Learning objectives.
After reading this article you should be able to:
-
•
Describe the process of preoperative assessment for lung transplant recipients.
-
•
Explain the challenges faced by the anaesthetist at different stages of lung transplant surgery.
-
•
Discuss the management of perioperative right ventricular failure and primary graft dysfunction.
-
•
Identify the indications for extracorporeal life support.
Key points.
-
•
In selected patients, lung transplantation is the definitive treatment for end-stage lung disease.
-
•
Potential transplant recipients undergo evaluation and optimisation by a multidisciplinary team.
-
•
Surgery may be performed with or without the use of extracorporeal life support.
-
•
The most common cause of perioperative cardiovascular failure is right ventricular dysfunction.
-
•
The most common cause of postoperative respiratory failure is primary graft dysfunction.
In suitable patients, lung transplantation is the definitive treatment for end-stage pulmonary disease. The most common conditions leading to this surgery are idiopathic interstitial pneumonia, chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF).
Each year, approximately 4000 patients worldwide undergo lung transplantation.1 In March 2019, the number of patients awaiting transplantation in the UK was 347 and the number of deaths on the waiting list was 57 during the previous year.2 In addition, for patients undergoing lung transplantation the outcomes are limited, with a median survival after primary transplant of 6.7 yrs.1 In the first year, approximately 20% of recipients die, most commonly from infection, primary graft dysfunction (PGD) and multiple organ failure. Thereafter, median survival is 8.9 yrs for patients who survive the first 12 months. Late causes of death include bronchiolitis obliterans caused by chronic graft failure, infection and malignancy.
Given the severity of disease and magnitude of surgery, anaesthesia for lung transplantation is challenging. In this review, we highlight the main issues, their assessment and implications for the anaesthetist during the pre-, intra- and postoperative phases of surgery. We consider areas such as selection of patients, impaired gas exchange, haemodynamic instability, imaging and communication.
Before surgery
Selection and evaluation of patients
Lung transplantation may be considered in patients with chronic end-stage lung disease who have a greater than 50% risk of death from lung disease within 2 yrs, but have a high probability (>80%) of surviving for 5 yrs after transplantation from a general medical perspective.3 Contraindications to lung transplantation are listed in Table 1.3,4
Table 1.
Contraindications to lung transplantation. Modified with permission from Table 2 of Martin and colleagues.6 ECLS, extracorporeal life support; GORD, gastroesophageal reflux disease; HIV, human immunodeficiency virus
|
Absolute Recent malignancy Significant dysfunction in another major organ system Acute unstable medical condition Uncontrolled bleeding Chronic uncontrolled multidrug resistant infection or active tuberculosis BMI >35 kg m−2 Significant chest wall or spinal deformity Alcohol or drug abuse Psychiatric or psychological conditions associated with lack of ability to cooperate with care Non-adherence to medical therapy Lack of a support team Limited rehabilitation potential |
|
Relative Age >65 yrs with a low physiologic reserve BMI 30–34.9 kg m−2 Severe malnutrition Mechanical ventilation or ECLS Infection with highly resistant organisms Hepatitis B or C without significant hepatic damage HIV infection with undetectable HIV-RNA Atherosclerotic disease with risk of end-stage heart disease Severe osteoporosis Inadequately controlled type 2 diabetes, hypertension, or GORD Extensive previous chest surgery with lung resection Psychiatric or psychological condition that has the potential to affect medical care |
The International Society for Heart and Lung Transplantation provides disease-specific thresholds for referring and listing patients for lung transplantation.3 Once referred, a thorough physical, social and psychological assessment of the patient and their support network is performed by a multidisciplinary team. Patients with end-stage pulmonary disease are frequently deconditioned. Therefore, completion of a pulmonary rehabilitation programme is mandatory to maximise exercise capacity and strength and to provide motivation and peer support. Ideally, a patient's BMI should be in the normal range at the time of surgery. Patients with low BMI should undergo nutritional support, which may involve enteral feeding. Patients with a high BMI should undergo a weight loss programme. Cardiovascular disease is a common accompaniment to end-stage pulmonary disease; consequently, assessment by a cardiologist is appropriate for at-risk patients.
In addition to standard pulmonary function testing, all patients should undergo a ventilation–perfusion (/) scan, CT scan of the chest and a transthoracic echocardiogram (TTE). A / scan identifies the least functional lung, which is important when considering single vs bilateral lung transplant and deciding which lung to explant first. A TTE is primarily used for evaluating right ventricular (RV) function and pulmonary artery (PA) pressure, but may also identify coexisting cardiac disease. A chest CT scan is used to rule out occult malignancy and may be required to identify the patency of the great vessels and to identify pulmonary collaterals (see below). Patients requiring lung transplantation because of pulmonary hypertension will have had a right heart catheter before referral. Other patients with evidence of severe pulmonary hypertension on TTE should be referred for a right-heart catheter study as part of their work-up for transplant. Once accepted onto the waiting list, ongoing clinical assessment and timely updates of key investigations are essential, as a patient's clinical condition may rapidly deteriorate while awaiting transplantation.
To facilitate perioperative planning and to provide informed consent, it is important to identify patients at high risk of adverse outcomes. Recipient characteristics indicating high risk include extracorporeal membrane oxygenation (ECMO) before surgery, oxygen requirement >5 L min−1, retransplantation, age >70 yrs, renal impairment, hepatic impairment, severe pulmonary hypertension, high or low BMI, and chronic use of corticosteroids.4, 5, 6 Donor characteristics indicating high risk include female donor–male recipient, donor/recipient weight ratio <0.7, donor–recipient cytomegalovirus mismatch (donor positive/recipient negative) and a history of smoking or diabetes.5,6
Disease-specific considerations
A minority of patients with COPD develop severe pulmonary hypertension and cor pulmonale. Patients with COPD are also at high risk for cardiovascular disease, and typically require additional cardiac investigations (e.g. CT coronary angiogram) before listing for transplant.
Patients with suppurative lung disease (CF, bronchiectasis) are frequently colonised or infected with multiresistant organisms, such as Burkholderia cenocepacia or Pseudomonas sp., and are on long-term antibiotic treatment via a chronic indwelling central venous catheter (CVC). Such patients are at risk of thrombotic obstruction or narrowing of the central veins and can develop adhesions and collateral vessels. Therefore, a CT angiogram or MRI scan is required before surgery to delineate these problems.
Patients with primary pulmonary hypertension or connective tissue diseases that are associated with pulmonary hypertension (e.g. scleroderma, mixed connective tissue disease) are at increased perioperative risk and have a high likelihood of requiring extracorporeal life support (ECLS) during or after surgery.7
Single vs bilateral lung transplant
The decision to perform a single or bilateral lung transplant is made during the assessment phase. Single lung transplant is technically simpler than bilateral transplant and makes the best use of a limited resource. Single lung transplant may be an appropriate choice for patients with COPD and interstitial lung disease. Patients with suppurative lung disease or severe pulmonary hypertension require bilateral transplant. Notwithstanding this distinction, bilateral lung transplant is increasingly preferred for all patients because of mounting evidence for an overall survival benefit.1,4 In 2017, bilateral transplant accounted for 80% of all lung transplants.1
On-pump vs off-pump surgery
The use of ECLS during lung transplantation varies considerably depending on the indication for surgery and institutional preference.6, 7, 8 In some centres, most transplants are performed off-pump, with the exception of patients with severe pulmonary hypertension or in those already receiving ECMO, who require on-pump surgery. In other centres, most transplants are electively performed on-pump. ECLS typically involves either standard cardiopulmonary bypass (CPB) or venoarterial (VA) ECMO. If ECLS is required after surgery, either venovenous (VV) or VA ECMO is used.
Assessment for anaesthesia
Patients are typically reviewed by an anaesthetist at the time of listing for transplant and again immediately before surgery. When assessing the patient, the anaesthetist should focus on aspects of the patient's disease that are likely to impact upon their perioperative management – in particular, the features defining a high-risk recipient described above. Note that all patients undergoing lung transplant for pulmonary hypertension should be considered high-risk. For patients with secondary pulmonary hypertension (e.g. caused by COPD), symptoms and signs of right heart failure indicate cardiac decompensation. As such, features of right heart failure are more important than the PA pressure per se. However, as a simple rule of thumb, a PA systolic pressure >60 mmHg, estimated on TTE, indicates clinically important pulmonary hypertension.
Aspects of the patient's systemic disease that should be considered include chronic corticosteroid use, renal dysfunction, oesophageal dysmotility necessitating extra care when inserting the TOE probe, and difficulty with tracheal intubation (e.g. scleroderma). The echocardiogram should be reviewed for evidence of RV dysfunction, as this problem predisposes to haemodynamic instability during and after surgery. Sites for vascular access should be considered in light of the patency of the central veins and the potential need for ECMO (see below).
Consent for anaesthesia should include discussing the potential for a prolonged ICU stay and multiple organ failure, the potential need for ECLS and options for postoperative analgesia, including the pros and cons of thoracic epidural (see below).
Patients are frequently apprehensive before lung transplantation. However, because of the risk of acute cardiorespiratory depression, sedative–hypnotic agents should be given under the direct supervision of the anaesthetist.
The anaesthetist should attend the multidisciplinary pretransplant meeting where the particular features of the donor and recipient are discussed. Issues such as the elective use of ECLS, single vs double lung transplant, expected arrival time of the donor lungs, along with any changes to standard protocols (e.g. immunosuppression, antimicrobial prophylaxis) are discussed and agreed upon.
During surgery
Timing
Appropriate timing of induction of anaesthesia is important to ensure the graft ischaemic time is minimised and the preimplantation anaesthesia time in the recipient is not unduly prolonged. Graft ischaemic time is the time from cross clamping the aorta in the donor to reperfusion of the allograft in the recipient. An ischaemic time longer than 5.5 h is associated with an increased incidence of PGD.9
The expected time of arrival of the donor lungs determines when the patient should be brought into the operating room. For a straightforward case, 1 h should be allowed for anaesthesia and 1 h for preimplantation surgery. The donor lungs should be accepted for transplantation before induction of anaesthesia in the recipient.
Routine anaesthesia management
In addition to the routine monitoring used for any major surgery, PA catheterisation, transoesophageal echocardiography (TOE) and bronchoscopy are appropriate for patients undergoing lung transplantation.8 External defibrillator pads, a fluid warmer, warming blanket and calf compressors should be used in all patients. Except in high-risk cases, our practice is to secure central venous access after induction of anaesthesia. We avoid the right internal jugular vein in case it is subsequently required for VV ECMO.
All patients presenting for lung transplantation should be considered extremely fragile and prone to hypotension (or even cardiac arrest) with induction of anaesthesia. The full theatre team – including the surgeon and perfusionist – should be immediately available during this time. Although we make no specific recommendation for the choice of drugs for anaesthesia, hypnotic and opioid drugs should be given slowly to avoid severe hypotension. Aggressive bag-mask ventilation can similarly cause severe hypotension. However, hypoxia and hypercarbia should also be avoided, as both can lead to acutely increased pulmonary vascular resistance, potentially precipitating RV failure.
The ability to perform one lung ventilation (OLV) and lung isolation is mandatory in all cases, which is most easily achieved with a left-sided double lumen tracheal tube. The correct position of the tube should be confirmed with bronchoscopy and any mucus plugs suctioned away. Once the tracheal tube is correctly positioned, a trial of OLV should be performed. Patients with COPD may benefit from OLV at a relatively low ventilatory frequency (≤12 bpm) and standard tidal volume (4-5 ml kg−1); patients with restrictive lung disease may benefit from a relatively higher ventilatory frequency (12–16 bpm) and lower tidal volume (3-4 ml kg−1).
For bilateral lung transplantation, either an anterolateral thoracosternotomy (clamshell) incision or bilateral anterior thoracotomy approach is used with the patient positioned supine. Single lung transplantation is performed via a lateral thoracotomy with the patient positioned on their side.
Antimicrobial prophylaxis and immunosuppressive agents are administered before the skin incision, and again at specific times during the case, according to the institution's protocol.
TOE provides useful information on RV function, volume status, vascular tone, intravascular air and the patency of the pulmonary veins.10,11 Baseline measurements of RV size and function, along with the severity of any tricuspid regurgitation, should be recorded for later comparison.
The transplant procedure
Single lung transplantation is commonly performed off-pump, similar to a standard open thoracotomy. Bilateral lung transplantation is performed as a sequential procedure, either on or off pump. The first native lung is excised and the first allograft is reperfused. Then, the second native lung is excised and the second allograft implanted. Each allograft has separate bronchial, pulmonary artery and pulmonary vein anastomoses.
Off-pump surgery
For surgery performed without elective ECLS, the least functional native lung is resected first. After surgical dissection and administration of low-dose unfractionated heparin, trial clamping of the PA is performed and patient's haemodynamics assessed clinically and with TOE. Marked hypotension may require initiation of ECLS. Progressive hypotension, acidosis, or hypoxia during pulmonary dissection may also necessitate ECLS, which is best initiated ‘semi-urgently’ rather than as an emergency procedure. Ius and colleagues have developed an algorithm to guide decision-making for the initiation of unplanned ECLS.12
Before reperfusion of the first allograft, bronchoscopy should be performed to assess the bronchial anastomosis and to clear any blood or secretions from the airways. Reperfusion predisposes to allograft pulmonary oedema and can cause marked hypotension. Clear communication between members of the theatre team and close anaesthetic vigilance are required during this time. The PA clamp should be released very slowly – over 10–15 min – to minimise the risk of allograft injury and hypotension. The anaesthetist should prepare for reperfusion by increasing the rates of vasoactive infusions and having bolus doses of fluid, calcium (3–6 mmol) and adrenaline (20–100 μg) ready. Gas embolism – particularly to the coronary arteries – can also occur with reperfusion, compounding haemodynamic instability and causing cardiac dysrhythmias.
After reperfusion of the first allograft, a trial of OLV of the newly implanted lung is undertaken to determine its ability to support gas exchange. Appropriate ventilator settings include a tidal volume of 3–4 ml kg−1 (donor weight) and PEEP 8–10 cmH2O. FIO2 and peak inflation pressure (PIP) should be as low as possible to achieve an SpO2 >90%. High PIP, hypoxia, acidosis, or hypotension may necessitate initiation of ECLS.
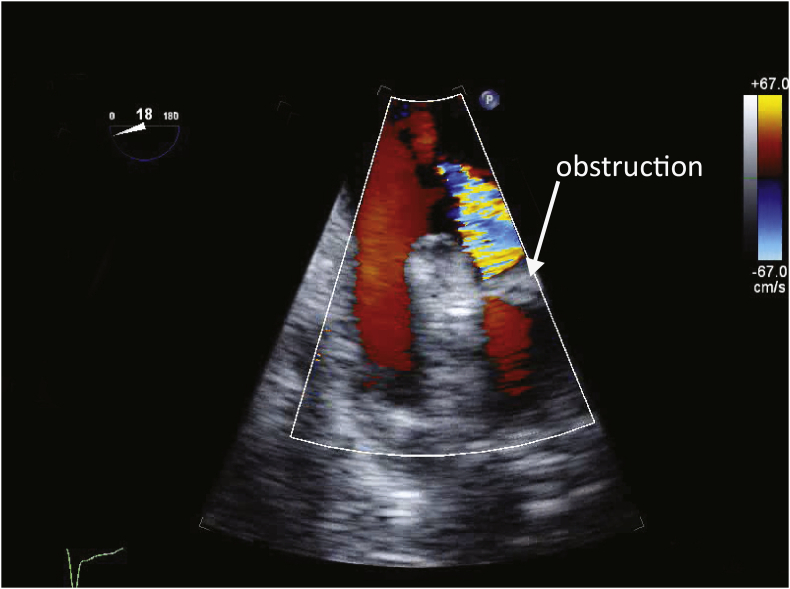
The patency of the pulmonary veins should be carefully assessed with TOE, as torsion of the pulmonary veins is an uncommon but well recognised problem (Fig. 1). A pulmonary vein diameter >0.5 cm, peak systolic velocity <1 m s−1 and a pulmonary vein-left atrial pressure gradient <12 mmHg are acceptable.11 Pulmonary venous obstruction usually necessitates surgical revision.
Fig 1.
Pulmonary venous obstruction. The image shows a transoesophageal echocardiography (TOE) view of the left-sided pulmonary veins after allograft reperfusion. An obstruction can be seen in one of the veins, possibly caused by torsion of the lung. With colour Doppler imaging, aliased, high-velocity flow is seen proximal to the obstruction. In contrast, flow in the other vein is laminar and of low velocity.
On-pump surgery
Elective ECLS is typically commenced when both lung hila are exposed and the PA is ready to be clamped. CPB requires full heparinisation, typically targeting an activated clotting time (ACT) >480 s. For VA ECMO, a target ACT of 160–200 s is appropriate. Anaesthetists and surgeons familiar with CPB need to be aware of the key differences when using VA ECMO. Unlike CPB, an ECMO circuit has no reservoir, which means there is no facility to rapidly administer fluid into the extracorporeal circuit. Centrifugal pumps used for ECMO generate a negative pressure (100–200 mmHg) in the drainage limb of the circuit. A loose stay suture around the venous ECMO cannulae or a loose cap on a CVC can lead to air entrainment into the circuit, which can cause an airlock in the pump head with abrupt cessation of circuit flow. We have introduced a formal ‘time out’ to eliminate the risk of gas embolism before initiation of VA ECMO.
Surgical retraction of the heart and great vessels can impair venous drainage during ECLS, resulting in an inability to maintain circuit flow. A common response to the resulting hypotension is to give a fluid challenge, which can lead to intravascular volume overload and impaired allograft function. Good communication between the theatre team helps mitigate this problem. Brief periods of low pump flow resulting from surgical manipulations should be tolerated.
Reperfusion during ECLS requires careful de-airing of the allograft. Controlled blood flow to the allograft helps mitigate the problems associated with reperfusion described for off-pump surgery. After reperfusion (but before discontinuation of ECLS), IPPV should be resumed at rest settings (Fio2 0.3, PEEP 8–10 cmH2O, PIP 20 cmH2O, frequency 8–10 bpm) to avoid allograft injury.
After reperfusion of the second allograft, two-lung ventilation is commenced and ECLS is discontinued. Assuming the patient's cardiac and respiratory functions are satisfactory, protamine should be (slowly) administered.
Management after allograft implantation
After allograft implantation, lung protective-ventilation and fluid restriction help minimise the risk of developing PGD.13 Ideal (two-lung) ventilator settings include a VT 4–6 ml kg−1, PIP <25 cmH2O, PEEP 8–10 cmH2O, Fio2 <0.5, targeting a SpO2 >90% and a pH >7.2.
Poor gas exchange or high PIP should be managed using a stepwise approach. Kinking or malposition of the tracheal tube should be excluded first. Blood and secretions should be suctioned from the airways. A gentle recruitment manoeuvre (PIP 30 cmH2O for 30 s) may be helpful. The patency of the pulmonary veins should again be inspected with TOE. Visible oedema fluid in the airways strongly suggests PGD or pulmonary venous obstruction. If PGD is suspected, a trial of inhaled nitric oxide (iNO, 10 ppm) may be beneficial. Persistent hypoxia (Fio2 >0.70, SpO2 <90%) should prompt consideration of VV ECMO.
Haemodynamic instability during this period is common and can be attributed to several causes including hypovolaemia, arrhythmias, vasoplegia, cardiac tamponade and increased intrathoracic pressure from aggressive mechanical ventilation. However, the most common cause is RV dysfunction.
RV failure presents with hypotension, low cardiac index (CI) and a high CVP. On TOE, the RV is typically dilated and poorly contractile, and tricuspid regurgitation has usually worsened compared with baseline. There may be marked leftward displacement of the atrial and ventricular septa (Fig. 2). Treatment includes maintaining RV perfusion pressure (i.e. MAP) with infusions of noradrenaline and vasopressin, avoiding further volume loading and attempting to reduce RV afterload with iNO or milrinone.14 Because of the risk of causing lactic acidosis, we avoid infusions of adrenaline. High intrathoracic pressure caused by aggressive mechanical ventilation further increases RV afterload. A rapid ventilatory frequency (16–20 bpm), low tidal volume (3–4 ml kg−1) and modest PEEP (<12 cmH2O) mitigates hypercarbia and reduces RV afterload. Persistent haemodynamic instability (MAP <65 mmHg, CI <2 L min−1 m−2) despite appropriate vasopressor therapy (noradrenaline >0.2 μg kg−1 min−1), particularly if associated with hypoxia, is an indication for VA ECMO.
Fig 2.
Right ventricular dysfunction. In this mid-oesophageal four-chamber transoesophageal echocardiography (TOE) view obtained during diastole, marked dilatation of the right atrium (RA) and ventricle (RV) can be seen. The atrial and ventricular septa are displaced leftward. The left atrium (LA) and ventricle (LV) are reduced in size compared with normal. These findings are typical of severe right ventricular volume overload. Not evident in this still frame, but obvious with real-time imaging, is severe RV systolic dysfunction.
After chest closure and insertion of drains, the double lumen tracheal tube is exchanged for a single lumen tube and the patient transferred to ICU.
Critical care management
Routine care
The principles of ICU care after lung transplantation involve lung protective ventilation, early tracheal extubation, fluid restriction, routine immunosuppressive and antimicrobial therapy, providing adequate analgesia, and prophylaxis against venous thromboembolism (VTE).
In the absence of significant cardiorespiratory dysfunction, patients should be weaned and extubated within 24 h. Low-dose noradrenaline (<0.05 μg kg−1 min−1) is helpful to support MAP and to avoid giving excessive volumes of fluids.
Thoracic epidural analgesia is the gold standard for pain management after lung transplantation.15 Typically, a thoracic epidural catheter is placed after surgery but before extubation to reduce the risk of epidural haematoma if systemic heparinisation is used for ECLS. However, a recent Cochrane review demonstrated comparable analgesic efficacy with paravertebral block.16 Intravenous opioid-based patient-controlled analgesia may be used for additional analgesia or when a regional technique is contraindicated.
Immunosuppression typically involves a calcineurin inhibitor (tacrolimus or cyclosporine), an antiproliferative agent (azathioprine or mycophenolate) and corticosteroids. Patients require ongoing antimicrobial therapy, either as prophylaxis or directed against pre-existing infection in the donor lungs.
Management of complications
Complications during the patient's ICU stay primarily relate to cardiorespiratory failure and the development of multiple-organ dysfunction. Recovery from multiple-organ dysfunction is often greatly prolonged because of pre-existing deconditioning and malnutrition.
The most common significant postoperative complication is PGD, affecting approximately 30% of patients.17 PGD is defined by impaired oxygenation and diffuse alveolar opacities on the chest radiograph (Table 2 and Fig. 3), and can occur at any time from allograft reperfusion up to 3 days after surgery.18 PGD is associated with increased early and late morbidity and mortality.19 PGD is more common in patients with severe pulmonary hypertension. Modifiable risk-factors include the use of ECLS, high intraoperative fluid and blood product administration, and high Fio2 during reperfusion.20 Treatment is supportive, and includes lung protective ventilation, iNO, fluid restriction and VV ECMO. In one study, 6% of patients undergoing lung transplantation required VV ECMO for PGD, with a 5 yr survival of 49%, which is worse – but not substantially so – than survival for all comers.21
Table 2.
Classification of primary graft dysfunction.20 Note, the use of extracorporeal membrane oxygenation (ECMO) in association with bilateral pulmonary oedema is classified as Grade 3
| Grade | Pulmonary oedema on chest radiograph | Pao2/Fio2 ratio (mmHg/%) |
|---|---|---|
| 0 | No | Any |
| 1 | Yes | >300 |
| 2 | Yes | 200–300 |
| 3 | Yes | <200 |
Fig 3.
Primary graft dysfunction. The image shows a chest radiograph from a patient who underwent lung transplantation 2 days earlier. The patient developed primary graft dysfunction requiring VV ECMO. Diffuse alveolar opacities are present throughout both lungs. The tips of the drainage and return ECMO cannulae are demonstrated. Other identifiable features include a tracheal tube (the tip is positioned at the origin of the right main bronchus and needs to be withdrawn slightly), four chest drains, two external defibrillator pads, a sternal wire at the base of the sternum (a characteristic finding after a ‘clamshell’ incision), and a small amount of subcutaneous air in the chest wall adjacent to the base of the left lung. VV ECMO, venovenous extracorporeal membrane oxygenation.
Other causes of early respiratory compromise include mucus plugging and atelectasis, a large haemothorax or pneumothorax (blocked or kinked drains), acute rejection, pneumonia, bronchial anastomotic leak, pulmonary embolism and dynamic hyperinflation.17 Pulmonary embolism is an especially feared complication, as, in the absence of a bronchial circulation, there is a high risk of pulmonary infarction. Consequently, routine VTE prophylaxis is essential once surgical bleeding has settled. In patients with COPD, dynamic hyperinflation (auto-PEEP) of the native lung is a particular risk after single lung transplantation, because of a (typically) higher compliance in the native lung compared with the transplanted lung. Very occasionally, independent lung ventilation using two ventilators and a double lumen tracheal tube is necessary to manage this problem.
Cardiovascular failure is most commonly attributable to RV dysfunction, and is exacerbated by hypoxia, acidosis, fluid overload and cardiac tamponade. Early, bedside TOE is essential to confirm the diagnosis and exclude other causes of hypotension. Treatment is as described above. Severe RV dysfunction that does not respond to more simple manoeuvres should be managed with VA ECMO.
Acute kidney injury occurs in about half of patients after lung transplantation with approximately one in eight patients requiring renal replacement therapy.22 It may be appropriate to withhold routine calcineurin inhibitor therapy in patients with evolving acute kidney injury.
To minimise the adverse consequences of fluid overload and acidosis, early institution of renal replacement therapy is appropriate.
The management of common problems encountered by the anaesthetist during the perioperative period is summarised in Table 3.
Table 3.
Assessment and management of common problems during lung transplantation. COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; ECLS, extracorporeal life support; ECMO, extracorporeal membrane oxygenation; PAH, pulmonary arterial hypertension; PAP,; PGD; primary graft dysfunction; PV, pulmonary venous; RV, right ventricular; TOE, transoesophageal echocardiogram; VA, venoarterial; VV, venovenous
| Problem | Assessment | Management |
|---|---|---|
| Before surgery | ||
| All patients | Fragile cardiorespiratory status. | Avoid sedative hypnotic drugs. |
| Preserve the right internal jugular vein for postoperative ECMO. | ||
| Suppurative lung disease (cystic fibrosis or bronchiectasis) | Chronic infection. | Specific antimicrobial prophylaxis or treatment. |
| Adhesions, collaterals vessels, central venous obstruction. | CT angiogram or MRI to determine patency of central veins and presence of collateral blood vessels. If central venous obstruction, plan alternative sites for central venous access or ECLS cannula. Plan for adequate venous access in case of excessive bleeding. |
|
| COPD or pulmonary fibrosis | Suitability for single lung transplantation. | Discuss ventilation–perfusion scan with the surgeon. |
| Primary pulmonary hypertension or severe secondary pulmonary hypertension | Right heart catheter study to assess magnitude and reversibility of PAP and pulmonary resistance. | ECLS during and after surgery. |
| Review TTE to assess RV function and severity of tricuspid regurgitation. | ||
| Connective tissue disease (e.g. scleroderma, mixed connective tissue disease) | Oesophageal problems. | Consider barium swallow or endoscopy to evaluate oesophageal function. Insertion of TOE probe may be difficult or contraindicated. |
| Difficult tracheal intubation. | Consider advanced airway management for intubation and placement of double-lumen tracheal tube. | |
| During surgery, before allograft reperfusion | ||
| Timing | Expected arrival of donor organs. | Allow at least 1 h for anaesthesia and 1 h for surgery before organ arrival. |
| Ensure native lungs are not excised before donor organ on site. | ||
| Positioning | Planned incision. | Position the patient appropriately. Care with pressure points and excessive abduction of upper limbs. |
| Haemodynamic instability | Surgical manipulations, auto-PEEP if COPD, RV dysfunction if PAH, hypovolaemia. | Integrate findings from TOE and haemodynamic monitors. |
| Be aware of the key differences between CPB and ECMO (see text). | ||
| ECLS if progressive. | ||
| Impaired gas exchange | Malposition of double lumen tracheal tube, mucus plugs, blood in airway, atelectasis, low cardiac output. | Check ventilator settings; hand ventilate. |
| Perform bronchoscopy and clear secretions. | ||
| Review haemodynamics and acid–base status. | ||
| ECLS if progressive. | ||
| During surgery, after allograft reperfusion | ||
| Haemodynamic instability | As above; additionally, consider reperfusion-induced vasoplegia. | ECLS (VA ECMO) if progressive. |
| Impaired gas exchange | As above; additionally, consider PV obstruction and PGD. | Assess pulmonary veins with TOE. |
| Pulmonary oedema in airways suggests PGD or PV obstruction. | Fluid restriction. | |
| Surgical revision if PV obstruction. | ||
| ECLS (VV ECMO) if progressive. | ||
| In the ICU | ||
| Haemodynamic instability | RV dysfunction, exacerbated by acidosis, fluid overload, tamponade, and haemothorax | VA EMCO if progressive. |
| Impaired gas exchange | PGD, large haemothorax; large pneumothorax because of kinked drain, pulmonary embolism, auto-PEEP associated with single lung transplant for COPD, obstructed pulmonary veins, pneumonia, bronchial anastomotic leak. | Check ventilator settings. Perform chest radiograph, chest ultrasound, bronchoscopy, TOE, CT angiogram if pulmonary embolism is suspected. VV ECMO if progressive. |
Conclusions
The perioperative care of a lung transplant requires a multidisciplinary team approach, bringing together knowledge from a range of specialists. Anaesthetists require expertise in several areas including cardiac and thoracic anaesthesia, TOE, ECLS and transplant medicine. Patients can deteriorate rapidly, requiring a coordinated response. Therefore, excellent communication between anaesthetists, surgeons and perfusionist is mandatory to ensure the optimal outcome for patients.
MCQs
The associated MCQs (to support CME/CPD activity) are accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Declaration of interests
The authors declare that they have no conflicts of interest.
Biographies
Emily Buckwell BA (Hons) FRCA is a senior fellow in cardiothoracic anaesthesia at Auckland City Hospital, Auckland, New Zealand.
Bevan Vickery FANZCA, FCICM is a consultant cardiothoracic anaesthetist and intensivist at Auckland City Hospital.
David Sidebotham FANZCA is a consultant cardiothoracic anaesthetist and intensivist at Auckland City Hospital.
Matrix codes: 1H02, 2A03, 3C00
References
- 1.Chambers D.C., Cherikh W.S., Harhay M.O. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult lung and heart–lung transplantation report—2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38:1042–1055. doi: 10.1016/j.healun.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conor S. Statistica.com. Lung transplants in the United Kingdom (UK) 2018/2019. Available from: https://www.statista.com/statistics/518774/number-of-lung-transplants-united-kingdom-uk/ (Accessed 14 April 2020).
- 3.Weill D., Benden C., Corris P.A. A consensus document for the selection of lung transplant candidates: 2014—an update from the pulmonary transplantation council of the international society for heart and lung transplantation. J Heart Lung Transplant. 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Martin A.K., Renew J.R., Jayaraman A.L., Murray A.W., Fritz A.V., Ramakrishna H. Analysis of outcomes in lung transplantation. J Cardiothorac Vasc Anesth. 2019;33:1455–1466. doi: 10.1053/j.jvca.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Russo M.J., Davies R.R., Hong K.N. Who is the high-risk recipient? Predicting mortality after lung transplantation using pretransplant risk factors. J Thorac Cardiovasc Surg. 2009;138:1234–1238 e1. doi: 10.1016/j.jtcvs.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin A.K., Yalamuri S.M., Wilkey B.J. The impact of anesthetic management on perioperative outcomes in lung transplantation. J Cardiothorac Vasc Anesth. 2019;34:1669–1680. doi: 10.1053/j.jvca.2019.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Shah P.R., Boisen M.L., Winger D.G. Extracorporeal support during bilateral sequential lung transplantation in patients with pulmonary hypertension: risk factors and outcomes. J Cardiothorac Vasc Anesth. 2017;31:418–425. doi: 10.1053/j.jvca.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Tomasi R., Betz D., Schlager S. Intraoperative anesthetic management of lung transplantation: center-specific practices and geographic and centers size differences. J Cardiothorac Vasc Anesth. 2018;32:62–69. doi: 10.1053/j.jvca.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Thabut G., Mal H., Cerrina J. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–791. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 10.McKee A. Heart and lung transplantation. In: Sidebotham D., Merry A., Legget M., Wright G., editors. Practical perioperative transoesophageal echocardiograhy. 3rd Edn. Oxford University Press; Oxford: 2019. pp. 259–264. [Google Scholar]
- 11.Tan Z., Roscoe A., Rubino A. Transesophageal echocardiography in heart and lung transplantation. J Cardiothorac Vasc Anesth. 2019;33:1548–1558. doi: 10.1053/j.jvca.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Ius F., Sommer W., Tudorache I. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: indications and midterm results. J Heart Lung Transplant. 2016;35:49–58. doi: 10.1016/j.healun.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 13.McIlroy D.R., Pilcher D.V., Snell G.I. Does anaesthetic management affect early outcomes after lung transplant? An exploratory analysis. Br J Anaesth. 2009;102:506–514. doi: 10.1093/bja/aep008. [DOI] [PubMed] [Google Scholar]
- 14.Murphy E., Shelley B. Clinical presentation and management of right ventricular dysfunction. BJA Educ. 2019;19:183–190. doi: 10.1016/j.bjae.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feltracco P., Barbieri S., Milevoj M. Thoracic epidural analgesia in lung transplantation. Transplant Proc. 2010;42:1265–1269. doi: 10.1016/j.transproceed.2010.03.109. [DOI] [PubMed] [Google Scholar]
- 16.Yeung J.H., Gates S., Naidu B.V., Wilson M.J., Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2:CD009121. doi: 10.1002/14651858.CD009121.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.C., Diamond J.M., Christie J.D. Critical care management of the lung transplant recipient. Curr Respir Care Rep. 2012;1:168–176. doi: 10.1007/s13665-012-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snell G.I., Yusen R.D., Weill D. Report of the ISHLT working group on primary lung graft dysfunction: Part I. Definition and grading—a 2016 consensus group statement of the international society for heart and lung transplantation. J Heart Lung Translant. 2017;36:1097–1103. doi: 10.1016/j.healun.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Christie J.D., Sager J.S., Kimmel S.E. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–165. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 20.Diamond J.M., Lee J.C., Kawut S.M. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwig M.G., Walczak R., Lin S.S., Davis R.D. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg. 2012;93:366–371. doi: 10.1016/j.athoracsur.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Atchade E., Barour S., Tran-Dinh A. Acute kidney injury after lung transplantation: perioperative risk factors and outcome. Transplant Proc. 2020;52:967–976. doi: 10.1016/j.transproceed.2020.01.018. [DOI] [PubMed] [Google Scholar]