Key points.
-
•
Local anaesthetic agents are amphipathic molecules.
-
•
They bind primarily to sodium channels but also to potassium and calcium channels, and G-protein-coupled receptors.
-
•
Structural modifications alter the physicochemical characteristics of a local anaesthetic.
-
•
Speed of onset, potency, and duration depend on the pKa, lipid solubility and protein binding, respectively.
-
•
All local anaesthetic agents carry a risk of toxicity.
Learning objectives.
By reading this article, you should be able to:
-
•
Describe the basic structure of local anaesthetic agents.
-
•
Illustrate the relationships between the structure, function and toxicity of local anaesthetic agents.
-
•
Identify the pharmacological profiles of commonly used local anaesthetics.
Introduction
Local anaesthetic agents suppress action potentials in excitable tissues by blocking voltage-gated Na+ channels. In doing so, they inhibit action potentials in nociceptive fibres and so block the transmission of pain impulses. This article describes nerve anatomy and physiology, the molecular site of action of local anaesthetics, and their pharmacology.
Nerve anatomy and physiology
Nerves rapidly transmit electrical signals to and from the central nervous system. Myelinated fibres conduct faster because the action potential jumps between nodes of Ranvier. Myelinated A fibres are categorised into four groups that have separate functions: Aα fibres supply skeletal muscle; Aβ fibres transmit tactile sensation; Aγ fibres provide innervation to muscle spindles; and Aδ fibres transmit nociception and cold. Myelinated B fibres are autonomic preganglionic nerves and the slower conducting, unmyelinated C fibres transmit dull pain from skin and viscera.1
Surgical incision or trauma creates a local ‘inflammatory soup’ that activates nociceptors, the free nerve endings of Aδ fibres found in skin, muscle, joints, bone and viscera. Stimulation of nociceptors results in depolarisation, which in turn activates voltage-gated sodium channels, proteins that reside in the cell membrane of nerves and myocardium.
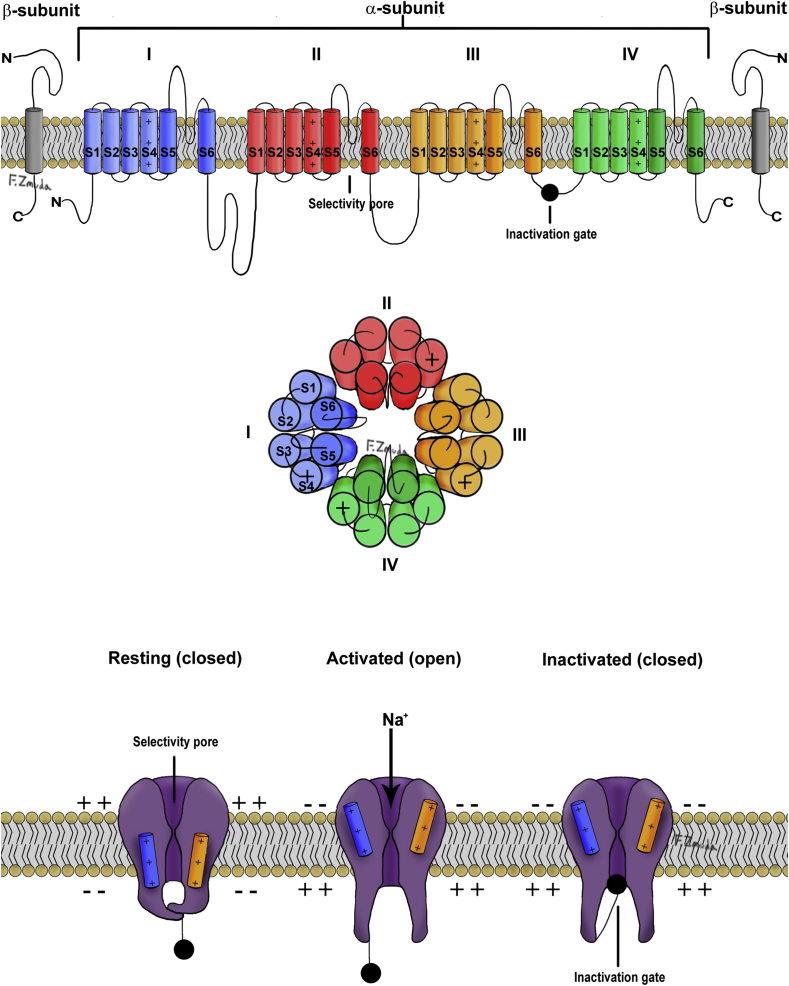
The voltage-gated Na+ channel is a complex structure composed of a large pore-forming α subunit associated with one or two β subunits. The α subunit is comprised of four domains (I–IV), each containing six segments (S1–S6) that wrap round a bell-shaped central channel (Fig. 1). The channel is formed by the S5 and S6 segments and the short loops of amino acids that link them. The inactivation gate is formed by a loop connecting domains III and IV. S4 in each domain has positively charged arginine or lysine amino acids and is the voltage-sensitive region of the Na+ channel.
Fig 1.
Structure and configurations of the voltage gated Na+ channel.
Three conformational states of the Na+ channel exist: resting, open, and inactivated. In the resting state, the membrane potential is approximately –70 mV and is generated by the outward movement of K+ ions along their concentration gradient, whereas the negatively charged anions (principally proteins) remain within the cell; this gerenates a transmembrane voltage or resting membrane potential. The S4 segments are in the ‘down’ position, making the channel non-conductive. The Na+ channels open during depolarisation by outward spiral rotation of the S4 segments allowing rapid influx of Na+ ions, down the electrical and chemical gradients. This exposes the receptor site of the inactivation gate, located between domains III and IV, leading to channel inactivation. From the inactivated state, the channel recovers to the resting state only by repolarisation of the cell membrane.2
Impulse generation along a nerve causes rapid movement of Na+ ions inwards and K+ ions outwards through selective ion channels. When the membrane potential increases to a threshold of –55 mV, an action potential is generated by the rapid influx of positive Na+ ions via the voltage-gated Na+ channels, peaking at +40 mV. Sodium channel inactivation and an efflux of K+ ions repolarises the nerve back to its resting state. Thereafter, the Na+/K+ pump restores the electrochemical gradients of the resting membrane potential.
Ten genes encode voltage-gated Na+ channels. All of the resultant channels are susceptible to local anaesthetic block.2 Different tissues express genes for Na+ channels differently. For example, NaV1.7 and NaV1.8 Na+ channels are highly expressed in sensory neurones, whereas NaV1.5 are found in cardiac cells and breast and colon cancer metastatic cells.
Mechanism of action of local anaesthetics
Lipophilic, unionised local anaesthetic molecules cross the phospholipid neuronal membrane. The molecules dissociate to reach a new equilibrium of ionised and un-ionised moieties, dependent on the intracellular pH and the pKa of the local anaesthetic. The ionised form binds to open voltage-gated Na+ channels in a reversible and concentration-dependent manner. The binding site for local anaesthetics is located in domain IV, loop S6 and is only accessible when the channel is open. The binding of local anaesthetics to open Na+ channels increases with the frequency of nerve depolarisation. This is known as a use-dependent or phasic block.
Bound local anaesthetic drug stabilises the inactivated receptor state, preventing further neuronal transmission. Local anaesthetic nerve block is concentration-dependent. With increased concentrations of local anaesthetic, the peak of the action potential is reduced, the firing threshold increases, impulse conduction is attenuated, and the refractory period lengthened. Increased concentrations inhibit all nerve conduction.
Both lidocaine and bupivacaine block cardiac Na+ channels. However, bupivacaine binds with higher affinity and dissociates more slowly. This causes it to accumulate during diastole, prolong conduction and induce re-entry-induced arrhythmias.
Local anaesthetics provide a differential block in a concentration-dependent manner. Aγ spindle efferents and the Aδ nociceptive fibres are most susceptible, whereas non-myelinated C fibres are relatively resistant. Differential sensitivity to local anaesthetics can be demonstrated during epidural block. Sympathetic fibres are most easily blocked, requiring the lowest concentration of local anaesthetic to block neuronal transmission. Sympathetic blockade usually reaches a higher dermatome than other modalities. Temperature (cold) and pain (pinprick), followed by proprioception and finally motor fibres are next most easily blocked, demonstrated by a descending dermatomal level. During epidural anaesthesia for Caesarean section, sensation of touch and proprioception (Aβ fibres) may therefore still occur despite adequate sensory block, which can be distressing for patients.
Pharmacology of local anaesthetics
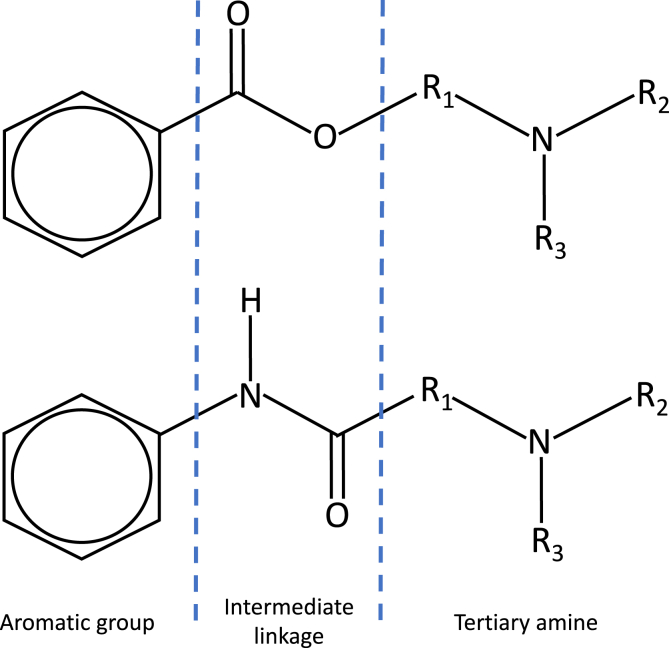
Local anaesthetic drugs are water-soluble salts of lipid-soluble alkaloids. The structure of local anaesthetics consists of three components: a lipophilic aromatic group, an intermediary link and a hydrophilic amine group (Fig. 2). The intermediary link categorises local anaesthetics into esters or amides (Table 1).3 For example, increasing the length of carbon chains attached to either the aromatic ring, amide linkage or the tertiary amine offers higher lipid solubility, potency and increased duration of action.
Fig 2.
Structure of ester (top) and amide local anaesthetics. Each contains an aromatic group, an intermediate linkage (ester/amide), and a tertiary amine.
Table 1.
| Structural classification | MW | pKa | Protein binding (%) | Partition coefficient | Onset | Elimination half-life (min) | Maximum dose without vasoconstrictor (mg kg−1) | Maximum dose with vasoconstrictor (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|---|
| Cocaine | Ester | 311 | 8.6 | 95 | – | Fast | 100 | 1.5 (topical) | – |
| Chloroprocaine | Ester | 271 | 9.1 | – | 17 | Fast | 6 | 11 | 14 |
| Prilocaine | Ester | 220 | 7.7 | 55 | 50 | Fast | 100 | 6 | 8 |
| Lidocaine | Amide | 234 | 7.8 | 70 | 110 | Fast | 100 | 3 | 7* |
| Mepivacaine | Amide | 246 | 7.7 | 77 | 42 | Fast | 115 | 5 | 7 |
| Bupivacaine | Amide | 288 | 8.1 | 95 | 560 | Moderate | 210 | 2 | 2 |
| Ropivacaine | Amide | 274 | 8.1 | 94 | 230 | Moderate | 120 | 3 | 3 |
| Levobupivacaine | Amide | 288 | 8.1 | 95 | – | Moderate | 210 | 2 | 2 |
9 for airway topicalisation.
Replacement of the tertiary amine by a piperidine ring increases lipid solubility and duration of action; the addition of a butyl group in place of the amine on the benzene ring of procaine gives tetracaine (amethocaine); and the addition of a propyl or butyl group to the amine end of mepivacaine results in ropivacaine or bupivacaine, respectively. Compared to bupivacaine, ropivacaine's propyl group gives a lower lipid solubility that causes it to penetrate large myelinated motor fibres to a lesser extent, giving a more selective sensory blockade.4
Bupivacaine exists in two enantiomers, which are mirror images of each other. Although structurally identical, enantiomers can exhibit clinical differences including potency and adverse effects. The discovery of a selective blockade of cardiac Na+ channels by the dextro-enantiomer of bupivacaine led to the creation and widespread use of two levo-enantiomers: levobupivacaine and ropivacaine.5 These exhibit lower potency at myocardial Na+ and K+ channels and have less effect on myocardial electrical conduction and contractility compared to bupivacaine.
Enantiomers were historically classified according to their ability to rotate the plane of polarised light. For example, the prefix dextro indicates clockwise rotation and the prefix levo indicates anticlockwise rotation of polarised light. Alternatively, enantiomers may be classified by the order of atoms around the central carbon molecule. For example, in a rectus (R) configuration, atomic mass reduces in a clockwise direction whereas the opposite occurs in the sinister (S) configuration.
Pharmacological properties of local anaesthetics
The speed of onset, potency and duration of local anaesthetics is dependant on the pKa, lipid solubility and protein binding, respectively.
pKa
The dissociation of amphipathic local anaesthetics is determined by their pKa and the pH of the tissue into which they are injected. The pKa is the pH at which the ionised and un-ionised forms are present in equal amounts. For bases, such as local anaesthetics, the higher the pKa, the greater the ionised fraction in solution. The ratio of the two states is described by the Henderson–Hasselbalch equation:
| log [A–]/[AH] = pKa – pH |
where [A–] is the ionised form and [AH] is the non-ionised form.
As rate of diffusion across the nerve sheath and nerve membrane is related to the proportion of non-ionised drug, local anaesthetics with low pKa have a fast onset of action, and local anaesthetics with a high pKa have a slow onset of action. For example, lidocaine (pKa=7.8) has a fast onset in comparison with bupivacaine (pKa=8.1), because at pH 7.4 a greater proportion of lidocaine exists in the non-ionised form. Inflamed and infected tissue is more acidaemic, and therefore more local anaesthetic exists in the ionised form, reducing the amount of un-ionised drug available to cross the nerve and provide analgesia. The pH of tissue can be affected by adjuvants, for example, some clinicians add bicarbonate to speed the onset of epidural anaesthesia.
Molecular weight
The smaller the molecular weight, the more rapidly molecules diffuse through membranes.
Lipid solubility
Lipid solubility and potency are closely related. The lipid solubility of local anaesthetics is expressed as the partition coefficient, which is defined as the ratio of concentrations when local anaesthetic is dissolved in a mixture of lipid and aqueous solvents. Greater lipid solubility enables more rapid diffusion through lipid membranes to reach their site of action, influencing the speed of onset, although—as outlined above—other factors are also important. In addition, greater lipid solubility gives a greater volume of distribution.
Protein binding
Local anaesthetics with high protein binding to α1-acid glycoprotein have a longer duration of action and lower bioavailability. Hypoxia, hypercarbia, and acidaemia all decrease protein binding, and increase the risk of toxicity. Children younger than 6 months have less protein binding capacity.
Vasoactivity
The vasoactivity of local anaesthetics influences potency and duration of action. For example, more rapid absorption occurs after lidocaine administration compared to bupivacaine. Levobupivacaine and ropivacaine have a bimodal vasoactive response. Both vasodilate at clinical doses and vasoconstrict at subclinical doses. Concentrations of adrenaline as low as 1:800,000 are sufficient to cause vasocontriction in tissues in the presence of local anaesthetics.
Routes of administration
Local anaesthetics are administered via a number of routes. These include topical, for example to the skin and airway, subcutaneous, intravenous, perineural, epidural and intrathecal. Further details of the routes of local anaesthetic delivery have been covered previously in this journal and are outside the scope of this article.
Pharmacokinetics
Absorption
The absorption of local anaesthetics is dependent on the site of injection, rate of injection, dosage and vasoactivity of the injectate. Typically, intrapleural block is associated with the highest absorption and subcutaneous infiltration with least absorption. The order of peak plasma concentration after a single dose is intrapleural > intercostal > lumbar epidural > brachial plexus > subcutaneous > sciatic > femoral.
Distribution
Esters local anaesthetic agents are less protein bound than amide local anaesthetics (Table 1). Tissue distribution tends to be proportional to the tissue/blood partition coefficient of the local anaesthetic, and the mass and perfusion of the tissue.
Metabolism and clearance
Ester and amide local anaesthetic agents differ concerning their metabolism and allergic potential. Esters are hydrolysed rapidly in plasma by pseudocholinesterase to the metabolite para-aminobenzoic acid (PABA), which can cause an allergic reaction. Plasma half-life varies from less than 1 min (chloroprocaine) to 8 min (tetracaine) and is prolonged in the presence of atypical cholinesterase. Cocaine, unlike other esters, undergoes hepatic hydrolysis followed by renal excretion.3
In the liver, amide local anaesthetics undergo aromatic hydroxylation, amide hydrolysis and N-dealkylation.3 Amide metabolism is much slower than plasma hydrolysis, and thus amide local anaesthetics are more prone to accumulation in the presence of hepatic dysfunction or reduced hepatic blood flow.3 Prilocaine undergoes metabolism in the lungs. Amides have a very low allergic potential themselves, and an observed reaction may be caused by an additive such as the stabilising agent methylparaben. In addition, response to vasoconstrictor adjuvants may be mistaken for allergy.
Clearance values and elimination half-times for amide local anaesthetics represent mainly hepatic metabolism because renal excretion of unchanged drug is minimal. Accumulation of metabolites may occur in renal failure. Lidocaine has a high hepatic extraction ratio: clearance is dependent on hepatic blood flow and is relatively unaltered by changes in hepatic enzyme activity. Owing to the efficiency of the drug in dissociating from plasma proteins, entering the hepatocyte, and undergoing metabolism, the rate limiting step is hepatic perfusion. This is important in critical illness, particularly in states of low cardiac output and reduced hepatic blood flow.6
Adjuvants
Adjuvants are used to influence the activity of the local anaesthetic, prolonging or enhancing its action. Those in clinical use include adrenaline, clonidine, opioids, ketamine, dexamethasone, dexmedetomidine and midazolam. Other than adrenaline, there is a weak evidence base for adding adjuvants to peripherally administered local anaesthetics. They are discussed in detail elsewhere in the BJA Education series.
Mixtures of local anaesthetics
When two compounds are mixed to produce a substance that has a single set of physical characteristics, it is said to be eutectic. The eutectic mixture of local anaesthetic (EMLA) contains a mixture of crystalline bases of 2.5% lidocaine and 2.5% prilocaine in an oil/water emulsion. The mixture has a lower melting point than separate local anaesthetics and allows for a higher concentration of local anaesthetic to be used.7
Anaesthetists often combine local anaesthetics in order to achieve adequate speed of onset and duration of block. In contrast to EMLA, the physicochemical properties of these mixtures are not well characterised. Clinical research has demonstrated that increasing the proportion of lidocaine in a lidocaine ropivacaine mixture may not necessarily have the anticipated effect of reduced time of block onset.8 Decisions regarding the mixture of local anaesthetic agents continue to be based on clinician preference rather than consensus.
Placental transfer
The rate and degree of diffusion of local anaesthetic across the placenta depends on protein binding, pKa, and maternal and fetal pH. Bupivacaine is highly protein bound (95%) and has an umbilical vein/maternal arterial ratio of 0.3. This contrasts with lidocaine (70%) with a ratio of 0.5–0.7. In prolonged labour, acidosis in the fetus can result in accumulation of local anaesthetic in the fetus by ion trapping. However, because of rapid hydrolysis, ester local anaesthetics do not cross the placenta in significant amounts.
Toxicity
Local anaesthetics cause both systemic and local toxicity. Systemic toxicity relates to the relatively narrow difference between therapeutic plasma levels and toxic levels (Table 1). Peak plasma levels are determined by the dose and rate of systemic absorption. The genes controlling the α subunit of Na+ channels give rise to different pharmacological and biophysical profiles of Na+ channels through the body. Ropivacaine and levobupivacaine have lower systemic toxicity than other amides because of their lower affinity for cardiac channels (NaV1.5).9 Systemic toxicity is covered elsewhere in the BJA Education series.10
Local toxicity caused by local anaesthetics to nerves (and other tissues) and occurs in a time-, concentration-, and drug-dependent manner. The precise order of events during local anaesthetic-induced neurotoxicity is not known, but possible cellular mechanisms identified include the intrinsic caspase, phosphoinositide 3-kinase and mitogen-activated protein kinase pathways. Local anaesthetic-induced neurotoxicity is rare, and the majority of perioperative nerve injuries are unrelated to regional anaesthesia.
O-toluidine, the metabolite of prilocaine, can oxidise haemoglobin causing methaemoglobinaemia. This shifts the oxyhaemoglobin dissociation curve to the left, reducing the ability of haemoglobin to release oxygen to the tissues. The risk of methaemoglobinaemia increases dramatically when the maximum recommended dose is exceeded (Table 1). Benzocaine and lidocaine can also cause methaemoglobinaemia.10,11
Intra-articular local anaesthetics are commonly used as part of multimodal analgesia in arthroscopy. However, they have been demonstrated to cause chondrotoxicity, and at a higher rate in patients with osteoarthritis. Ropivacaine is associated with a lower risk than bupivacaine or mepivacaine.12
Additional actions of local anaesthetics
Anti-inflammatory and antibacterial
Local anaesthetics have anti-inflammatory effects, mediated by a variety of mechanisms. They decrease polymorphonuclear leukocyte adherence, migration and accumulation at the site of inflammation, and altering macrophage and monocyte function.13 Although anti-inflammatory effects can be beneficial, it is also possible that reducing the function of such cells perioperatively could increase the risk of bacterial infection. At high concentrations, local anaesthetics conversely have anti-bacterial effects in laboratory studies. However, systemic concentrations during regional or local anaesthesia are low, and a theoretical risk of increased susceptibility to infection therefore exists.13 This has not been borne out, however, in vivo.
Antimetastatic properties
Surgery is associated with an initial proinflammatory phase followed by a period of immunosuppression. The antitumour activity of natural killer cells and CD8 T cells are inhibited and protumour effects of regulatory T cells and type 2 T helper cells are promoted. This provides an opportunity for tumour cells to metastasise.14
Local anaesthetics may reduce cancer recurrence by attenuation of this stress response, a reduction in pain (through regional techniques), which in turn reduces opioid and volatile requirements (both of which may negatively influence the immune system), and through a direct anti-tumour effect. However, in vivo data are limited to retrospective observational studies and post hoc analysis of randomised control trials originally of non-cancer outcomes.14 A Cochrane review concluded that evidence for a benefit of regional anaesthesia on tumour recurrence is currently inadequate.15
Neuropathic pain
The mechanism of action of intravenous lidocaine in neuropathic pain cannot be explained by blockade of voltage gated Na+ channels alone. The clinical effects include reduction of spontaneous pain, allodynia, and hyperalgesia.16
Acute postsurgical pain and postoperative ileus after colorectal surgery
Intravenous lidocaine is increasingly used to manage acute postsurgical pain and reduce postoperative ileus after colorectal surgery despite the available evidence being conflicting.6,17
Novel local anaesthetic agents
Liposomal preparations
Prolonged postoperative analgesia can be achieved via placement of a catheter to allow continuous or repeated administration of local anaesthetic. However, technical complications are relatively common and include leakage, migration and infection.
Liposomes are amphipathic molecules that form lipid bilayer spherical vesicles when suspended in an aqueous solution. They are used as a sustained release drug delivery system without the need for continuous infusion or risk of toxicity. However, Cochrane reviews have commented on the ‘low quality and volume of evidence’ and the lack of benefits of liposomal bupivacaine over bupivacaine for infiltration and peripheral nerve blockade to treat postsurgical pain.18
Development of other longer-acting local anaesthetics has focused on derivatives of natural toxins, use of additional receptors on nerve membranes, or attachment of bupivacaine to biodegradable polymers.
Animal toxins
Many venoms in nature contain toxins that inhibit Na+ channels, either as pore blockers or gate modifiers.19 A shellfish saxitoxin derivative, neosaxitoxin, binds to the extracellular portion of Na+ channels. Its potential benefits include better affinity for NaV1.7 and NaV1.8 sensory channels than NaV1.5 cardiac Na+ channels, but it still exhibits severe toxic reactions. Combination of venom analogues with local anaesthetic and adrenaline has been suggested as a means of optimising benefits and adverse effects.
Vanilloids and quaternary lidocaine
Quaternary lidocaine is a charged molecule that cannot pass through lipid barriers. The transient receptor potential (TRP) ion channels in primary afferent nociceptive neurones are activated by noxious heat and by the vanilloid capsaicin,20 and potentially provide an aqueous route for the entry and binding of quaternary lidocaine to the voltage-gated Na+ channel. Animal studies have demonstrated prolonged analgesia with less motor block than equivalent doses of lidocaine. However, the use of capsaicin is limited by painful irritation.21
Polymers
Sucrose acetate isobutyrate extended-release bupivacaine (SABER-bupivacaine) is a biodegradable depot drug that has the capacity to hold high concentrations of bupivacaine up to 660 mg.22 Similarly, a biodegradable polymer mixture of bupivacaine and meloxicam, an anti-inflammatory, termed HTX-011, has been investigated as a means of delivering long-lasting infiltrative pain relief after hernia and bunion surgery. Phase 3 infiltration studies show better pain relief compared to bupivacaine and extended time to first analgesic rescue.23 However, SABER-bupivacaine contains benzyl alcohol and cannot be used for perineural use. Trials of HTX-011 for nerve block are awaited.
Local anaesthetic reversal
Persistent numbness, drooling and inability to eat after local anaesthesia for dental surgery are unpleasant. Phentolamine mesylate is a non-selective alpha-adrenergic antagonist that causes vasodilation. This increases the blood flow and is reported to halve the reversal time of local anaesthesia.24
Conclusions
All local anaesthetics target the voltage-gated Na+ channel and carry the risk of toxicity. Therefore, knowledge of local anaesthetic drug pharmacology is essential for the safe use of these agents. New developments in the development of peripheral analgesics include toxins, and polymer-based compounds providing extended slow release.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Declaration of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors would like to acknowledge the contribution of the illustrator for Fig. 1, Dr Filip Zmuda MPharm PhD; research associate, University of Dundee.
Biographies
Alasdair Taylor BSc Med Sci FRCA is a specialty trainee in anaesthesia and a previous fellow in regional anaesthesia at Ninewells Hospital, Dundee. He is coauthor of the book Anatomy for the FRCA. His interests are in regional anaesthesia, global health, and medical education.
Graeme McLeod FRCA FFPMRCA MD is a consultant anaesthetist at Ninewells Hospital and honorary professor at the University of Dundee. He is co-lead of the MSc in Regional Anaesthesia and co-director of the annual regional anaesthesia mastery learning cadaver course at the University of Dundee; and also honorary senior lecturer at the University of East Anglia. He conducts translational multidisciplinary research and has published widely in the field of regional anaesthesia.
Matrix codes: 1A02, 2B03, A09
References
- 1.Colvin L.A. Physiology and pharmacology of pain. In: Thompson J.P., Wiles M.D., Moppett I.G., editors. Smith and Aitkenhead's textbook of anaesthesia. 7th edn. Elsevier; St Louis: 2019. pp. 100–121. [Google Scholar]
- 2.Atterall W.A., Swanson T.M. Structural basis for pharmacology of voltage-gated sodium and calcium channels. Mol Pharmacol. 2015;88:141–150. doi: 10.1124/mol.114.097659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peck T.E., Hill S.A., Williams M. 3rd edn. Cambridge University Press; Cambridge: 2008. Pharmacology for anaesthesia and intensive care. [Google Scholar]
- 4.Kuthiala G., Chaudhary G. Ropivacaine: a review of its pharmacology and clinical use. Indian J Anaesth. 2011;55:104–110. doi: 10.4103/0019-5049.79875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nau C., Strichartz G.R. Drug chirality in anesthesia. Anesthesiology. 2002;97:497–502. doi: 10.1097/00000542-200208000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Boucher B.A., Wood G.C., Swanson J.M. Pharmacokinetic changes in critical illness. Crit Care Clin. 2006;22:255–271. doi: 10.1016/j.ccc.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenström Reiz G.M., Reiz S.L. EMLA — a eutectic mixture of local anaesthetics for topical anaesthesia. Acta Anaesthesiol Scand. 1982;26:596–598. doi: 10.1111/j.1399-6576.1982.tb01822.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama M., Sakuma Y., Imamura H., Yano K., Kodama T., Ikari K. A comparison of the dose of anesthetic agents and the effective interval from the block procedure to skin incision for ultrasound-guided supraclavicular brachial plexus block in upper extremity surgery. Asian J Anesthesiol. 2017;55:83–86. doi: 10.1016/j.aja.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 9.El-Boghdadly K., Pawa A., Chin K.J. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth. 2018;11:35–44. doi: 10.2147/LRA.S154512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christie L.E., Picard J., Weinberg G.L. Local anaesthetic systemic toxicity. Continuing education in anaesthesia. Crit Care Pain. 2015;15:136–142. [Google Scholar]
- 11.Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837–845. doi: 10.1213/ane.0b013e318187c4b1. [DOI] [PubMed] [Google Scholar]
- 12.Breu A., Rosenmeier K., Kujat R., Angele P., Zink W. The cytotoxicity of bupivacaine, ropivacaine, and mepivacaine on human chondrocytes and cartilage. Anesth Analg. 2013;117:514–522. doi: 10.1213/ANE.0b013e31829481ed. [DOI] [PubMed] [Google Scholar]
- 13.Cruz F.F., Rocco P.R.M., Pelosi P. Anti-inflammatory properties of anesthetic agents. Crit Care. 2017;21:67. doi: 10.1186/s13054-017-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall T., Sherwin A., Ma D., Buggy D.J. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123:135–150. doi: 10.1016/j.bja.2019.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cakmakkaya O.S., Kolodzie K., Apfel C.C., Pace N.L. Anaesthetic techniques for risk of malignant tumour recurrence. Cochrane Database Syst Rev. 2014;11 doi: 10.1002/14651858.CD008877.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermanns H., Hollmann M.W., Stevens M.F. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019;123:335–349. doi: 10.1016/j.bja.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Weibel S., Jelting Y., Pace N.L. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;4 doi: 10.1002/14651858.CD009642.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton T.W., Athanassoglou V., Trivella M. Liposomal bupivacaine peripheral nerve block for the management of postoperative pain. Cochrane Database Syst Rev. 2016;8 doi: 10.1002/14651858.CD011476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maatuf Y., Geron M., Priel A. The role of toxins in the pursuit for novel analgesics. Toxins. 2019;11:131. doi: 10.3390/toxins11020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Messeguer A., Planells-Cases R., Ferrer-Montiel A. Physiology and pharmacology of the vanilloid receptor. Curr Neuropharmacol. 2006;4:1–15. doi: 10.2174/157015906775202995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenneis C., Kistner K., Puopolo M. Bupivacaine-induced cellular entry of QX-314 and its contribution to differential nerve block. Br J Pharmacol. 2014;171:438–451. doi: 10.1111/bph.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadj A., Hadj A., Hadj A. Safety and efficacy of extended-release bupivacaine local anaesthetic in open hernia repair: a randomized controlled trial. ANZ J Surg. 2012;82:251–257. doi: 10.1111/j.1445-2197.2011.05754.x. [DOI] [PubMed] [Google Scholar]
- 23.Viscusi E., Gimbel J.S., Pollack R.A., Hu J., Lee G.C. HTX-011 reduced pain intensity and opioid consumption versus bupivacaine HCl in bunionectomy: phase III results from the randomized EPOCH 1 study. Reg Anesth Pain Med. 2019;44:700–706. doi: 10.1136/rapm-2019-100531. [DOI] [PubMed] [Google Scholar]
- 24.Prasanna J.S. OraVerse: reverses numbness after dental procedures. J Maxillofac Oral Surg. 2012;11:212–219. doi: 10.1007/s12663-011-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]