Learning objectives.
By reading this article you should be able to:
-
•
Explain the key physiological differences between the left (LV) and right ventricle (RV).
-
•
Define preload, and afterload in relation to RV function.
-
•
Explain the phenomenon of ventricular interdependence.
-
•
Describe the fundamental principles of RV assessment using transthoracic echocardiography.
Key points.
-
•
There are key physiological distinctions between the RV and LV.
-
•
The RV is tolerant of preload but intolerant of raised afterload.
-
•
In the normal RV, perfusion occurs throughout the cardiac cycle.
-
•
Size, function, septal position and estimated pulmonary artery pressure are key to assessment of the RV using TTE.
-
•
RV dysfunction may occur with few clinical findings and requires a high index of suspicion for diagnosis.
In 1616, Sir William Harvey was the first physician to realise the importance of the right ventricle (RV) and its interactions with the pulmonary circulation, yet up until the mid-20th century little emphasis had been placed on the RV. Before the 1950s, the main focus was on the left ventricle (LV), the RV being thought of as little more than a passive structure with the sole purpose of providing a conduit between the systemic and pulmonary circulations. During the 1950s, cardiac surgeons began to understand the importance of the RV as they attempted to develop techniques for palliation of right-heart hypoplasia. The crucial role of the RV is now well recognised in a wide variety of cardiac and non-cardiac conditions.
The anatomy and physiology of the RV differ significantly from that of the LV. As a result, a different approach is required in terms of assessment and management. In order to manage patients with RV dysfunction it is important to have a good understanding of RV anatomy and physiology and their clinical application. The aim of this article is to provide an understanding of the structure and function of the RV for the anaesthetist and intensivist.
Anatomy of the RV
The body of the RV receives blood from the right atrium (RA), whilst the outflow tract, transfers the blood to the pulmonary artery (PA). These two areas are separated by a ridge, the crista supraventricularis, which extends in to the ventricular cavity. The RV is the most anterior chamber in the normal heart residing immediately behind the sternum. The RV is crescent-shaped in cross-section and triangular in side-profile. The ventricle is composed of two layers of muscular fibres—superficial circular fibres that are continuous with the subepicardial fibres of the LV and deeper longitudinal fibres. In contrast, the LV has a more complex structure and movement pattern, with three layers of fibres compared with the two of the RV. The thin, free wall of the RV wraps around the more muscular wall of the LV.
Perfusion of the RV
The RV's blood supply is dependent on the dominance of the coronary system for each individual. Eighty percent of the population are known to have an RV supplied by the right coronary artery. Unlike the LV, perfusion of the RV occurs during both systole and diastole providing myocardial oxygen delivery throughout the cardiac cycle. This phenomenon only occurs in healthy hearts; however, in the diseased state such as in patients with pulmonary hypertension, increased intracavity pressure during systole means the distribution of blood to the RV during the cardiac cycle is more like that of the LV, occurring only during diastole.
Although, the RV ejects the same cardiac output (CO) as the LV, RV stroke work is only around a quarter that of the LV. The RV is thin-walled, with approximately one sixth of the muscle mass of the LV. Under normal conditions, ejection is maintained despite lesser muscle mass as the RV is coupled with the pulmonary circulation which has a lower vascular resistance and greater distensibility in comparison with the systemic circulation. As such, both pulmonary artery pressure and vascular resistance are approximately a fifth of that of the systemic circulation (Table 1). The ability of the lung to recruit partially collapsed or unused vessels as CO increases, for example during exercise, serves to maintain coupling and accounts for the minimal changes demonstrated in pulmonary arterial pressure and reduction in pulmonary vascular resistance (PVR) seen on exercise.
Table 1.
Comparison of the features of the normal left and right ventricules
| Right ventricle | Left ventricle | |
|---|---|---|
| Shape | Crescent | Ellipsoidal |
| Structure | Two layers of fibres | Three layers of fibres |
| Free wall thickness (mm) | 1–5 | 8–10 |
| Circulation | Low-pressure, low-resistance | High-pressure, high-resistance |
| Stroke volume (ml) | 70–90 | 70–90 |
| Ejection fraction (%) | 65 | 70–80 |
| Ventricular pressure (diastole; mm Hg) | 0–8 | 4–12 |
| Ventricular pressure (systole; mm Hg) | 15–30 | 90–140 |
| Afterload (dynes-s cm−5) | Pulmonary vascular resistance <250 | Systemic vascular resistance 800–1200 |
| Adaptation to disease | Tolerant of preload | Tolerant of afterload |
Ejection of blood from the RV occurs after a reduction in free wall surface area and shortening of longitudinal fibres, beginning at the inflow tract and moving in a ‘peristaltic’ manner towards the RV outflow tract. Compare this with the LV where blood is ejected because of a concentric contraction of the LV free wall and septum, combined with a twisting movement of the heart.1
RV cardiodynamics
The performance of the RV is affected by variety of interacting factors including preload, afterload, and ventricular interdependence.2 The LV pressure–volume loop has a familiar rectangular shape, with parallel sides because of well-defined isovolumetric contraction and relaxation phases, and sharp ‘corners’ where the beginning and end of both systole and diastole can be easily identified. In comparison, RV isovolumetric contraction time is shorter than that of the LV as during early systole, the pulmonary valve opens when RV pressure exceeds that of the low-pressure PA. The point of end-systole is less well defined in the RV leading the RV pressure–volume loop to appear almost triangular in shape. This occurs as ejection of blood from the RV can continue despite decreasing RV pressure because of the momentum of blood in the low-pressure system; as a result, the isovolumetric ventricular relaxation phase is shortened or absent in the RV (Fig. 1).1
Fig 1.
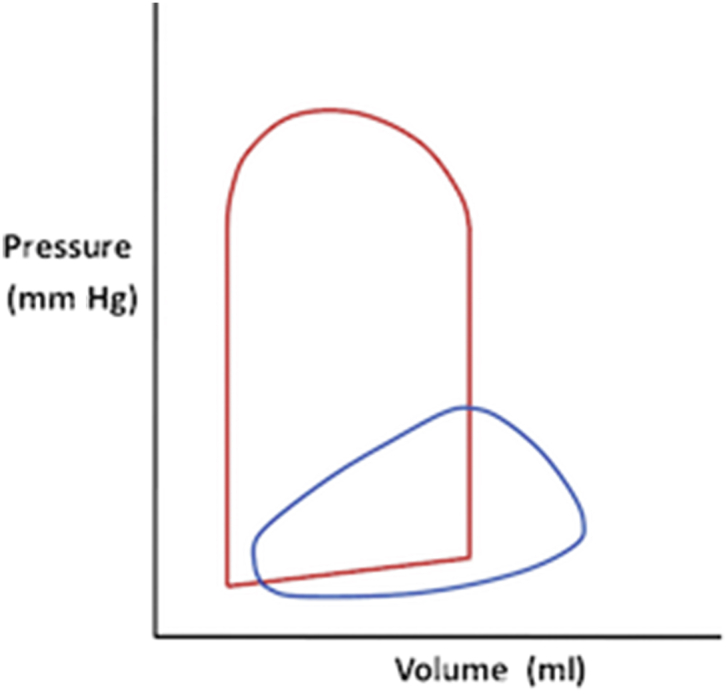
Pressure-volume curves of the left and right ventricle. Note the lower pressures and more triangular appearance of the PV loop for the right ventricle (blue) compared with the more rectangular left ventricle (red).
Preload
Preload is defined as the initial stretching of the cardiac fibre before contraction and is influenced by a variety of factors including atrial contractility, ventricular compliance, venous return, wall tension, and heart rate. Conceptually, RV end-diastolic volume and end-diastolic pressure (EDP) are considered to be indices of preload. The RV is described as being ‘tolerant’ of preload; low muscular mass means the comparatively compliant RV is able to dilate (up to a point) in the face of excessive volume. With ongoing distention, however, decompensation occurs and failure ensues. Excessive RV dilatation is commonly accompanied by dilation of the tricuspid valve annulus and the development of tricuspid regurgitation (TR). Significant TR leads to further volume overload and reduces forward flow, reducing CO. Volume overload of the RV can distort the LV shape and impair LV filling and function (see below: ‘ventricular interdependence’).2
Afterload
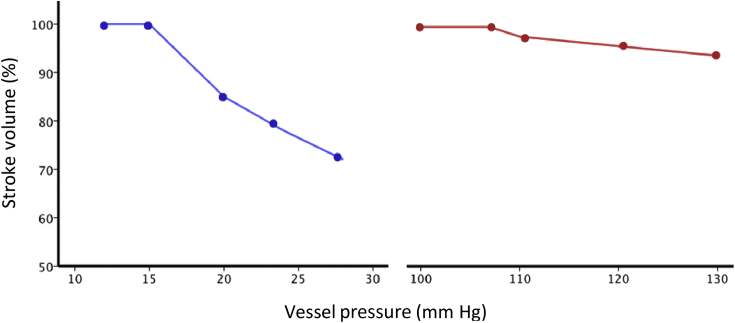
RV afterload is the load the RV has to overcome during the ejection phase of the cardiac cycle and in its most complete sense is influenced by pulmonary vascular resistance, distensibility and pulse wave reflections taking place within the pulmonary vascular bed. The RV is less well equipped to deal with acute changes in afterload because of a reduction in cardiac muscle mass compared with the LV; as such, the RV is considered to be intolerant of afterload (Fig. 2). In clinical practice, PVR is used as an index of afterload (Fig. 3). In chronically increased afterload, the RV pressure–volume loop gradually shifts to become rectangular in shape and appear similar to the normal LV pressure–volume loop.
Fig 2.
Comparison of RV (blue) and LV (red) adaptation to afterload. Note how the RV stoke volume falls rapidly in response to increased afterload compared to the LV.
Fig 3.
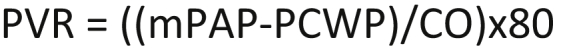
Equation for pulmonary vascular resistance (PVR). Measured in dynes-sec cm−5. MPAP = mean pulmonary artery pressure (mmHg). PCWP = pulmonary capillary wedge pressure (mmHg). CO = cardiac output (L/min). 80 = conversion term to equalise the units.
Ventricular interdependence
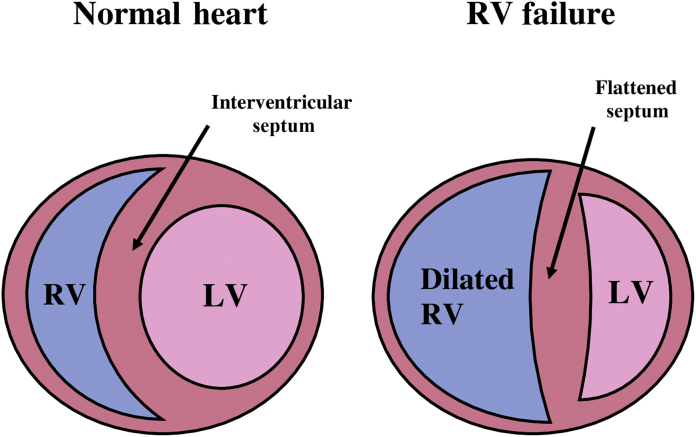
Ventricular interdependence describes the phenomenon whereby the function, volume or pressure in one ventricle can directly influence that of the other.3 Both the LV and the RV are interdependent as they are contained within the relatively non-distensible pericardial sac with a shared ventricular septum. Whilst these interactions are present continuously, it is in times of dysfunction that interdependence becomes most evident.4 When RV pressure or volume overload occurs, the RV can affect LV performance and result in a decreased LV preload and contractility. In normal hearts, LV-EDP usually exceeds RV-EDP. In times of RV overload, RV-EDP may exceed LV-EDP forcing the ventricular septum towards the LV during diastole. This distorts the normal LV shape (creating a so called ‘D-shaped’ ventricular cavity; Fig. 4), reducing LV diastolic compliance and impairing LV filling.5
Fig 5.
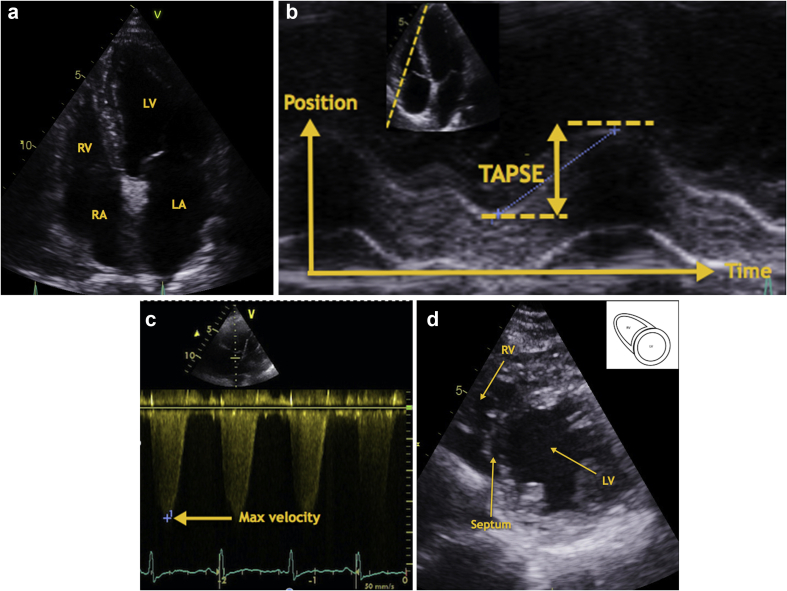
Transthoracic echocardiography images. Images to be used in conjunction with Table 2. 5a: Normal Apical 4 chamber TTE view. 5b: Calculation of right ventricle systolic function using TAPSE. 5c: Calculation of right ventricle systolic pressure using the maximum velocity of the TR jet. 5d: Normal Short axis TTE view.
Fig 4.
Graphical representation of ventricular interdependence.
In addition, these alterations in LV geometry have a direct effect on LV systolic function, by reducing the mechanical efficiency of LV contraction.6 The clinician must be wary of the apparently empty, well contracting LV, which may appear reassuring when seen on echocardiography; in conditions of RV failure, the LV can be underfilled because of compression by, and reduced delivery from the, interdependent RV.
The haemodynamics of the right heart are affected by respiratory effort.7 During spontaneous breathing, inspiration leads to a negative intrathoracic pressure ‘sucking’ blood into the thoracic cavity and improving RV filling whilst simultaneous expansion of the pulmonary vasculature leads to a reduction in RV afterload, promoting right-sided CO. Contrast this to mechanical ventilation, where there is a cyclical increase in intrathoracic pressure during inspiration. As intrathoracic pressure increases, filling is impeded and extra-alveolar capillaries are compressed, increasing afterload. These phenomena can lead to the exhibition of marked systolic pressure/stroke volume variation during mechanical ventilation in patients with RV dysfunction; caution must be exercised not to interpret these changes as being solely reflective of hypovolaemia.
Assessment of the RV
Clinical assessment should focus on evaluating signs and symptoms of RV dysfunction and establishing the precipitating event or underlying aetiology. Not all of the investigations discussed may be readily available, particularly in the emergency setting.
Jugular venous pressure and central venous pressure
Assessing the jugular venous pressure (JVP) can be of enormous value in a patient with RV failure. A raised JVP reflects raised atrial pressure and is therefore a specific sign of right-sided failure. Kussmaul's sign, an increase in JVP on inspiration, can be indicative of the cause of RV failure. Increased venous return combined with impaired RV diastolic compliance, occurring in RV infarction and constrictive pericarditis, results in disproportionately increased RAP on inspiration.
The limited validity of central venous pressure (CVP) in identifying patients who will respond positively to a ‘fluid challenge’ means that CVP monitoring, for this purpose, has been falling out of favour in the general intensive care patient. The utility of CVP monitoring as a surrogate monitor of RV function, however, is often under-appreciated. Increasing CVP in a patient with RV dysfunction is an ominous sign. The trend of the CVP in response to fluids and inotropes can provide useful information as can interpetation of the waveform. In the presence of severe TR, a dominant v-wave occurs during ventricular systole as a result of retrograde RA filling. This is followed by an associated sharp y descent occurring as a result of high RA volume. Pulmonary hypertension can lead to a dominant a-wave as the RA is contracting against increased resistance.
Laboratory investigations
Investigations should include full blood count, renal and liver function tests, troponin, and lactate, which may demonstrate evidence of organ hypoperfusion as a result of RV dysfunction. Increased brain natriuretic peptide (BNP) and troponin may be caused by a variety of aetiologies of RV failure. Studies have demonstrated the role of increased BNP in diagnosing RV dysfunction and predicting morbidity in patients with pulmonary hypertension.8 Troponin and BNP have been shown to be predictors of RV dysfunction in acute pulmonary embolus.9
ECG
The ECG can often be normal in patients with RV dysfunction. There may, however, be evidence of right axis deviation, right bundle branch block or RV hypertrophy. The classic triad of a deep S-wave in lead 1, Q-wave, and an inverted T-wave in lead 3 may be present, demonstrating evidence of RV strain and acute cor pulmonale. This pattern is traditionally described in the context of ECG findings in PE but occurs in <10% of patients.
CXR
The value of CXR when assessing the RV is limited as a result of its position as an anterior cardiac structure that therefore only contributes to a small portion of the heart border. The RV is normally best viewed on a lateral CXR where an enlarged RV will fill the retrosternal space and occupy >50% of the area between the diaphragm and sternal angle. The LV may also displace backwards, which results in cardiomegaly. There may be evidence of the underlying aetiology.
Transthoracic echocardiography
The mainstay of RV imaging in clinical practice is transthoracic echocardiography (TTE). TTE is widely available and can be used to assess both structure and function. Echocardiographic imaging of the RV is challenging, however, with both the complex shape of the RV and its retrosternal position contributing to these challenges.
Whilst the LV is broadly circular in cross-section, therefore volumes can be estimated by summing the volumes of multiple slices of a known thickness (‘Simpson's method of discs'); in contrast, the RV cannot be easily modelled geometrically, leading to difficulty in assessing volumes and function on TTE. As a result, numerous surrogate echocardiographic indices of RV function have been described; all have significant limitations and vary in their performance when compared with (gold standard) cardiovascular magnetic resonance techniques.
TTE is nonetheless the most widely used clinical tool for bedside assessment of RV function. Whilst LV function is commonly described quantitatively in terms of ejection fraction, it can be appreciated that because of the difficulties in measuring RV volumes with TTE, RV TTE reporting tends to be more qualitative in nature. The guidelines for TTE examination of the RV recommend both qualitative and quantitative assessments.10 Apical four-chamber, parasternal long and short axis and subcostal views provide images for a comprehensive assessment of RV function and RV systolic pressure (RVSP). Discussion of the comprehensive echocardiographic assessment of RV function is outside the scope of this article; however, there are a number of parameters, relevant to RV function that are commonly reported in any formal echocardiography report. Table 2 discusses these parameters with the aim of better informing the non-echocardiographer on the interpretation of such reports. Some of the more complex parameters are discussed in more detail below.
Table 2.
Quick reference guide for important TTE measures of the RV. Detailed explanation of each measure is given in the main body of the article. Please see Figure 5 and online videos for TTE images
| TTE measurement | How to measure | Values |
|---|---|---|
| RV size (Fig. 5a) |
Two ways to determine: Qualitative comparison of the relative sizes of the RV and LV. (The normal RV should be 2/3 the size of the LV, only if the LV size is NORMAL) Diameter at the base, mid-level and length. If values higher than those given, RV dilatation present |
Qualitative: Mild dilation: RV >2/3 LV Moderate dilation: RV=LV Severe dilation: RV larger than LV Diameter: Base >42 mm Mid-level >35 mm Length >83 mm |
| RV systolic function (Fig. 5b) |
Variety of parameters may be used. TAPSE is the most common: ‘M-mode’ cursor through the tricuspid annulus provides a graphical representation of annular position against time |
Displacement of the annulus towards the apex during systole is indicative of systolic function <17 mm=RV systolic dysfunction |
| RVSP (Fig. 5c) |
RVSP=4v2+CVP where v is velocity Max velocity of the TR jet (highlighted in Fig. 5c) is converted to a trans-valvular pressure gradient with Bernoulli's equation (p=4v2) |
Normal RVSP: <35 mm Hg |
| Septal position (Fig. 5d) |
Visual assessment of ventricular septal position in parasternal short axis view In Fig. 5d, a parasternal short axis is shown. The LV, RV and interventricular septum are highlighted |
As the RV dilates, the ventricular septum will flatten and the LV will lose its characteristic circular shape and become D-shaped in cross-section (see Fig. 3) Flattening in diastole only suggests volume overload Flattening in systole and diastole suggests volume and pressure overload |
RV size
Qualitative assessment of the RV allows for a comparison of the relative size of the RV and the LV when viewed in an apical four-chamber view. Caution should be used when using this method as it is dependent on the LV being normal in shape and size. Quantitative assessment measures the diameter at the base, mid-level, and length and the RV/LV basal diameter ratio.
RV systolic function
RV systolic function has been determined using a variety of measures; tricuspid annular plane systolic excursion (TAPSE) and fractional area change are two of the variables used most traditionally. TAPSE is easily obtainable, derived from the apical four-chamber view using M-mode. The M-mode curser is placed through the tricuspid annulus and measures the amount of longitudinal motion of the annulus at peak systole. Impaired RV systolic function has a TAPSE value of <17 mm.11
Fractional area change is obtained from a four-chamber view by tracing the RV endocardium in systole and diastole, beginning at the annulus, along the free wall to the apex, and back along the interventricular septum to the annulus. It is expressed as a percentage change in the RV area between end-diastole and end-systole. Normal value is >35%.11 It has been shown to correlate well with RV ejection fraction measured by cardiac magnetic imaging.
Assessment of pulmonary artery pressure
Systolic pulmonary artery pressure is considered the same as RVSP in the absence of stenotic pulmonary valves disease (very rare outside the congential cardiac population). RVSP is derived by the addition of the pressure gradient between the RV and the RA, to the pressure in the RA. To measure the RV-RA pressure gradient, the maximum velocity of the TR jet must be measured. The maximum velocity measured as the peak regurgitation is converted to pressure using Bernoulli's law [pressure (p) =4×volume (v)2]. The maximum velocity of the TR jet should be measured in either an apical four-chamber or a short-axis view.
RA pressure is estimated from the inferior vena cava (IVC) diameter and the presence of inspiratory collapse, in spontaneously breathing patients, in a subcostal view. The measurement should be made at end-expiration and just proximal to the hepatic veins that lie proximal to the ostium of the RA.10 An IVC<17 mm with a collapsibility index of 50% suggest an RA pressure of 5 mm Hg. An IVC>17 mm with collapsibility >50% suggests an RA pressure of 10 mm Hg whereas the same IVC diameter but with an index <50% suggests an RA pressure of 15 mm Hg.
In patients whose lungs are ventilated using positive pressures, the degree of collapsibility of the IVC cannot be used as a reliable marker of RA pressure. In these patients, however, RA pressure is commonly measured by central venous cannulation.
Septal morphology
Chronic RV dilatation, for example resulting from isolated volume overload as occurs in TR, results in the RV apex progressively replacing the LV as the true apex of the heart. If viewed in a parasternal short axis window, the LV will appear D-shaped because of flattening of the ventricular septum.10 The geometry of the LV will also alter during RV pressure overload as a result of shifting of the septum to the left away from the centre of the RV and towards the centre of the LV. The LV cavity will appear D-shaped in the short-axis view, predominantly during systole.10
Transoesophageal echocardiogram
Intraoperative transoesophageal echocardiogram (TOE) may be used for high risk surgical procedures and is commonplace in cardiac anaesthesia. It is also useful in haemodynamically unstable patients both in the theatre and in intensive care to guide management strategies. TOE has the advantage over TTE of better quality imaging, particularly in patients who are ventilated with positive-pressure ventilation, chest wall injuries, or during cardiothoracic surgery.12 Consideration should be given to high risk patients for an expert intra-operative TOE RV assessment.
Pulmonary artery catheter
The pulmonary artery catheter (PAC) is the diagnostic gold standard for the diagnosis of pulmonary hypertension. The PAC has fallen out of favour in the management of the general ICU patient; however, it may aid the diagnosis of RV failure and allow appropriate management. The PAC allows right-sided measurements to be performed and thermodilution allows RV (cardiac) output to be measured. The PAC allows for the accurate measurement of CO which in turn allows the clinician to calculate PVR, as a surrogate marker for afterload. Acute RV dysfunction is suggested by an CVP greater than pulmonary capillary wedge pressure, a low cardiac index and stroke volume index and mixed venous oxygen saturations <55%.13
Conclusion
A sound understanding of RV anatomy and physiology is essential for the anaesthetist and intensivist. Consideration of the basic principles of preload, afterload, contractility, coronary perfusion, and ventricular interdependence will inform a structured approach to clinical aspects of RV failure and its management.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Emma Murphy MRCP is a clinical research fellow based in cardiothoracic anaesthesia and intensive care at the Golden Jubilee Hospital, Glasgow.
Ben Shelley FRCA, FFICM, MD is a consultant in cardiothoracic anaesthesia and intensive care at the West of Scotland Heart and Lung Centre, which includes the Scottish Pulmonary Vascular Unit, the Scottish National Advanced Heart Failure Unit (including mechanical circulatory support and cardiac transplantation) and the Scottish Adult Congenital Cardiac Service. He has an established research programme examining right ventricular function after lung resection.
Matrix codes: 1A01, 2C01, 3G00
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bjae.2018.05.001.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Dilated RV with deviated intraventricular septum throughout systole and diastole which demonstrates RV pressure overload.
Dilated RV with poor RV function.
References
- 1.Greyson C.R. The right ventricle and pulmonary circulation: basic concepts. Rev Esp Cardiol. 2010;63:81–95. doi: 10.1016/s1885-5857(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 2.Haddad F., Hunt S.A., Rosenthal D.N. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 3.Santamore W.P., Gray L. Significant left ventricular contributions to right ventricular systolic function. Mechanism and clinical implications. Chest. 1995;107:1134–1145. doi: 10.1378/chest.107.4.1134. [DOI] [PubMed] [Google Scholar]
- 4.Naeije R., Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res. 2017;113:1474–1485. doi: 10.1093/cvr/cvx160. [DOI] [PubMed] [Google Scholar]
- 5.Bleeker G.B., Steendijk P., Holman E.R. Acquired right ventricular dysfunction. Heart. 2006;92(Suppl 1):i14–i18. doi: 10.1136/hrt.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookes C., Ravn H., White P. Acute right ventricular dilatation in response to ischemia significantly impairs left ventricular systolic performance. Circulation. 1999;100:761–767. doi: 10.1161/01.cir.100.7.761. [DOI] [PubMed] [Google Scholar]
- 7.Caplin J.L., Flatman W.D., Dyke L. Influence of respiratory variations on right ventricular function. Br Heart J. 1989;62:253–259. doi: 10.1136/hrt.62.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fijalkowska A., Torbicki A. Role of cardiac biomarkers in assessment of RV function and prognosis in chronic pulmonary hypertension. Eur Heart J Suppl. 2007;9:H41–H47. [Google Scholar]
- 9.Choi H.S., Kim K.H., Yoon H.J. Usefulness of cardiac biomarkers in the prediction of right ventricular dysfunction before echocardiography in acute pulmonary embolism. J Cardiol. 2012;60:508–513. doi: 10.1016/j.jjcc.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Rudski L.G., Lai W.W., Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 86–8. [DOI] [PubMed] [Google Scholar]
- 11.Lang R., Badano L., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults, An update from the American Society of echocardiography and the European association of cardiovascular imaging (vol 16, pg 233, 2015) Eur Heart J Cardiovasc Imaging. 2016;17 doi: 10.1093/ehjci/jev014. 969–969. [DOI] [PubMed] [Google Scholar]
- 12.Hashmi M. Perioperative and acute care transesophageal echocardiography (TOE) Anaesth Pain Intens Care. 2015;19:297–302. [Google Scholar]
- 13.Hrymak C., Strumpher J., Jacobsohn E. Acute right ventricle failure in the intensive care unit: assessment and management. Can J Cardiol. 2017;33:61–71. doi: 10.1016/j.cjca.2016.10.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dilated RV with deviated intraventricular septum throughout systole and diastole which demonstrates RV pressure overload.
Dilated RV with poor RV function.