Learning objectives.
By reading this article you should be able to:
-
1.
Describe the specific indications for one-lung ventilation in adults.
-
2.
Choose and position correctly a double lumen tube to achieve lung isolation.
-
3.
Manage one-lung ventilation in patients with a difficult airway.
-
4.
Effectively and safely manage hypoxaemia during one-lung ventilation.
Key points.
-
1.
With the increase in number of minimally invasive thoracic procedures, intraoperative one-lung ventilation is being used more often than before.
-
2.
Double-lumen tubes are the most commonly used devices for lung isolation.
-
3.
A fibreoptic bronchoscope is essential to aid placement, confirm position and troubleshoot problems that arise with use of lung isolation devices.
-
4.
A clear plan is essential in managing patients with difficult airway (including tracheostomy) who require one-lung ventilation.
-
5.
Hypoxaemia with initiation of one-lung ventilation is common and should be managed in a stepwise sequential manner.
Apart from their traditional role in lung resection surgeries, lung isolation techniques have been extensively used to allow one-lung ventilation (OLV) in patients undergoing surgeries of the oesophagus, aorta or thoracic spine.1 With the increase in the number of minimally invasive combined laparoscopic–thoracoscopic procedures for upper gastrointestinal surgeries, the use of intraoperative OLV has spread beyond the thoracic surgery operating theatres. A good knowledge of the various techniques available for initiating and maintaining OLV is therefore essential for general anaesthetists. This article will discuss the practical aspects of lung isolation in adults.
Indications for OLV
Lung isolation techniques have the following applications:
Absolute indications:
-
1.To prevent damage or contamination of the healthy lung
-
•lung abscess and pulmonary haemorrhage
-
•
-
2.To control distribution of ventilation
-
•bronchopleural fistula, major cyst or bulla, traumatic bronchial disruption
-
•
-
3.To facilitate single lung lavage
-
•Cystic fibrosis, pulmonary alveolar proteinosis
-
•
Relative indications:
-
1.To improve surgical access (strong)
-
•Thoracic aortic aneurysm
-
•Pneumonectomy
-
•Lung volume reduction surgery
-
•Minimally invasive cardiac surgery
-
•Upper lobectomy
-
•Video assisted thoracoscopic surgery
-
•
-
2.To improve surgical access (weaker)
-
•Oesophageal surgery
-
•Middle and lower lobectomy
-
•Mediastinal mass reduction
-
•
Techniques available for OLV
There are three different methods that can be used to achieve lung isolation and OLV: double lumen tubes (DLTs), bronchial blockers (BBs), and single lumen tubes (SLTs) advanced into either of the right or left main-stem bronchus. Each of these devices have their own advantages and disadvantages and are discussed below.
Double-lumen tubes
The introduction of the Carlen's design of DLT in the 1950s was a landmark event in the practice of thoracic anaesthesia worldwide as it allowed anaesthetists, for the first time, to reliably achieve lung isolation. Several decades later and after multiple modifications, DLTs are still the most widely used devices for achieving safe OLV.2
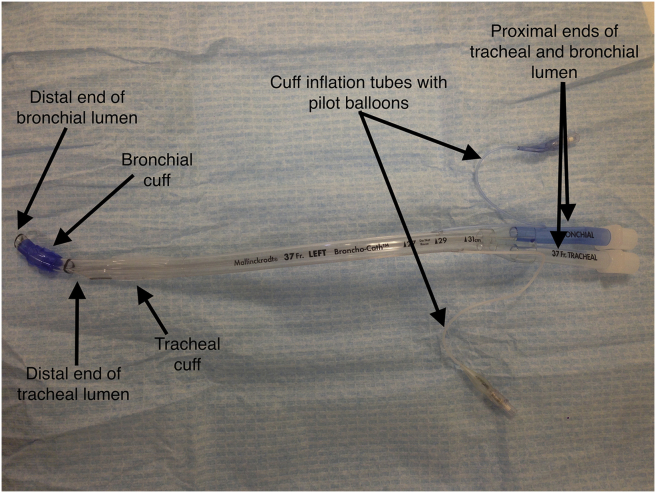
The DLT is a bifurcated tube with separate tracheal and endobronchial lumens, that can be used to ventilate either lung independently. The tracheal lumen is designed to terminate above the carina, while the bronchial lumen is angled to fit into the appropriate main-stem bronchus. The bronchial cuff and its pilot balloon inflation line is usually coloured blue for easy identification (Fig. 1). Right- and left-sided DLTs differ in their basic design because of the crucial anatomical differences between the right and left main-stem bronchi. The right main bronchus is shorter, straighter and wider than the left main bronchus. Additionally, the right upper lobe bronchus take off is very close to the origin of the right main-stem bronchus. It is for these reasons that right sided DLTs have a modified cuff and side opening (Murphy's eye) in the endobronchial tube to allow ventilation of the right upper lobe (Fig. 2).3 Although the polyvinyl chloride-based modified Robertshaw tubes are the most widely used DLTs, disposable rubber DLTs based on the original design of Robertshaw are available as well.
Fig 1.
The main features of a left-sided double lumen tube.
Fig 2.
Murphy's eye and oblique bronchial cuff of a right-sided double lumen tube.
Before and while using a DLT, the following questions need to be answered.
-
1.
What type (i.e. side) of DLT is needed?
Although most elective thoracic procedures can be successfully managed with a left-sided DLT,2 there are some specific indications for use of a right-sided DLT (Table 1).
Table 1.
Indications for a right sided double lumen tube
Surgery involving the left main bronchus
|
Distorted anatomy of left main bronchus
|
See main text for explanation.
Thoracoscopic surgery involves the use of long instruments and the presence of a left-sided tube in left lung surgery can make surgical manipulation of the bronchus difficult. Some surgeons, therefore, prefer the operative (i.e. left) lung to be tube free. In addition, with a patient in the lateral position and laterally flexed, there can be partial obstruction of the distal trachea. Therefore, ventilating the dependent lung via the tracheal lumen may be difficult, requiring higher pressures. There is also a risk of air trapping in that lung.
-
2.
What size DLT do we choose?
Adult DLTs commonly come in sizes 35, 37, 39, and 41 Fr.
The French scale is the external diameter of the tracheal segment (in mm) multiplied by three. A common problem with the use of DLTs is the lack of objective guidelines to choose properly the appropriate size of the DLT. A properly sized DLT is one that passes easily through the glottis and advances without resistance within the trachea, with the bronchial component passing into the intended bronchus without difficulty. The most accurate method of selecting the size of the DLT uses measurements of the left main-stem bronchial diameter from a computed tomography scan.4 A traditional approach would be to use 37 and 39 Fr DLTs for average sized female and male patients respectively. A size up or down is used for larger and smaller patients respectively. However, since the bronchocath type of DLTs are considerably longer than required, some thoracic anaesthetists now advocate the use of smaller sized DLTs to minimize the risk of airway trauma and to use a fibreoptic bronchoscope (FOB) to confirm tube position every time.
-
3.How is the DLT inserted?
-
•Check the integrity of the tracheal and bronchial cuffs.
-
•Lubricate the outside of the DLT.
-
•Lubricate and insert an intubating stylet (usually provided in the pack) into the endobronchial lumen before insertion.
-
•The stylet can be preshaped to aid placement of the DLT.
-
•Perform direct laryngoscopy and visualize the glottis.
-
•Advance the DLT till the endobrochial cuff has passed beyond the vocal cords and then remove the stylet.
-
•Rotate the DLT 90° clockwise or anticlockwise (depending on the side of DLT placement).
-
•Pass the tracheal cuff beyond the glottis until resistance is encountered.
-
•The depth of insertion of the DLT correlates to the height of an average sized patient and is given by the formula 12 + (patient height)/10 cm, measured at the teeth.5
-
•
-
4.
How is successful positioning of the DLT confirmed?
Sequential clamping and auscultation: the ‘three-step’ method
-
•
Step 1: Inflate the tracheal cuff with the minimal volume to seal glottic air leak. Perform positive pressure ventilation and auscultate to confirm bilateral air entry. Obtain an acceptable capnography trace.
-
•
Step 2: Clamp the tracheal limb of the breathing circuit connector and disconnect this from the tracheal lumen of the DLT. Inflate the bronchial cuff with 1–3 ml and ventilate through the bronchial lumen. Auscultate to confirm unilateral ventilation and no audible air leak (Fig. 3).
-
•
Step 3: Release the tracheal lumen clamp and close the port. Auscultate to confirm resumption of bilateral air entry.
Fig 3.
Disconnection of tracheal lumen from breathing circuit and clamping of tracheal limb of the connector for one-lung ventilation through the bronchial lumen.
While the sequential clamping and auscultation technique described above is usually sufficient to confirm appropriate position of left-sided DLTs, FOB is usually used to confirm the same. For right-sided DLTs, FOB use is essential for accurate placement (see below).
For left-sided DLTs:
-
•
Insert the FOB through the tracheal lumen and visualize the carina.
-
•
Identify the blue coloured endobronchial cuff as a thin crest within the left main bronchus but not herniating over the carina after inflation.
For right-sided DLTs:
-
•
In addition to the above steps, insert the FOB through the endobronchial lumen and ensure the Murphy's eye is aligned with the right upper lobe bronchus.
-
5.What are the problems related to the use of DLTs?
-
a.Malposition: This is the most common problem encountered with the use of DLTs. While small malpositions are clinically insignificant, a majorly malpositioned DLT may partially collapse the ventilated lung or fail to permit collapse of the intended lung, causing hypoxaemia and inadequate surgical access.6 Common causes of malposition include dislodgement of the endobronchial cuff because of overinflation, extension or flexion of the head and neck during or after patient positioning, and surgical manipulation of the bronchus.
-
b.Airway trauma:7 An oversized DLT or an undersized DLT that has migrated distally, can rupture the membranous portion of the trachea or bronchus. Iatrogenic airway trauma can present as unexplained airleaks, subcutaneous emphysema, massive bleed into the DLT lumen or protrusion of the endobronchial or endotracheal cuff into the surgical field.
-
c.Tension pneumothorax in the dependent lung during OLV caused by high ventilating pressures or large tidal volumes, especially in patients with pre-existing emphysematous lung disease.8
-
a.
Bronchial blockers
An alternate technique to achieve lung isolation involves the use of BBs to occlude the main-stem bronchus, thereby preventing ventilation distal to the occlusion. In addition, BBs can be used to provide selective lobar collapse as well.1 Collapse of the right upper lobe can be difficult due to the proximal take off of the right upper lobe bronchus, which is easily occluded by the bronchial cuff. BBs are placed either intraluminal within a SLT (i.e. coaxial) or placed separately adjacent and outside the SLT (i.e. independent). In general, they are more prone to movement and displacement than DLTs.
Some of the currently available BBs are:
-
•
Torque control blocker univent
-
•
Arndt wire-guided endobrochial blocker
-
•
Cohen tip-deflecting endobronchial blocker
-
•
Fuji Uniblocker
-
•
Rusch Bifid EZ-blocker
The characteristics of the BBs are provided in Table 2.9
Table 2.
Features of different bronchial blockers
| Cohen blocker | Fuji uniblocker | Arndt blocker | |
|---|---|---|---|
| Size (Fr) | 9 | 5, 9 | 5, 7, 9 |
| Guidance mechanism | Wheel device | None, preshaped | Nylon wire loop |
| Smallest recommended tracheal tube (for coaxial use) (mm) | 9 Fr (8.0 ETT) | 9 Fr (8.0 ETT) | 5 Fr (4.5 ETT), 7 Fr (7.0 ETT), 8 Fr (8.0 ETT) |
| Central channel (mm ID) | 1.6 | 2.0 | 1.4 |
ETT, endotracheal tube.
While the BBs mentioned above have a similar principle of use, they vary slightly with respect to each other in terms of their technique of insertion. Anaesthetists unfamiliar with the routine use of BBs are advised to refer to the manufacturer manual for details regarding size selection and technique of insertion. Regardless of their design, all BBs require the use of a FOB to confirm position.
Single-lumen tubes
The last option available for lung isolation is to use a SLT or an endobronchial tube and advance it into the main bronchus of the non-operative lung to selectively ventilate it, while permitting slow collapse of the contralateral lung. This technique is hardly used in adult patients except in rare cases of emergency surgery or extremely difficult airways.
The advantages and disadvantages of the various options available for OLV are presented in Table 3.
Table 3.
Comparison between the various options available for lung isolation
| Options | Advantages | Disadvantages |
|---|---|---|
| Double lumen tubes |
|
|
| Bronchial blockers |
|
|
| Endotracheal tube advanced into bronchus |
|
|
CPAP, continuous positive airway pressure; OLV, one-lung ventilation; FOB, fibreoptic bronchoscopy.
Lung isolation in special scenarios
Difficult airway
Patients with an anticipated difficult airway requiring OLV can present extreme challenges to the anaesthetist.10 These patients must have a careful detailed preanaesthetic evaluation including a thorough airway assessment. Some could also benefit from multidisciplinary input involving thoracic surgeons, radiologists, and ear, nose, and throat surgeons. Several options are available in these situations to increase the chance of safe and successful lung isolation:
-
•
Optimal patient positioning and adequate dose of muscle relaxant with sufficient time prior direct laryngoscopy to obtain ideal intubating conditions.
-
•
Direct laryngoscopy with the use of specially designed bougies for DLTs.
-
•
Videolaryngoscopes (e.g. McGrath™).
-
•
Rigid optical stylets (e.g. BONFILS intubating endoscope).
-
•
Standard orotracheal intubation with SLT followed by exchange for a DLT using specifically designed soft tipped DLT exchange catheters (Cook exchange catheter; Cook Critical Care, Bloomington, USA).
-
•
FOB (awake or asleep; while standard textbooks of thoracic anaesthesia describe the use of FOB for insertion of DLTs, in reality, the use of FOB with DLTs can be extremely difficult owing to the bulky nature of the tube and its increased length compared to standard endotracheal tubes).
Patients with a tracheostomy in place or postlaryngectomy
Placing lung isolation devices through a tracheostomy stoma is fraught with dangers of malposition and potential complete airway loss.11 The options available to obtain OLV in this unique patient subgroup are:
-
•
Removal of the tracheostomy tube (TT) and inserting a conventional DLT through the stoma.
-
•
Removal of the TT and insertion of a SLT through the stoma followed by a BB.
-
•
Passing a BB coaxially through the cuffed TT.
-
•
Replacing the TT with a specially designed short DLT (Naruke DLT).12
-
•
Removal of the TT and standard orotracheal placement of a DLT or BB (not possible postlaryngectomy).
Patients with intraoperative OLV requiring postoperative mechanical ventilation
Although less than ideal, some patients who have had prolonged and often complicated thoracic surgeries may require postoperative mechanical ventilation. The challenges presented by this scenario include:
-
•
A potentially oedematous upper airway at the end of a prolonged surgery.
-
•
Requirement of continued lung isolation in the immediate postoperative period, either to protect the healthy lung from contamination or to avoid stump dehiscence due to undue positive pressure ventilation.
-
•
Unfamiliarity among nursing staff in the intensive care unit regarding the management of DLTs and BBs.
-
•
Increased chances of hypoxaemia and airway trauma postoperatively.
-
•
Increased pressure on suture lines due to positive pressure ventilation, thereby increasing risk of dehiscence and pneumothorax.
The management options available are:13
-
•
If the surgical procedure has not been a lung resection surgery, double-lung ventilation can be safely resumed in the postoperative period. The DLT is exchanged with a standard SLT over an exchange catheter under direct laryngoscopy at the end of the surgery.
-
•
Both lungs could be ventilated in the ICU with the DLT in situ. The bronchial cuff should be deflated as soon as permanent two-lung ventilation is established, to reduce bronchial mucosal damage caused by the low volume high pressure bronchial cuff and any potential airflow obstruction that could be caused from the bronchial cuff herniating over the carina.
Hypoxaemia during OLV
Immediately after the initiation of OLV, there is a fall in arterial oxygenation and saturation which gradually picks up as hypoxic pulmonary vasoconstriction (HPV) increases. HPV is characteristically biphasic, with an early response beginning within the first few seconds to reach a maximum in about 15 min, followed by a second phase that begins about 30–40 min later to peak at 2 h.14 The pathophysiology of HPV in health and disease has been reviewed in detail in a recent article in the BJA Education.15 While there is no accepted figure for the safest lower limit of oxygen saturation during OLV, a value ≥90% is recommended. Hypoxaemia during OLV in most cases respond well to some of the following simple interventions:16
-
•
Increase FiO2 to 1.0. This can be employed in all patients except those who have received bleomycin for malignancy.
-
•
Recheck the position of DLT/BB. A correctly positioned tube is crucial for the development of a adequate and appropriate HPV response.
-
•
Ensure that patient haemodynamics are acceptable and cardiac output is optimal. Treat with fluid, vasopressors, or inotropes as appropriate.
-
•
Perform recruitment manoeuvre to the ventilated lung. This may, however, cause transient hypotension and transient worsening of hypoxemia if more blood is diverted to the non-ventilated lung.
-
•
Adjust PEEP to the ventilated lung (caution in patients with chronic obstructive pulmonary disease).17
-
•
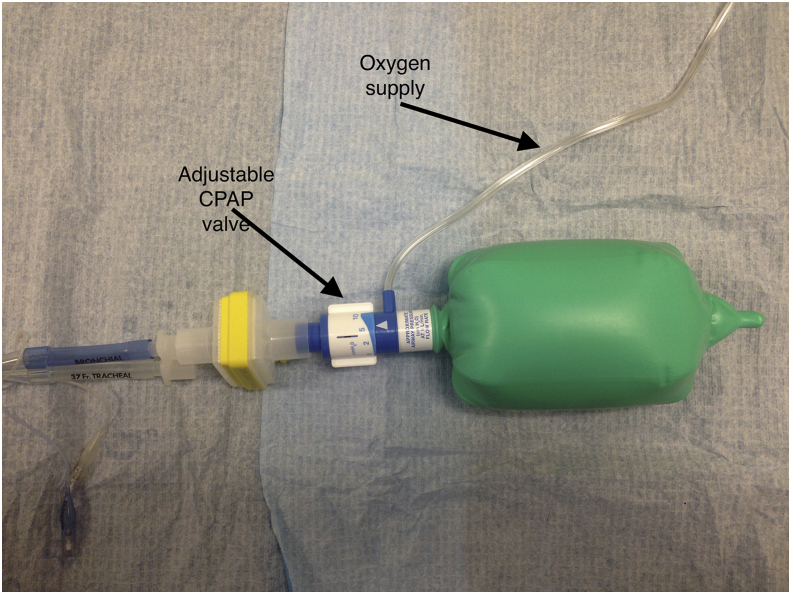
Insufflation of oxygen followed by application of 1–5 cm H2O of CPAP to the non-ventilated lung (Fig. 4).17
-
•
Intermittent reinflation of the non-ventilated lung (preconditioning of the HPV reflex, that becomes better with repeated hypoxic exposures).18
-
•
Mechanical restriction of blood flow to the non-ventilated lung by clamping of the pulmonary arteries, by the surgeons.
-
•
In case of sudden severe desaturation, resume two-lung ventilation as soon as possible.
Fig 4.
Continuous positive airway pressure (CPAP) circuit for non-ventilated lung. Used for the treatment of hypoxaemia during one-lung ventilation.
OLV and acute lung injury
Patients requiring OLV are at an increased risk of developing acute lung injury in the immediate postoperative period either due to the pathology itself or due to the surgical procedure they have undergone. Anaesthetic management, particularly mechanical ventilation can influence the extent of perioperative acute lung injury and it is for this reason that specific lung protective strategies are recommended to mitigate the extent of lung damage caused by positive pressure ventilation.19 The suggested ventilator strategies as part of lung protective strategies include.
-
•
Maintain FiO2 as low as possible
-
•
Low tidal volumes (6 ml kg−1 predicted body weight) to maintain peak airway pressures as low as possible, not more than 35 cm H2O
-
•
PEEP 5–8 cm H2O (except in patients with chronic obstructive pulmonary disease where no added PEEP is used)
-
•
Frequent recruitment manoeuvres
-
•
Permissive hypercapnia
To conclude, the optimal technique of lung isolation will depend on several factors, including the indication for OLV, the patient's airway, the expertise of the anaesthetist and the availability of equipment. Whatever technique is ultimately used, it is crucial to understand the patient's laryngotracheobronchial anatomy, review relevant imaging preoperatively and gain expertise in the use of FOB to confirm position and troubleshoot issues that could occur with the use of OLV devices.
Declaration of interest
None declared.
MCQs
The associated MCQs (to support CME/CPD activity) can be accessed at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Vighnesh Ashok FRCA is an International Training Fellow in Anaesthetics at Norfolk and Norwich University Hospital NHS Trust
Jonathon Francis FRCA is a Consultant Anaesthetist at Norfolk and Norwich University Hospital whose specialist interests include anaesthesia for Thoracic and Upper GI surgery
Matrix codes: 1A01, 2A01, 3A01
References
- 1.Campos J.H. Progress in lung separation. Thorac Surg Clin. 2005;15:71–83. doi: 10.1016/j.thorsurg.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky J.B., Lemmens J.M.H. Left double lumen tubes: clinical experience with 1,170 patients. J Cardiothorac Vasc Anesth. 2003;17:289–298. doi: 10.1016/s1053-0770(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 3.Campos J.H., Gomez M.N. Pro: right sided double lumen endotracheal tubes should be used routinely in thoracic surgery. J Cardiothorac Vasc Anesth. 2002;16:246–248. doi: 10.1053/jcan.2002.31091. [DOI] [PubMed] [Google Scholar]
- 4.Eberle B., Weiler N., Vogel N. Computed tomography based tracheobronchial image reconstruction allows selection of individually appropriate double lumen tube size. J Cardiothorac Vasc Anesth. 1999;13:532–537. doi: 10.1016/s1053-0770(99)90003-4. [DOI] [PubMed] [Google Scholar]
- 5.Yasumoto M., Higa K., Nitahara K. Optimal depth of insertion of left sided double lumen endobronchial tubes cannot be predicted from body height in below average sized adult patients. Eur J Anaesthesiol. 2006;23:42–44. doi: 10.1017/S0265021505001742. [DOI] [PubMed] [Google Scholar]
- 6.Inoue S., Nishimine N., Kitaguchi K. Double lumen tube location predicts tube malposition and hypoxemia during one-lung ventilation. Br J Anaesth. 2004;92:195–201. doi: 10.1093/bja/aeh055. [DOI] [PubMed] [Google Scholar]
- 7.Yuceyar L., Kaynak K., Canturk E. Bronchial rupture with a left sided polyvinylchloride double lumen tube. Acta Anaesthesiol Scand. 2003;47:622–625. doi: 10.1034/j.1399-6576.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 8.Weng W., DeCrosta D.J., Zhang H. Tension pneumothorax during one-lung ventilation: a case report. J Clin Anesth. 2002;14:529–531. doi: 10.1016/s0952-8180(02)00405-1. [DOI] [PubMed] [Google Scholar]
- 9.Campos J.H. Which device should be considered the best for lung isolation: double lumen endobronchial tube versus bronchial blocker. Curr Opin Anaesthesiol. 2007;20:27–31. doi: 10.1097/ACO.0b013e3280111e2a. [DOI] [PubMed] [Google Scholar]
- 10.Hagihara S., Takashina M., Mori T. One-lung ventilation in patients with difficult airways. J Cardiothorac Vasc Anesth. 1998;12:186–188. doi: 10.1016/s1053-0770(98)90330-5. [DOI] [PubMed] [Google Scholar]
- 11.Tobias J.D. Variations on one-lung ventilation. J Clin Anesth. 2001;13:35–39. doi: 10.1016/s0952-8180(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 12.Saito T., Naruke T., Carney E. New double intrabronchial tube (Naruke tube) for tracheostomized patients. Anesthesiology. 1998;89:1038–1039. doi: 10.1097/00000542-199810000-00037. [DOI] [PubMed] [Google Scholar]
- 13.Anantham D., Jagadesan R., Tiew P.E.C. Clinical review: independent lung ventilation in critical care. Crit Care. 2005;9:594–600. doi: 10.1186/cc3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumb A., Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiol. 2015;122:932–946. doi: 10.1097/ALN.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 15.Tarry D., Powell M. Hypoxic pulmonary vasoconstriction. BJA Educ. 2017;17:208–213. [Google Scholar]
- 16.Ng A., Swanevelder J. Hypoxaemia during one-lung anaesthesia. BJA Educ. 2010;10:117–122. [Google Scholar]
- 17.Fugiwara M., Abe K., Mashimo T. The effects of positive end-expiratory pressure and continuous positive airway pressure on the oxygenation and shunt fraction during one-lung ventilation with propofol anesthesia. J Clin Anesth. 2001;13:473–477. doi: 10.1016/s0952-8180(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 18.Wang J.Y., Russel G.N., Page R.D. A comparison of the effects of desflurane and isoflurane on arterial oxygenation during one-lung anesthesia. Anaesth. 2000;55:167–173. doi: 10.1046/j.1365-2044.2000.055002167.x. [DOI] [PubMed] [Google Scholar]
- 19.Kilpatrick B., Slinger P. Lung protective strategies in anaesthesia. Br J Anaesth. 2010;105:108–116. doi: 10.1093/bja/aeq299. [DOI] [PMC free article] [PubMed] [Google Scholar]