Key points.
-
•
Video-assisted thoracic surgery (VATS) and robot-assisted thoracic surgery (RATS) are minimally invasive surgical procedures.
-
•
VATS and RATS have suggested benefits in terms of reduced perioperative complications and shorter lengths of stay compared with open surgery.
-
•
VATS is the preferred surgical approach for patients with poor cardiorespiratory reserve undergoing lung resection.
-
•
The success of VATS and RATS depends on optimal lung isolation.
-
•
RATS is a novel surgical technology that necessitates a different approach for the whole operating team.
Learning objectives.
By reading this article, you should be able to:
-
•
Summarise the thoracic surgical procedures that can be performed by a minimally invasive technique.
-
•
Explain the considerations for anaesthesia specific to video-assisted thoracic surgery and robot-assisted thoracic surgery (RATS), including the need for successful lung isolation and the challenges of managing hypoxaemia during one-lung ventilation.
-
•
Describe the important features of providing anaesthesia for RATS procedures, including positioning, limited access to the patient, and the need for well-rehearsed emergency drills.
The traditional approach for thoracic surgery is thoracotomy, which allows good surgical access but involves an extensive incision and rib spreading.1 In minimally invasive thoracic surgery (MITS), thoracotomy is not performed and surgery takes place with the operator's hands outside the chest. In video-assisted thoracic surgery (VATS) procedures, the surgeon directly manipulates instruments, whereas in robot-assisted thoracic surgery (RATS) the surgeon is a step further removed, at a separate console, controlling robotically held instruments. These techniques have been developed as less invasive approaches to thoracic surgery, with proponents suggesting benefits in terms of less pain, reduced inflammation, less impairment in pulmonary function, reduced postoperative morbidity, and shorter hospital stays.2
The main current use of MITS is with VATS for lung resection. Although there are no published RCTs comparing true VATS resection with thoracotomy, there is a suggestion of benefit: small studies have shown reduced perioperative morbidity, shorter hospital stays and recovery, and even potentially improved long-term survival.3,4 In addition, there are data demonstrating that some of these benefits may be more pronounced in those patients with poor lung function (FEV1 <60% predicted).5 The Video Assisted Thoracoscopic Lobectomy Versus Conventional Open Lobectomy for Lung Cancer study (VIOLET), a large pragmatic RCT comparing VATS and thoracotomy for lung cancer resection, has recently finished recruiting in the UK with results expected in 2020.
The proportion of lung resections performed by VATS in the UK increased from 15.7% in 2005 to 47.3% in 2015 (the most recent data available).6 In appropriate patients, particularly those with poor cardiorespiratory reserve, VATS is advocated as the preferred surgical approach.7 In addition to an increased proportion of lung resections performed by minimally invasive methods, there is an increased absolute number of lung resections being performed.6 This is driven by efforts to improve outcomes in lung cancer. Clinical guidelines now advocate offering lung resection to patients previously considered unsuitable for surgery, as long as they are willing to accept the higher risks. This includes patients with disease that would have been considered inoperable, or patients previously considered too unfit for surgery. Therefore, increasing numbers of higher-risk patients will continue to present for lung resection, with a larger proportion of minimally invasive procedures, and safe perioperative management is paramount. There is also a growing desire to minimise the length of hospital stay and associated costs after thoracic surgery: enhanced recovery protocols have been developed, with VATS being a vital component.7
As a development from VATS, there has been an increased use of robotic-assisted operations in thoracic surgery, in keeping with trends in other surgical specialties. The use of narrower, high-precision instruments with 360° articulation, placed through smaller ports, and with better (three-dimensional and high definition) visualisation and increased surgical comfort, are all thought to contribute to better outcomes.
Similar to VATS, there are no RCTs comparing outcomes after RATS with either VATS or thoracotomy. Despite the lack of high-quality evidence, there is a suggestion from retrospective cohort studies that RATS is safe, with similar outcomes to VATS in terms of perioperative morbidity, mortality, and cancer outcomes.4 Currently, the lack of robust evidence means that NHS England will not routinely commission RATS for resection of primary lung cancer.8
Video-assisted thoracic surgery
Surgical considerations
VATS describes the group of surgical procedures where a video camera and instruments are inserted via ports to avoid open surgery and associated rib spreading. The term can be applied to a number of similar but not identical procedures. This means that VATS is usually performed with a 4–8 cm ‘utility’ port for camera insertion, access, and specimen removal, along with two further incisions for instrument insertion.9 Alternatively, the camera and all instruments can be inserted through a single port—referred to as ‘uniportal’ VATS. The specific set-up depends on the surgery performed and operator preference.
Although the majority of thoracic surgical operations can be performed using VATS (Table 1), there are a number of procedures that would usually be carried out by thoracotomy. The central location of structures and the angulation required with VATS mean pneumonectomy, tracheal resection, and sleeve resections are challenging, and are often carried out via an open approach.
Table 1.
Procedures performed with VATS
| Procedure type | Indication |
|---|---|
| Diagnostic | Pleural biopsy and thoracocentesi |
| Staging of lung, pleural and oesophageal malignancies | |
| Diagnosis of parenchymal disease: fibrosis, nodules and pneumonitis | |
| Diagnosis of mediastinal tumours: thymoma, sarcoma and germ cell | |
| Pericardial disease: pericarditis and tumours | |
| Therapeutic | Pleural disease: pleurodesis and decortication |
| Parenchymal disease: wedge resection, segmentectomy, lobectomy, pneumonectomy, bullae resection and lung-volume reduction surgery | |
| Mediastinal disease: tumour excision, thymectomy and chylothorax | |
| Oesophageal surgery: vagotomy, myotomy and oesophagectomy | |
| Sympathectomy |
The detailed review of the selection of patients, and assessment of suitability to undergo thoracic surgery is beyond the scope of this article, but is based on factors related to the patient and factors related to the surgery being performed. Factors related to the patient include pulmonary function and the presence of comorbidities whilst factors related to surgery include tumour type, anatomical location and tumour, node and metastasis status.
For VATS, there are very few absolute contraindications, but factors considered include the following:10
-
(i)
previous surgery or radiotherapy (with associated adhesions)
-
(ii)
extensive pleural disease
-
(iii)
anatomical considerations (such as central or endobronchial lesions)
-
(iv)
large tumours (>6 cm).
The inability to tolerate one-lung ventilation (OLV) would be an absolute contraindication for longer procedures, but short procedures, including biopsies and bullectomies, may be possible with a VATS approach.
Conversion to open lobectomy may be required during any VATS procedure and can happen for a number of reasons:
-
(i)
intraoperative complications (mainly bleeding)
-
(ii)
technical problems (e.g. poor visualisation)
-
(iii)
anatomical problems (e.g. poor interlobar fissure or diffuse pleural adhesions)
-
(iv)
oncological considerations (e.g. upstaging of tumours and unexpected chest wall involvement).
The potential for injury to major vessels (aorta and pulmonary artery) with massive haemorrhage and the inability to immediately obtain control remain significant risks to MITS. Emergency conversion most frequently occurs for bleeding that cannot be definitively treated by a VATS approach. In reality, control of haemorrhage can usually be achieved thoracoscopically whilst a thoracotomy is performed in a controlled manner.
Considerations for anaesthesia
Anaesthesia for VATS is similar in many respects to anaesthesia for open thoracic surgery. Lung-protective ventilation and judicious fluid management are essential to try and minimise postoperative pulmonary complications (PPCs).7,11,12 However, there are a number of key differences.
General intraoperative management, including positioning
Conduct of anaesthesia will vary depending on the VATS procedure being performed. Smaller procedures may be performed with peripheral i.v. access, non-invasive arterial pressure monitoring, and short-acting agents. More prolonged or major procedures will require larger-gauge i.v. access and intra-arterial cannulation for monitoring and sampling. In those at increased risk of bleeding (previous surgery, extensive hilar resections, etc.), two large-bore cannulae should be considered mandatory. Central venous cannulation is infrequently used in VATS, but should be considered for individual patients, for example, those in which i.v. access is challenging, or cardiac function is poor and vasopressors or inotropic drugs may be required.
The choice of anaesthetic technique depends on the experience and preference of the anaesthetist, but neither TIVA nor volatile anaesthesia has been shown to be superior for patients undergoing OLV.13 However, a recent large retrospective study suggests the benefit for TIVA in patients undergoing lung resection with a reduced incidence of unplanned admission to the ICU.14
As with open surgery, positioning is frequently in the lateral decubitus position. In contrast to thoracotomy, in MITS, the operating table is adjusted to promote extreme lateral flexion, opening intercostal spaces and improving surgical access. This means care must be taken to ensure the patient is secured to the operating table; the combination of back and arm supports and ‘suction bean bag’ have both been described. Whatever method is chosen, the priorities are to ensure there is no scope for movement, that pressure points are padded effectively, and that the head and neck are adequately supported for a potentially long procedure. Caution must be taken to ensure airway devices, vascular cannulae, and monitors are not displaced at this stage. The double-lumen tracheal tube (DLT) can be displaced proximally with the bronchial cuff above the carina, or distally with the tracheal lumen in the bronchus, meaning it is essential that final confirmation of correct placement occurs once the patient is in the final position for surgery.
Lung isolation
Successful VATS is dependent on having a clear view of the operative field, with good lung isolation facilitating better exposure. Details of lung isolation have been described recently in this journal, and the techniques used often depend on local preference and expertise.12 It has been suggested that a DLT is the preferred technique for lung isolation in VATS, with bronchial blockers seen as less suitable because of slower lung collapse resulting from their narrower calibre. However, this is not borne out in clinical practice, with bronchial blockers widely utilised and studies demonstrating no clinically relevant differences between the two techniques.15
There are several reasons why adequate lung collapse may not be achieved:
-
(i)
misplaced DLT or bronchial blocker
-
(ii)
pleural adhesions or disease
-
(iii)
chronic bronchitis: inflamed narrowed airways, copious thickened secretions and impaired function of the mucociliary elevator, all contributing to gas trapping in the alveoli
-
(iv)
emphysema: loss of lung tissue and reduced elasticity
-
(v)
fibrosis
-
(vi)
tumour with extrinsic or intrinsic narrowing of airways promoting gas trapping.
Unfortunately, many of these conditions are evident in patients requiring thoracic surgery. Factors specific to VATS include the need for prompt and effective lung isolation, usually once the patient is positioned for surgery and placement of the DLT has been confirmed with the fibreoptic bronchoscope. Although the lung will not ‘collapse’ until the pleura is breached, volume loss can be encouraged before surgical incision, increasing pleural retraction pressure and theoretically reducing the risk of direct lung injury at the time of surgical port insertion. This period, in which the lung is isolated and not collapsed, can lead to increased shunt and hypoxia before commencing surgery, which will not improve until the pleura is incised and the lung collapses. Efforts must be made to avoid considerably prolonging OLV time; in thoracic surgery, there is a direct association between duration of OLV and risk of PPC.16 If significant delays are expected, then two-lung ventilation should be performed until surgery is ready to commence.
Techniques for improving lung collapse include:
-
(i)
ventilating the lungs with 100% O2 before lung isolation to remove nitrogen from the operative lung
-
(ii)
application of suction to the operative lung: if collapse is difficult in individual lobes, suction can be directed using the fibreoptic bronchoscope to ensure the large airways are clear of secretions.
As in open surgery, although there is no absolute level of hypoxia that is permitted, an oxygen saturation ≥90% is generally accepted.12 The options for management of hypoxia during OLV are similar to the patient undergoing thoracotomy, but caution needs to be exercised when applying them in these patients.12 Similar to open surgery, initial management includes the optimisation of ventilation to the dependent, non-operative lung and ensuring cardiovascular function is optimised with targeted use of fluids or vasoactive medications as appropriate to the clinical situation. As it can interfere with surgical exposure, the use of CPAP on the operated lung should be the final step in managing hypoxia. Good communication is essential to ensure the surgeon is not at a point in the operation where an impeded view could have catastrophic consequences. However, the judicious use of CPAP, starting low and increasing slowly, can be tolerated.
Analgesia
Pain after all thoracic surgery can be severe and may arise from retraction, fracture/dislocation of ribs, injury to intercostal nerves, or irritation resulting from chest drains. Even with MITS, a large wound is still required for removal of a surgical specimen, and trying to minimise this size can increase distraction forces resulting in rib fractures. Despite lower levels of pain compared with thoracotomy, pain after MITS should still be considered moderate to severe.17
Good analgesia is essential to allow mobilisation and prevent PPCs. Inadequate analgesia can further compromise impaired respiratory status by impeding cough and reducing secretion clearance. The incidence of chronic pain after VATS is 25% (compared with 33% after thoracotomy) and, given the potential role of poorly controlled acute pain in its development, good analgesia is vital.18
A multimodal approach, incorporating regional and systemic analgesia, is required. The selection needs to balance the risks and benefits, is tailored to the patient, and depends on the extent of surgery. After thoracotomy, despite the historical preference for thoracic epidural analgesia (TEA), there is no clear analgesic benefit between TEA and paravertebral blockade (PVB) with a lower incidence of minor adverse effects with PVB.19 Likewise, there is no established gold-standard regional analgesic technique advocated for VATS procedures, and the choice often depends on factors specific to the patient and the surgery. As in thoracotomy, PVB has been shown to be non-inferior to thoracic epidural blockade in VATS.20 This may account for the increased use of PVB with opioids in these patients.21
Novel fascial plane techniques, such as serratus anterior and erector spinae blocks, are increasingly being described after VATS procedures. They may offer a less invasive alternative for analgesia, although their benefit has yet to be proved in large, well-conducted studies.22,23
Spontaneously breathing and awake VATS
There are a number of potential anaesthetic techniques for VATS that do not involve tracheal intubation and positive pressure ventilation. These include awake surgery with regional anaesthesia; and general anaesthesia with spontaneous ventilation but without intubation. The majority of VATS procedures can be performed in awake patients with regional anaesthesia along with sedation and local anaesthetic (topically to the lung, topically to the airway, stellate ganglion block, or vagus nerve block) to suppress the cough reflex.24 Case series describing procedures that have been performed in awake patients range from simple pleural procedures to major lung resections (including lobectomy, pneumonectomy, and lung volume reduction), thymectomy and tracheal resections.24 The potential benefits of awake thoracic surgery come from avoidance of the morbidity associated with general anaesthesia (mechanical OLV, residual neuromuscular block, and haemodynamic instability) along with the potential benefits of regional anaesthesia (improved analgesia, reduced thrombotic complications and decreased surgical stress response). Proponents believe these will translate into reduced perioperative morbidity and reduced hospital stay.24 Experience of the technique is essential with careful selection of patients being key. Although the technique shows potential, recent guidance by the European Society of Cardiothoracic Surgeons states that ‘non-intubated’ anaesthesia for lung resection cannot currently be recommended.7
Robot-assisted thoracic surgery
RATS is a novel technique in the UK and involves a totally new style of operating with implications for the whole operating team. All procedures performed by VATS could be performed by RATS (Table 1). The robot's high-precision instruments with 360° angulation means that some surgical procedures, such as sleeve resections, pneumonectomy and mediastinal surgery, may even be easier with a robotic approach than with VATS.
Surgical factors
In RATS, the principal operating surgeon is at a console remote from the patient with a trained surgical assistant scrubbed at the table for positioning of instruments and the deployment of manual devices. Surgery is usually performed through up to four ports in a single intercostal space with the potential for an additional access port.
During RATS, there is a greater team responsibility with a large number of team members needing to be trained in their specific roles. The components of an operating team for RATS are described in Table 2, and although the operating theatre staff can perform multiple tasks, it is important that each person's specific roles are identified at the preoperative briefing. The allocation of roles in emergencies is particularly important, meaning if there are personnel changes during surgery, a detailed handover of responsibilities must occur.
Table 2.
RATS operating theatre team
| Team member | Role |
|---|---|
| Lead surgeon | Operates remote console controlling robotic arms |
| Surgical assistant | Inserts/changes port access; changes robotic arms; deploys manual instruments |
| Scrub nurse | Provides instruments and support to surgeons |
| Floor nurse | Assists scrub staff |
| Robotic assistant | Charged with docking and undocking the robot (including emergency) |
| Gown assistant | Provides gown and gloves for lead surgeon in emergency |
| Instrument assistant | Provides and opens instrument trays for emergency thoracotomy |
| Anaesthetist | Responsible for anaesthesia, lung isolation and patient positioning |
| Anaesthetic assistant | Supports the anaesthetist |
| Operating theatre assistant | Supports the anaesthetic, surgical and scrub staff |
Similar to VATS, there is a steep learning curve recognised for RATS procedures with increasing numbers translating to shorter operating times; decreased conversion rates; and improved surgical metrics, such as nodal upstaging rate (a measure of the completeness of lymph node dissection).4 This can mean that, in the early phase of a programme, operative times can be expected to be much longer than the equivalent VATS procedure.
Selection of patients is important to ensure they are anatomically suitable for robotic resection, and have minimal comorbidities. Although the aim was that all patients would be suitable for RATS, the consideration of comorbidities is particularly important when setting up a service and when in the steep part of the learning curve, as operative times can be particularly prolonged. This may expose patients to increased risk of complications and delay recovery.
Logistics
A number of logistical factors need to be considered when incorporating a RATS programme. Although there are potential cost benefits of reduced length of stay and less perioperative morbidity, there are large capital and consumable costs to be considered. A retrospective review from the USA in 2014 indicated that there was an increased hospital cost of approximately $4,500 per RATS lobectomy case when compared to a similar operation by VATS. In addition to the financial cost, robotic systems have a large equipment footprint that needs to be considered when incorporating them into existing facilities. The operating theatre needs to be large enough to accommodate the robot and ancillary equipment, and leaving space for access to the patient, space for nursing and support staff, and for essential anaesthetic and operating theatre apparatus (such as anaesthetic machine, monitoring, fibreoptic bronchoscope, fridges, etc.). It is essential that a pathway to drive and dock the robot is kept clear at all times so that it can be removed in an emergency without obstruction.
Anaesthetic factors
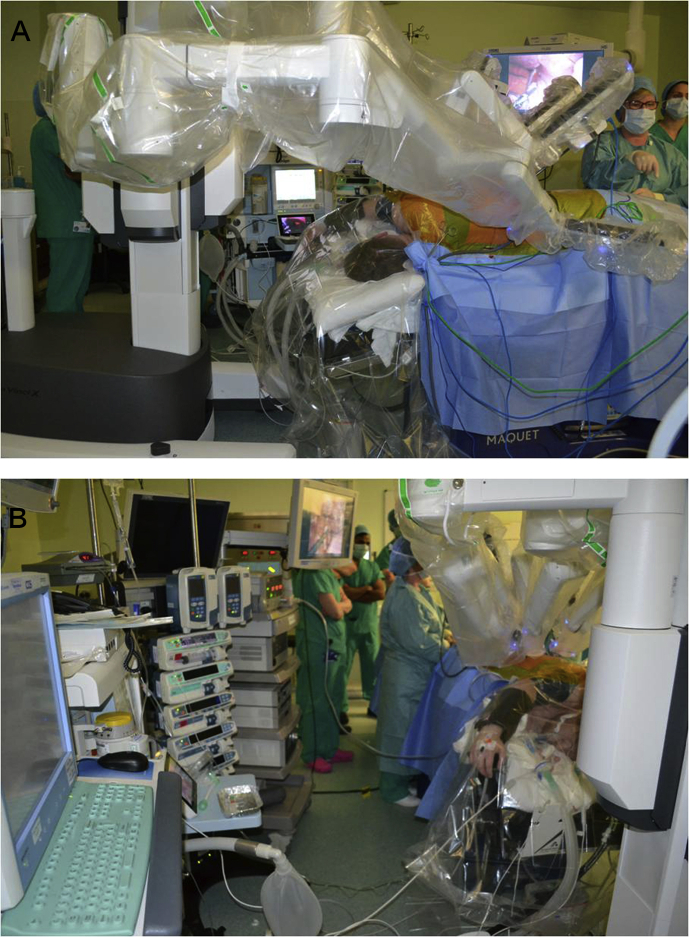
The delivery of anaesthesia is similar to VATS, and many of the factors, such as induction, maintenance, lung isolation and postoperative analgesia, can be directly applied to patients undergoing RATS. However, there are a number of key considerations. There is severely restricted access to the patient, and positioning of equipment needs to be considered when the arms of the robot are engaged (see Fig. 1a and b). This will include the fibreoptic bronchoscope with limited ability to access the airway during the procedure. The use of a left-sided DLT where possible is preferred, given the lower chance of displacement compared with a right-sided DLT or bronchial blocker. The use of a left-sided DLT will have less risk of displacement during the procedure, but this needs to be balanced against the potential for obstruction of the tracheal lumen during extreme lateral flexion when a left-sided tube is used in a right-sided surgical procedure. Whatever airway isolation device is chosen, confirmation of its correct placement once the patient is in the final position for surgery is of paramount importance. Venous and arterial access needs to be positioned to enable access but to prevent snag/trip hazards. The use of a clear head drape allows direct visualisation of the patient.
Fig 1.
Patient undergoing right-sided robotic-assisted thoracic surgery. (A) The da Vinci robotic operating system in position at the head end of a patient in the left lateral decubitus position. This illustrates the competition for space and limited access to the patient. The clear head drape allowing direct visualisation of the patient can be seen. (B) The view from the anaesthetist's perspective at the head end of the patient. The limited space means that positioning of equipment required during surgery needs to be carefully considered.
In contrast to VATS, in which the chest cavity is open to atmospheric pressure, during RATS, carbon dioxide is insufflated through sealed ports to create positive intrathoracic pressure of 5–10 cmH2O; this allows better operating conditions by encouraging lung deflation and flattening the diaphragm. However, this can affect cardiovascular stability with compression of mediastinal vessels leading to hypotension and bradycardia when higher pressures are used. These cardiovascular effects may be more prominent in those who are unable to compensate for the raised intrathoracic pressure, such as those with poor ventricular function or hypovolaemia. Carbon dioxide insufflation can contribute to hypercapnia, which may be challenging to manage during OLV. This can be accepted as part of a permissive approach to hypercapnia as long as it is tolerated haemodynamically and the patient has no comorbidities precluding it, such as those at risk for raised intracranial pressure where cerebral vasodilatation could have catastrophic effects.
The robot works from a fixed position and lacks compensatory movement, meaning movement of the patient or table could result in serious injury as the robotic arms will not move in response. Ensuring the table is locked and not moved is essential, and should be part of any preoperative checklist. Likewise, involuntary movements, such as coughing or diaphragmatic excursion, could be catastrophic when operating close to great vessels. This means that an adequate depth of anaesthesia and neuromuscular block is essential throughout the procedure, and many therefore see the use of neuromuscular blocking agents by infusion, ideally with quantitative monitoring of neuromuscular block, as a key component.
Prolonged procedures may mean that the use of a urinary catheter (no longer widely used in VATS) is required. Intermittent pneumatic compression boots, for the prevention of thromboembolism and to encourage venous return, should be mandatory.
Operating theatre layout and positioning of the patient
Planning of the operating theatre layout is key to allow placement of equipment and to enable robot access and docking. There has to be consideration for movement of the robot arms, as they can exert significant pressure on the patient; without haptic feedback to the operating surgeon, there is the risk of injury to the patient. The surgeon is reliant on staff at the table to inform them that the arms are clashing or touching the patient. The majority of cases will be in an extremely flexed, lateral decubitus position, and additional care needs to be taken to ensure pressure areas are padded and the patient is securely fixed to the operating table. Depending on the operative side, the position of the robot may be altered to allow the patient's face to be directed towards the anaesthetist, which can help optimise access to the airway during surgery.
The need for accurate dissection planes and angulation means RATS is particularly suited to mediastinal surgery. Here, the patient is positioned supine, with the side of the patient from which surgical access is required, being placed at the edge of the operating table. On the operative side, the patient's arm needs to be positioned below the horizontal plane of the thorax so as not to obstruct the surgical approach. The addition of a sandbag under the torso helps facilitate this.
Postoperative analgesia is similar to VATS procedures with all techniques previously described being suitable. Although the ports inserted are smaller compared to VATS and are usually in one intercostal space, there are often more of them, meaning analgesic requirements can be similar. The size of the surgical sample will continue to dictate the minimum wound size and the risk of rib fracture. Placement of paravertebral blocks at the start of surgery, when ports are inserted, allows regional analgesia to be provided throughout. This can reduce anaesthetic requirement and is advantageous during prolonged cases.
Emergency protocols
With the principal surgeon not scrubbed at the table, any emergency that cannot be controlled by robotic instruments, and therefore, requires immediate surgical intervention, has two critical delays built into the resuscitation pathway:
-
(i)
safe removal of the robot to allow resuscitation
-
(ii)
expeditious movement of the surgeon to be scrubbed at the patient.
In emergency events, such as catastrophic bleeding, short delays can be critical and every attempt should be made to minimise them.
The use of emergency protocols is necessary with clear communication between all team members essential. Given the proximity of surgery to the great vessels, surgical emergencies are primarily for bleeding. If bleeding is being controlled by a robotic instrument, the operating surgeon needs to communicate to the scrub nurse which instruments are in use and which can safely be removed. Bleeding can then be controlled by the surgical assistant with a manual instrument, and once all robotic instruments are removed, the robot is undocked from the patient and a thoracotomy performed. In uncontrolled bleeding, all surgical instruments should be immediately released and the scrub nurse should undock all robotic arms. The robot is then removed and a thoracotomy performed immediately.
Anaesthetic emergencies can include loss of airway, cardiac arrest, anaphylaxis or malignant hyperthermia. In this situation, an emergency should be declared, all instruments should be released and removed, and then the robot is undocked. The table should be flattened to a neutral position with resuscitation continuing as normal.
Clear communication and awareness of roles are essential to ensure the robot is undocked safely and efficiently. Rehearsing and simulation of these emergencies as a team will help safely manage them when they occur.
Conclusions
The increasing use of MITS procedures means the safe provision of perioperative care is becoming essential. VATS numbers are increasing, with older and sicker patients presenting for surgery. RATS is a novel surgical technique that has implications for the whole operating theatre team.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Philip McCall FRCA MD is a clinical lecturer in anaesthesia, pain and critical care medicine at the University of Glasgow, and an advanced trainee in cardiothoracic anaesthesia in the West of Scotland, based at the Golden Jubilee National Hospital. He has a research interest in cardiothoracic anaesthesia and has published on outcomes after thoracic surgery.
Mark Steven FRCA MRCP is a consultant in cardiothoracic anaesthesia and lead thoracic anaesthetist at the Golden Jubilee National Hospital. He is the thoracic representative on the Association for Cardiothoracic Anaesthesia and Critical Care committee.
Ben Shelley FRCA FFICM MD is a consultant in cardiothoracic anaesthesia and intensive care at the Golden Jubilee National Hospital. He is an honorary clinical associate professor at the University of Glasgow.
Matrix codes: 1H02, 2A07, 3G00
References
- 1.Cao C., D’Amico T., Demmy T. Less is more: a shift in the surgical approach to non-small-cell lung cancer. Lancet Respir Med. 2016;4:e11–e12. doi: 10.1016/S2213-2600(16)00024-2. [DOI] [PubMed] [Google Scholar]
- 2.Walker W.S., Carnochan F.M., Pugh G.C. Thoracoscopic pulmonary lobectomy. Early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg. 1993;106:1111–1117. [PubMed] [Google Scholar]
- 3.McCall P.J., Macfie A., Kinsella J., Shelley B.G. Critical care after lung resection: CALoR 1, a single-centre pilot study. Anaesthesia. 2015;70:1382–1389. doi: 10.1111/anae.13267. [DOI] [PubMed] [Google Scholar]
- 4.Upham T.C., Onaitis M.W. Video-assisted thoracoscopic surgery versus robot-assisted thoracoscopic surgery versus thoracotomy for early-stage lung cancer. J Thorac Cardiovasc Surg. 2018;156:365–368. doi: 10.1016/j.jtcvs.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 5.Ceppa D.P., Kosinski A.S., Berry M.F. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons Database analysis. Ann Surg. 2012;256:487–493. doi: 10.1097/SLA.0b013e318265819c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Society for Cardiothoracic Surgery . 2019. Thoracic outcomes.https://scts.org/outcomes/thoracic/ Available from: [Google Scholar]
- 7.Batchelor T.J.P., Naidu B., Rasburn N.J. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (ERAS®) society and the European society of thoracic surgeons (ESTS) Eur J Cardiothorac Surg. 2018;55:91–115. doi: 10.1093/ejcts/ezy301. [DOI] [PubMed] [Google Scholar]
- 8.NHS England . 2016. Clinical commissioning policy: robotic assisted lung resection for primary lung cancer.https://www.england.nhs.uk/wp-content/uploads/2018/07/Robotic-assisted-lung-resection-for-primary-lung-cancer.pdf Available from: [Google Scholar]
- 9.Rocco G., Internullo E., Cassivi S.D., Van Raemdonck D., Ferguson M.K. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin. 2008;18:235–247. doi: 10.1016/j.thorsurg.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Hanna J.M., Berry M.F., D’Amico T.A. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis. 2013;5(Suppl. 3):S182–S189. doi: 10.3978/j.issn.2072-1439.2013.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam N., Park B.J., Wilton A. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg. 2007;84:1085–1091. doi: 10.1016/j.athoracsur.2007.05.053. discussion 91. [DOI] [PubMed] [Google Scholar]
- 12.Ashok V., Francis J. A practical approach to adult one-lung ventilation. BJA Educ. 2018;18:69–74. doi: 10.1016/j.bjae.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modolo N.S.P., Modolo M.P., Marton M.A. Intravenous versus inhalation anaesthesia for one-lung ventilation. Cochrane Database Syst Rev. 2013;7:CD006313. doi: 10.1002/14651858.CD006313.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shelley B.G., McCall P.J., Glass A. Association between anaesthetic technique and unplanned admission to intensive care after thoracic lung resection surgery: the second Association of Cardiothoracic Anaesthesia and Critical Care (ACTACC) National Audit. Anaesthesia. 2019;74:1121–1129. doi: 10.1111/anae.14649. [DOI] [PubMed] [Google Scholar]
- 15.Bussieres J.S., Somma J., Del Castillo J.L. Bronchial blocker versus left double-lumen endotracheal tube in video-assisted thoracoscopic surgery: a randomized-controlled trial examining time and quality of lung deflation. Can J Anaesth. 2016;63:818–827. doi: 10.1007/s12630-016-0657-3. [DOI] [PubMed] [Google Scholar]
- 16.Licker M., de Perrot M., Spiliopoulos A. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- 17.Nagahiro I., Andou A., Aoe M., Sano Y., Date H., Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg. 2001;72:362–365. doi: 10.1016/s0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 18.Wildgaard K., Ravn J., Nikolajsen L., Jakobsen E., Jensen T.S., Kehlet H. Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesthesiol Scand. 2011;55:60–68. doi: 10.1111/j.1399-6576.2010.02357.x. [DOI] [PubMed] [Google Scholar]
- 19.Shelley B., Macfie A., Kinsella J. Anesthesia for thoracic surgery: a survey of UK practice. J Cardiothorac Vasc Anesth. 2011;25:1014–1017. doi: 10.1053/j.jvca.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 20.D’Ercole F., Arora H., Kumar P.A. Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth. 2018;32:915–927. doi: 10.1053/j.jvca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Kotemane N.C., Gopinath N., Vaja R. Analgesic techniques following thoracic surgery: a survey of United Kingdom practice. Eur J Anaesth. 2010;27:897–899. doi: 10.1097/EJA.0b013e32833d1259. [DOI] [PubMed] [Google Scholar]
- 22.Semyonov M., Fedorina E., Grinshpun J. Ultrasound-guided serratus anterior plane block for analgesia after thoracic surgery. J Pain Res. 2019;12:953–960. doi: 10.2147/JPR.S191263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson J.M.B., Lohser J., Klaibert B. Erector spinae plane block for postoperative rescue analgesia in thoracoscopic surgery. J Cardiothorac Vasc Anesth. 2018;32 doi: 10.1053/j.jvca.2018.06.026. e5–7. [DOI] [PubMed] [Google Scholar]
- 24.Kiss G., Castillo M. Nonintubated anesthesia in thoracic surgery: general issues. Ann Transl Med. 2015;3:110. doi: 10.3978/j.issn.2305-5839.2015.04.21. [DOI] [PMC free article] [PubMed] [Google Scholar]