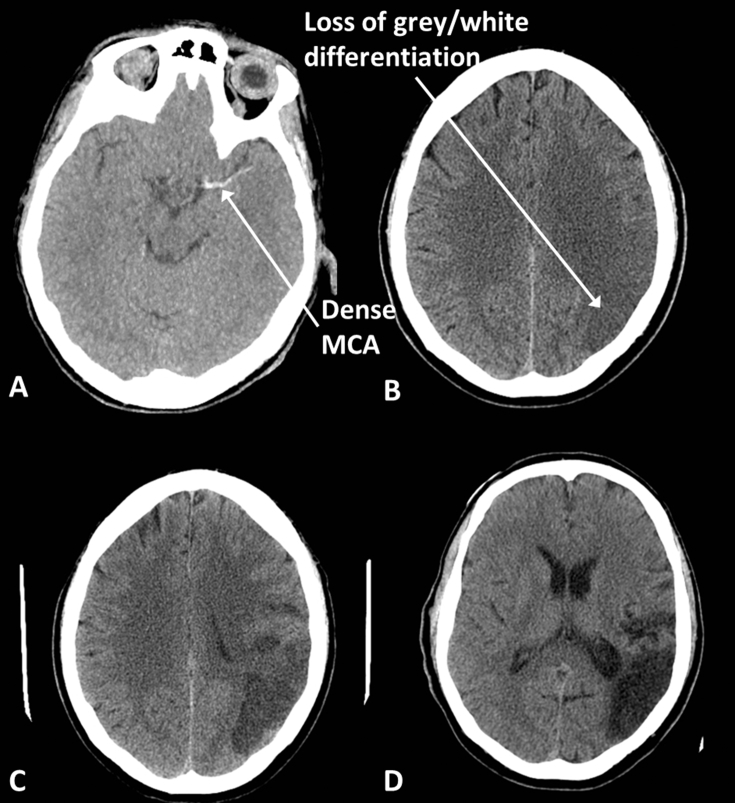
The earliest signs of infarction (potentially visible within the first few hours) are loss of the distinction between grey and white matter and swelling within the affected arterial territory (Fig. 9). Haemorrhagic transformation can take place resulting in high-density foci within the area of infarction. If a large vessel is thrombosed, the vessel itself may appear hyperdense. With time the density of the infarcted tissue reduces and the infarct is usually more obvious by 12 h. At the chronic stage (>3 weeks) there will be a dark area with evidence of volume loss.
Fig 9.
Middle cerebral artery (MCA) infarction. (A) Acute thrombus within the MCA appears dense. (B) Early infarcts, here 3 h after symptom onset in a different patient to (A), are seen as loss of grey/white differentiation and swelling; notice that fewer sulci are visible on the left compared with the right. (C) With time the infarcted tissue becomes less dense and more obvious, here at 36 h. (D) Chronic infarcts are black and associated with volume loss; note the enlargement of the sulci and occipital horn of the lateral ventricle.
Malignant infarction describes infarcts involving large areas of the brain, for example the entire middle cerebral artery (MCA) territory or combined MCA and anterior cerebral artery (ACA) territories resulting in significant mass effect and herniation. Younger patients and those whose brains have not undergone significant atrophy are at greater risk, as there is proportionally less extra-axial space available to accommodate the swelling. Decompressive craniectomy may be beneficial within the first 48 h after onset.4
Venous sinus thrombosis
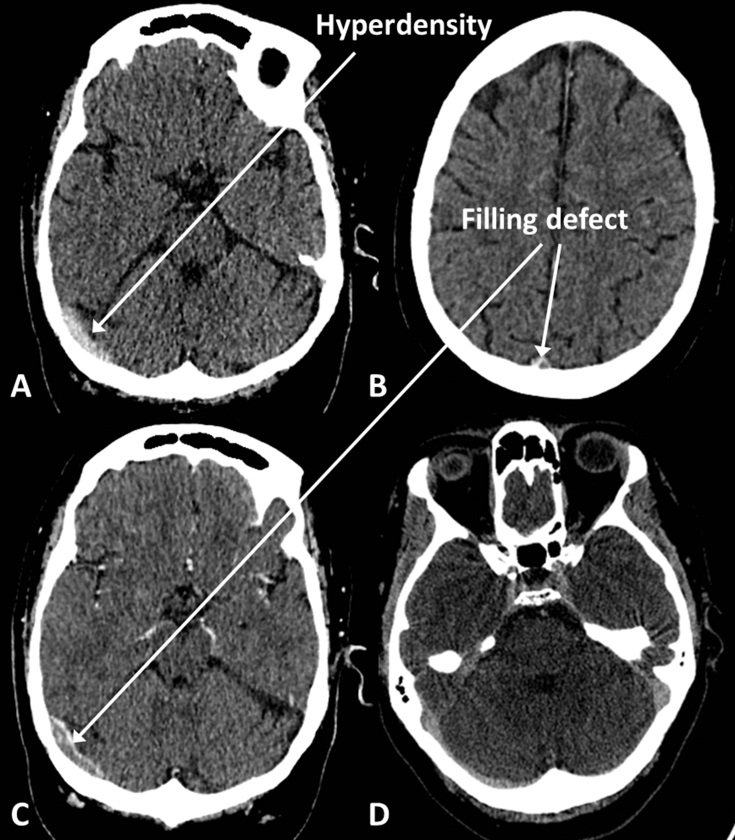
Acute thrombosis will result in hyperdensity within the affected sinus on unenhanced CT (Fig. 10). CT venography (CTV) involves injection of a contrast agent, and the scan is timed so that the venous structures should be filled with hyperdense contrast. Clot is seen as a hypodense ‘filling defect’ within the otherwise contrast-filled sinus. The brain parenchyma within the territory drained by the affected vessel may be hypodense and swollen because of venous hypertension and is prone to haemorrhage and so-called ‘venous infarction’.
Fig 10.
Venous sinus thrombosis. (A) Hyperdense clot in right transverse sinus on unenhanced CT. (B) Filling defect in superior sagittal sinus on CT venogram (CTV). (C) Filling defect in right transverse sinus on CTV. (D) Normal CTV for comparison; notice that the sinuses should opacify homogenously.
Infection
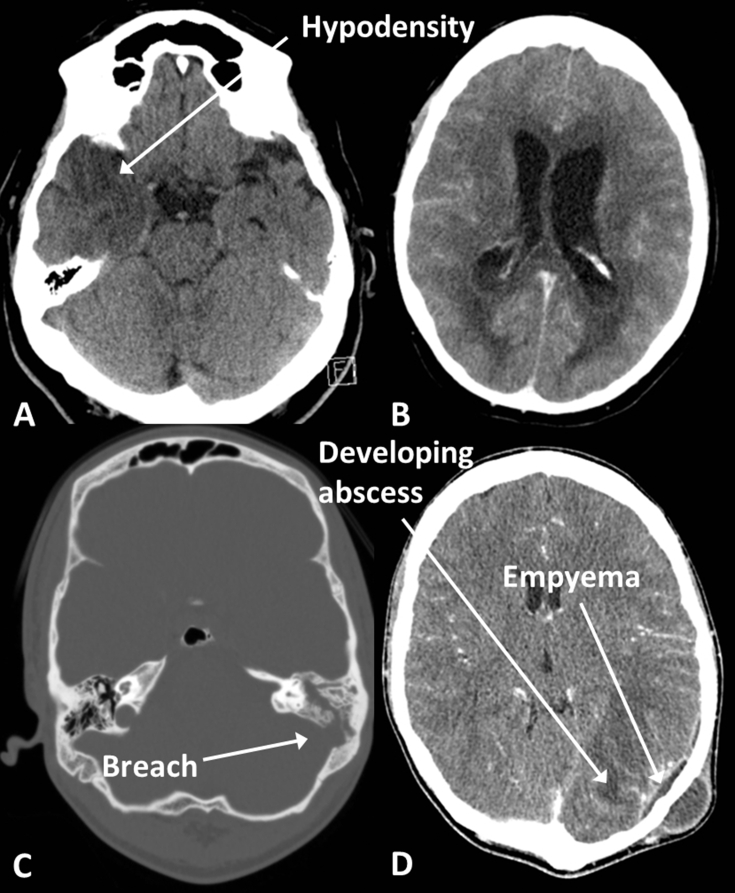
Infection may involve any of the intracranial compartments (Fig. 11). The parenchyma is involved in encephalitis which is often caused by herpes simplex virus and affects the limbic system with swelling and low density in the medial temporal lobes, insula, and cingulate gyri, often bilaterally but asymmetrically. In meningitis, the scan is often normal but enhancement may be seen over the surface of the brain. Imaging is not always necessary in meningitis but can be useful in detecting suspected complications such as infarction or hydrocephalus and suspected local causes such as sinusitis or mastoiditis.
Fig 11.
Infection. (A) Herpes encephalitis, with low density in the right temporal lobe. (B) Ventriculitis in a different patient; note the enhancement of the ventricle walls and the fluid levels in the occipital horns. (C) Mastoiditis with fluid in the left mastoid air cells (compare with the aerated right mastoid air cells) and a breach in the bony wall of the mastoid. (D) Empyema and abscess in the same patient as (C) secondary to the mastoid infection; note also the scalp abscess.
Abscesses are ring enhancing lesions with surrounding low-density oedema. These may rupture into the ventricles causing ventriculitis where debris may be seen within the ventricular system and the lining of the ventricles may enhance.
Extradural and subdural empyema have the same morphology as haemorrhage into these compartments but will generally be of low density and exhibit peripheral enhancement.
Hydrocephalus
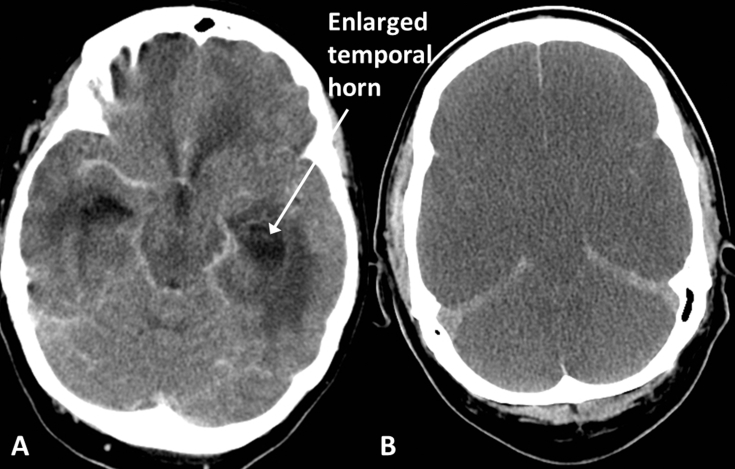
Enlargement of the ventricles is seen in hydrocephalus and, where the lateral ventricles are involved, the temporal horns are an important site for review as they often enlarge first (Fig. 12A). Low-density oedema may appear in the periventricular white matter because of the raised intraventricular pressure. The walls of the third ventricle are usually parallel but with hydrocephalus involving the third ventricle they may bulge laterally when viewed in the axial plane.
Fig 12.
(A) Hydrocephalus, in a patient with ventriculitis; note the enlarged temporal horns and surrounding low density oedema. (B) Diffuse hypoxic brain injury; note the diffuse loss of grey/white differentiation and generalised swelling causing effacement of the sulci and cisterns in this patient after an out-of-hospital cardiac arrest. There is no haemorrhage but the tentorium and falx appear dense relative to the adjacent hypodense oedematous brain.
Hydrocephalus is not always clear-cut on imaging because normal aging, or indeed pathological neurodegeneration, result in loss of brain tissue and ventricular enlargement without raised intraventricular pressure. Comparing the degree of ventricular enlargement with the size of the sulcal spaces can help, as can comparison with previous imaging.
Communicating hydrocephalus may result from previous subarachnoid haemorrhage or meningitis and causes enlargement of all ventricles. Non-communicating hydrocephalus results from an obstruction within the ventricular system, for example at the fourth ventricle because of a cerebellar infarct, haemorrhage, or mass. Only the ventricles above the obstruction will enlarge.
Diffuse hypoxic brain injury
Depending on the degree of injury, the brain will be generally swollen with effacement of cisterns, sulci and ventricles (Fig. 12B). As with infarction, the distinction between grey and white matter is lost but instead of this being within the territory of a single artery, it is widespread and bilateral. The deep grey nuclei are often affected with variable involvement of the cortical grey matter.
Tumours
As a general rule, high-grade primary brain tumours are irregular mass lesions, often with a solid rim and lower density centre (Fig. 13). They are more likely to be solitary than metastases, which may be solid or cystic but are often less irregular. Lymphoma is classically hyperdense to grey matter and has an affinity for the periventricular regions. All will usually enhance, but lymphoma tends to produce solid enhancement whereas the others tend to produce peripheral, ring, or nodular enhancement.
Fig 13.
Tumours. (A) Metastases, hyperdense lesion in left basal ganglia and right frontal oedema related to a second lesion; (B) high-grade glioma, large heterogenous mass containing areas of haemorrhage and enhancement, crossing the corpus callosum; and (C) lymphoma, uniformly enhancing periventricular mass.
Summary
CT imaging of the brain is a vital tool in the diagnosis of acute intracranial pathology. It often provides the basis for important early management decisions and is very effective at identifying patients who need urgent surgical intervention. The increasing availability of scanners has resulted in increased usage over time but as a modality that uses potentially harmful ionising radiation, it is important to guard against inappropriate use. Documents such as the NICE head injury guideline seek to reduce unnecessary use and should be followed. Whereas CT is excellent for bone detail, acute haemorrhage, acute surgical pathology, and vascular imaging, MRI provides superior soft tissue detail to CT and often becomes necessary where more subtle pathology is present.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Nicholas Skipper FRCR EDiNR is a consultant neuroradiologist at Brighton and Sussex University Hospitals NHS Trust. He completed a fellowship in adult and paediatric neuroradiology in Sheffield.
Mark Igra FRCR EDiNR is a consultant neuroradiologist at Leeds General Infirmary. He completed his neuroradiology fellowship in Sheffield and has a subspecialty interest in neuro-oncology and spinal intervention.
Andrew Davidson FRCA FFICM is a consultant in neuroanaesthesia and neurointensive care at the Royal Hallamshire Hospital, Sheffield.
Matrix codes: 2A02, 3A10, 2A12, 2C01, 3F00
References
- 1.Whiting P., Singatullina N., Rosser J. Computed tomography of the chest: I. Basic principles. Br J Anaesth Educ. 2015;15:299–304. [Google Scholar]
- 2.American College of Radiology, American Society of Neuroradiology and Society for Paediatric Radiology . ACR, ASNR & SPR; 2015. Practice parameter for the performance of computed tomography (CT) of the brain.https://www.acr.org/-/media/ACR/Files/Practice-Parameters/CT-Brain.pdf Available from: [Google Scholar]
- 3.National Institue for Health and Care Excellence . NICE Guideline (CG176); 2014. Head injury: assessment and early management.https://www.nice.org.uk/guidance/cg176 Available from: [PubMed] [Google Scholar]
- 4.Hofmeijer J., Kappelle L.J., Algra A., Amelink G.J., van Gijn J., van der Worp H.B., HAMLET investigators Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open randomised trial. Lancet Neurol. 2009;8:326–333. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]