Key points.
-
•
Shoulder surgery can be associated with severe postoperative pain.
-
•
Interscalene brachial plexus blockade is used to provide anaesthesia, analgesia, or both for shoulder surgery.
-
•
The beach chair position carries risks of compromising haemodynamic and cerebral function.
-
•
Selective peripheral nerve blockade provides alternative analgesia to interscalene blockade.
-
•
Operative pathways should integrate preoperative patient education, intraoperative regional anaesthesia, and multimodal postoperative analgesia.
Learning objectives.
By reading this article you should be able to:
-
•
Plan the intraoperative conduct of shoulder surgery with the patient either awake, sedated, or using general anaesthesia.
-
•
Describe the steps required to safely perform interscalene brachial plexus blockade for shoulder surgery.
-
•
Describe the complications and adverse effects of interscalene nerve block.
-
•
Discuss the differences between regional anaesthetic techniques performed for anaesthesia to facilitate awake surgery and techniques used to provide postoperative analgesia.
Anatomy
The shoulder receives sensory innervation from the cervical (C3,4) and brachial plexuses (C5,6). The major motor and sensory innervation to the shoulder is from the suprascapular nerve (upper trunk of the brachial plexus) and axillary nerve (posterior cord of the brachial plexus). Minor sensory innervation is from the lateral pectoral, musculocutaneous, and subscapular nerves. The cutaneous supply to the cape of the shoulder, upper thoracic region and also a sensory contribution to the acromioclavicular and sternoclavicular joints is from the supraclavicular nerves (descending branches of the cervical plexus; C3,4). The cutaneous supply distal to the glenohumeral joint is from the superior lateral cutaneous nerve of the arm (from the axillary nerve), the medial cutaneous nerve of the arm (from the medial cord of the brachial plexus), and the lateral cutaneous branch of the second intercostal nerve.
Surgical context
The prevalence of shoulder pain in the population is 7% overall, increasing to 26% in those aged >70 yrs.1 Acromioplasty (sub-acromial decompression), stabilisation, adhesiolysis (release of frozen shoulder) and rotator cuff repair are the most common procedures undertaken, and these are usually performed arthroscopically. Open procedures include arthroplasty of the glenohumeral joint, which can be performed as a total or partial joint replacement, open stabilisation (e.g. Latarjet–Bristow procedures), open rotator cuff repair, and most trauma procedures. Total joint replacements are divided into anatomical or reverse procedures. In the latter, the ball-and-socket arrangement of the joint is reversed prosthetically in the presence of a deficient rotator cuff to provide a mechanical advantage to the remaining deltoid muscle.
Shoulder arthroscopy is conducted via two or three ports, the position of which depends on the surgery being conducted. Typically, a posterior port is sited inferior and medial to the posterior-lateral aspect of the acromion and serves as a primary viewing portal, with anterior port, lateral port, or both (whose position is variable depending on the proposed surgery) gaining access to the joint for instrumentation.
The standard approach for shoulder arthroplasty is an anterior skin incision running from the coracoid process along the deltopectoral line towards the deltoid tuberosity of the humerus. The beach chair position improves surgical access and reduces intraoperative venous pressure and associated bleeding. The lateral position allows the application of traction to the arm to improve surgical access.
Both arthroscopic (particularly rotator cuff repair and stabilisation) and open shoulder surgery are associated with moderate to severe postoperative pain; surgery to the shoulder is one of the most painful procedures undertaken as a day case.
Preoperative considerations
Patients attending for shoulder surgery range from young adults with sporting injuries to older patients presenting with arthritic complications and trauma. Patients with diabetes mellitus have a higher incidence of frozen shoulder compared with the general population.
Consistent preoperative verbal and written communication outlining the expected anaesthetic technique should be provided. Patients can be counselled about the regional anaesthetic; undergoing surgery while conscious; the anticipated postoperative recovery period and plan for pain management.
Regional anaesthetic techniques
Given that shoulder surgery generally results in significant postoperative pain requiring opioids, regional anaesthesia forms an important part of the anaesthetic technique by improving patient experience and increasing the success of day case pathways. Regional anaesthesia reduces operating theatre time, allows earlier discharge from (or bypassing of) the postanaesthesia care unit, and reduces postoperative complications such as pain, sedation, nausea, and vomiting, and the need for overnight stay.2 Regional anaesthesia can be used as a sole anaesthetic technique or it can be combined with general anaesthesia. At the authors' institutions, arthroscopic cases are performed routinely under regional blockade in conscious or sedated patients, whereas open surgery is usually performed using regional blockade supplemented with general anaesthesia.
Undertaking shoulder surgery in conscious patients offers several advantages, summarised in Box 1.
Box 1. Potential advantages of conducting shoulder surgery in conscious patients.
| Avoidance of potential airway, respiratory, and cardiovascular complications of general anaesthesia (including reduction of hypotension in the ‘beach-chair’ position) |
| Reduced postoperative nausea and vomiting |
| Faster return to normal diet and medications in the postoperative period |
| Efficiency savings in time to institute and conduct general anaesthesia safely |
| Increased engagement of patient in their care (patients able to see their pathology and observe their treatment on the monitor in real time) |
Alt-text: Box 1
Despite the paucity of evidence to support the safety of performing nerve blocks in conscious compared with anaesthetised patients, nerve blocks for shoulder surgery at our institutions are performed with the patient awake, unless factors related to the patient render this inadvisable (e.g. a movement disorder). Anxiety is effectively allayed by preoperative education and communication from the anaesthetist during block performance. Midazolam 1–2 mg i.v. also exerts a useful anxiolytic effect while preserving the ability of the patient to communicate warning signs of impending nerve injury or local anaesthetic toxicity.
Interscalene brachial plexus blockade
Blockade of the brachial plexus at the interscalene groove has evolved substantially since Winnie's original landmark description and subsequent modifications (which were designed to reduce the risk of neuraxial injury and improve ease of catheter insertion). Elicited paraesthesiae and nerve stimulation are now rarely used as sole methods of localising nerves, having been superseded by visualisation of the relevant anatomy, needle-tip position and local anaesthetic spread using ultrasound. Peripheral nerve stimulation with or without pressure monitoring may be combined with ultrasound, but there is no direct evidence that any single or combined technique reduces the risk of peripheral nerve injury after regional anaesthesia.3 Ultrasound does, however, allow fewer needle passes, lower volumes of local anaesthetic, and better postoperative analgesia compared with nerve stimulation.4
Technique and benefits
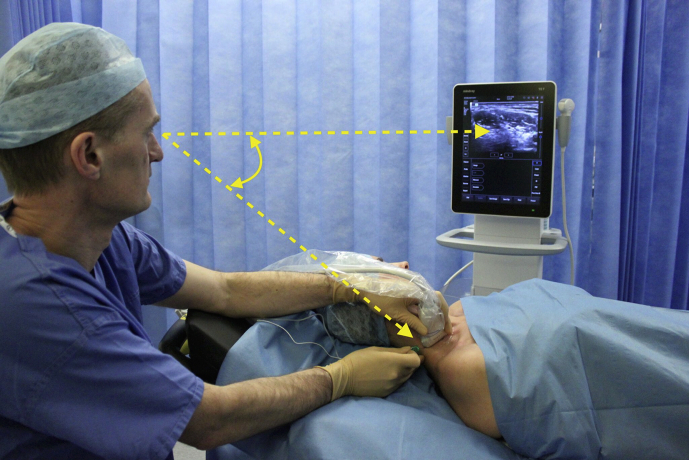
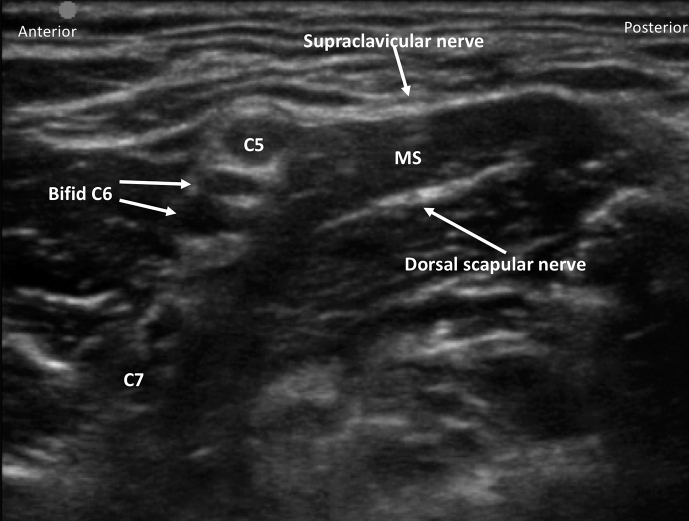
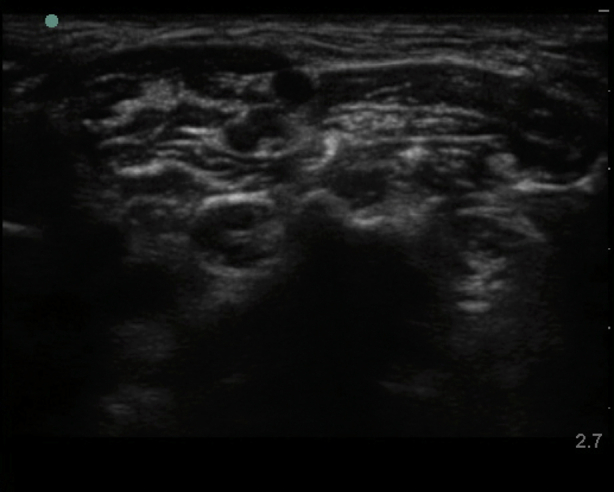
Interscalene brachial plexus blockade (ISB) is typically conducted in an awake patient after verbal consent, institution of routine monitoring, and contralateral peripheral i.v. access. With the patient positioned 30° supine and the head slightly turned to the opposite side, expose the ipsilateral neck and perform skin asepsis. The anaesthetist, patient, and ultrasound machine should be ergonomically arranged to facilitate scanning and needling (Fig. 1). A high-frequency (e.g. 15–6 MHz) linear array ultrasound transducer is ideally suited to identify the superficial interscalene groove and relevant anatomy as it is rare that the structures of interest lie deeper than 4 cm. First, identify the subclavian artery lying in the supraclavicular fossa and obtain a short-axis view of the trunks of the brachial plexus lying posterior and superficial to the artery. Then scan superiorly to trace the plexus between the anterior and middle scalene muscles deep to the prevertebral fascia. The sternocleidomastoid muscle lies superficially, and the phrenic nerve may be seen passing over the anterior scalene muscle away from the C5 root scanning superior to inferior. More medial scanning reveals the internal jugular vein, carotid artery, thyroid gland, and trachea. Scanning superiorly from the subclavian artery, the upper and middle trunks of the plexus give way to the roots which appear as hypoechoic round structures, with the superficial C5 nerve root overlying the typically bifid C6 nerve root, and the C7 root passing onto its characteristic transverse process (Fig. 2). The vertebral artery can be identified anterior to the C7 transverse process using colour Doppler. The dorsal scapular nerve arises from the C5 nerve root and can often be seen as a hyperechoic structure traversing through the middle scalene muscle (Fig. 2), sometimes accompanied by the long thoracic nerve. Both must be avoided when needling. The characteristic ultrasound shapes of the cervical transverse processes can be successively demonstrated by scanning inferiorly to superiorly (Supplementary Figs S1a-g). Our preference is an in-plane posterior-lateral to anterior-medial approach using a 50 mm echogenic short-bevel needle, positioning the tip initially deep to the C6 nerve root or upper trunk and seeking spread of local anaesthetic anterior and posterior to the nerves within the interscalene groove. Re-positioning of the needle superficial to the C5 nerve root or upper trunk is often required to obtain satisfactory spread of local anaesthetic. We do not routinely needle between C5 and C6 (and certainly not between the bifid heads of C6) unless muscular bridges are preventing adequate spread. The ability to observe in real time the spread of local anaesthetic around the roots or upper trunk is a clear advantage of ultrasound guidance. After the injection of local anaesthetic (ropivacaine 0.75% 10–15 ml produces surgical anaesthesia in approximately 20 min) in the interscalene groove, the needle is withdrawn and passed superficial to the pre-vertebral fascia overlying the scalene muscles into the superficial cervical fascia deep to the sternocleidomastoid muscle. The supraclavicular nerve trunk can be traced superiorly to inferiorly from the C4 root, passing superficially and posteriorly over the middle scalene muscle, through the pre-vertebral fascia, before dividing into terminal branches within the superficial cervical fascia deep to the sternocleidomastoid muscle (Fig. 2).5 A further injection of 2–3 ml local anaesthetic (without need to make a second skin puncture) targeting these nerves provides cutaneous anaesthesia and blocks their sensory contribution to the acromioclavicular joint for surgery.
Fig 1.
Ergonomic arrangement of anaesthetist, patient and ultrasound machine for interscalene blockade.
Fig 2.
Interscalene groove including dorsal scapular nerve in middle scalene muscle (MS) and supraclavicular nerve before division.
Adverse effects and complications
The phrenic nerve lies on the superficial surface of the anterior scalene muscle, close to the brachial plexus at the level of C5/6 before moving away from the plexus over the muscle surface inferiorly in the neck. Consequently, it is often blocked during the performance of an ISB. The duration is related to the type and mass of local anaesthetic administered. The incidence is reported as 100% with a traditional landmark based approach using volumes greater than 20 ml.6 Even with local anaesthetic dose reduction, the incidence of phrenic nerve palsy is approximately 25–50%.7 Most healthy individuals remain asymptomatic and compensate for the unilateral reduction in diaphragmatic activity by using their intercostal and accessory muscles to maintain tidal volume. Forced expiratory volume in 1 s (FEV1) may be reduced by up to 40%, however, and patients with comorbidities, particularly obesity and respiratory disease, may develop troublesome dyspnoea. In rare cases some degree of ventilatory support may be needed.
Physiological compensation means that pulse oximetry is not a sensitive test for identifying phrenic nerve dysfunction. Diaphragmatic ultrasound scanning, performed 15–30 min after the block, is a relatively simple bedside test that is more accurate.8 Identification of patients at high risk of deterioration from phrenic nerve palsy is essential. These patients are often the same group who would benefit from the avoidance of perioperative opioids and general anaesthesia. Although phrenic nerve palsy can be reduced by modifying the ISB technique, plans for possible postoperative respiratory support in a high dependency unit should be made for high risk patients. Intraoperative continuous positive pressure ventilation (CPAP) or high flow nasal oxygenation can be beneficial, and the authors have had success with these modalities prophylactically in patients with poor respiratory function and in the treatment of symptomatic dyspnoea. Persistent phrenic nerve dysfunction has been reported with a suggested incidence of approximately 1:2000.9 The most likely pathophysiology is considered to be compressive neuropathy, and this may be amenable to surgical decompression.10
ISB has previously been reported to be associated with a higher incidence of neurological dysfunction than many other peripheral blocks, with an incidence of temporary dysfunction up to 14% at 10 days in some series.11 In addition to peripheral nerve injury, neurological injury from cervical cord trauma has been described after landmark ISB was performed in an anaesthetised patient.12 Although ultrasound guidance may mitigate this risk, the performance of ISBs in deeply sedated or anaesthetised patients should only be performed after an individual patient risk/benefit assessment.
The spread of local anaesthetic to surrounding structures will produce predictable adverse effects such as transient Horner's syndrome (in approximately 50% of patients) and recurrent laryngeal nerve blockade. Although these are usually minor, they may be distressing to patients and it is prudent to mention them during the consent process. Horner's syndrome is common and produces ipsilateral ptosis, miosis, nasal congestion, and anhydrosis. Spontaneous resolution occurs after a few hours, and reassurance is all that is required. Recurrent laryngeal nerve blockade, caused by local anaesthetic spread over the anterior scalene muscle, results in a hoarse voice that is usually of no significant clinical consequence.
Systemic local anaesthetic toxicity cause by absorption is rare, but the presence of vessels including the vertebral artery near to the roots of the plexus means that toxicity from intravascular injection is a possibility. The rapid delivery of local anaesthetic from the vertebral artery to the brain means that the presenting features are CNS adverse effects and convulsions. Careful needle visualisation (maintaining a superficial trajectory in relation to the C7 nerve root), aspiration before injection, incremental injection, and direct visualisation of local anaesthetic spread within tissues, all help prevent this complication.
The incidence of hypotensive and bradycardic events is up to 20% during shoulder surgery.13 These typically occur in the sitting position around 30 min after the placement of an ISB. The origin is multifactorial and may be a vasovagal response but the Bezold–Jarisch reflex is often implicated; high circulating concentrations of catecholamines and an underfilled, hypercontractile ventricle (induced by venous pooling in the sitting position) stimulates intramyocardial mechanoreceptors, resulting in an abrupt reduction in sympathetic tone together with increased vagal tone. Prompt treatment with an antimuscarinic (ideally atropine because of its rapid onset) with or without sympathomimetic drugs is indicated.
Alternative blocks for awake shoulder surgery
Although ISB is the most commonly used regional anaesthetic technique for shoulder surgery, a number of other approaches have been investigated in an attempt to avoid unwanted adverse effects such as phrenic nerve blockade.
Superior trunk block
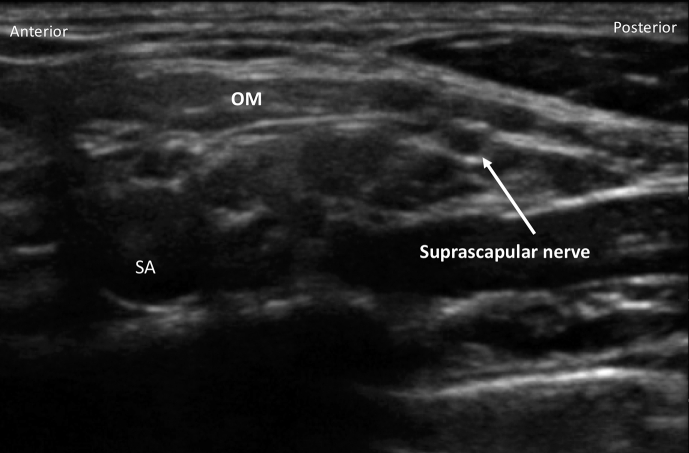
The C5 and C6 nerve roots may be tracked distally in the interscalene groove, where they fuse to form the superior trunk. As the course of the suprascapular and transverse cervical arteries is highly variable, these vessels should be sought and their position noted before needling. Using a posterior-lateral to anterior-medial in-plane approach and a hydrodissection technique, the superior trunk may be surrounded with 10–15 ml local anaesthetic. This will effectively block the major nervous innervation to the shoulder joint. The suprascapular nerve originates from the superior trunk and moves laterally deep to omohyoid muscle (Fig. 3); the block is therefore performed proximal to this point. Although case reports indicate effective analgesia and absence of phrenic nerve blockade (because of the more distal approach), there are no prospectively randomised data to support this technique. We will often perform a superior trunk block if this provides a superior view (e.g. in a patient with a short neck).
Fig 3.
Suprascapular nerve deep to the omohyoid muscle (OM) moving distally from subclavian artery (SA) and plexus.
Supraclavicular brachial plexus block
The supraclavicular brachial plexus block has also produced shoulder anaesthesia with similar analgesic effect to that of ISB, with a reduced incidence of adverse effects such as Horner's syndrome.14 However, the risk of phrenic nerve block is not appreciably different to that of ISB, limiting its benefits in clinical practice.15 In addition, a supraclavicular brachial plexus block risks missing the more proximally departing suprascapular nerve.
Intraoperative conduct
Having placed an ISB, efficacy can easily be assessed by verifying motor weakness in the deltoid and biceps muscles (C5/6). Loss of sensation over the cape of the shoulder will confirm blockade of the supraclavicular nerves. Patients may then self-position on the operating table in either beach chair or in the lateral position with traction applied to the arm. We favour the beach chair position as it provides superior patient comfort during awake surgery.
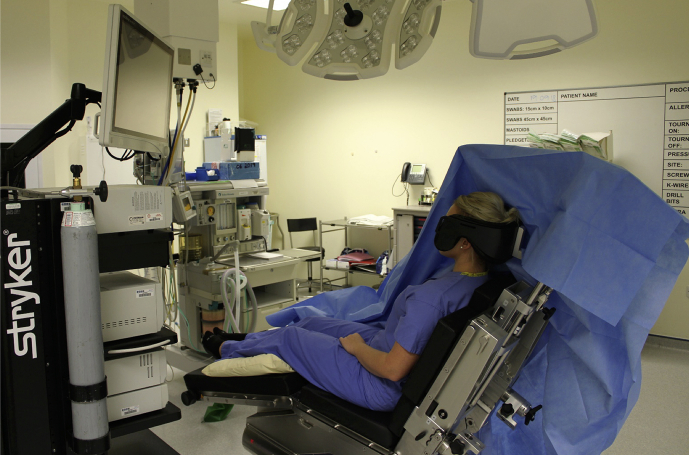
The drapes should be supported so that they do not lie on the patient's face, providing an option to view the surgical monitor during awake surgery. A pillow positioned under the knees also reduces stretch on the hamstrings and increases comfort in awake patients (Fig. 4). A proportion of patients' supraclavicular nerve distribution will not cover the posterior port site, so subcutaneous infiltration of long-acting local anaesthetic should be placed before starting surgery. Once started, the anaesthetist or surgeon can engage and inform the patient by describing the surgery. A proportion (in our experience, 10–20%) of patients will experience intraoperative pain at some point. Having discussed this with patients before operation, a dose of alfentanil i.v. will allow surgery to continue in virtually all patients by providing analgesia without disinhibition. Bradycardia and hypotension (described above) must be managed rapidly to prevent severe bradycardia, syncope secondary to decreased cerebral perfusion, or both. For those patients requesting sedation, the authors prefer to use midazolam boluses and propofol via target-controlled infusion with supplemental oxygen and monitoring of exhaled CO2. At the end of surgery, awake patients can reposition (with support) onto the bed.
Fig 4.
Typical posture in beach chair position. Large anaesthetic tube support keeps the surgical drape away from the patient's face.
If general anaesthesia is planned in the beach chair position, care should be taken to pad and support the heels and arms. The head is placed and secured in a specific support with the neck optimally positioned. One benefit of the beach chair position is that airway management with a supraglottic airway is often sufficient (the authors prefer second-generation devices); these also have the advantage of being less stimulating during head movement that occurs during surgical traction on the arm. Spontaneous respiration or pressure-support ventilation decreases the reduction of venous return and resulting hypotension associated with the beach chair position and mandatory positive pressure ventilation. The head-up nature of the beach chair position has been implicated in producing cerebral ischaemia secondary to hypotension or thromboembolic events;16 therefore, the authors prefer to maintain blood pressure during general anaesthesia close to baseline values, appreciating the vertical distance of the head above the heart. A vasopressor infusion may be used to achieve this. Careful surgical dissection is also vital to avoid air embolus. In addition to standard monitoring, titration of anaesthetic depth using depth of anaesthesia monitoring may be advantageous to reduce hypotension secondary to deep anaesthesia. Although it is not part of our routine practice, cerebral oxygen saturation monitoring has also been used in the beach chair position to try and ensure adequate cerebral blood flow during surgery.
Postoperative management
Most patients who have undergone arthroscopic shoulder surgery can be discharged home on the day of surgery.
Limb care
After shoulder surgery, most patients will be discharged with their arm in an adjustable supporting sling. All patients should be instructed to protect the insensate limb from accidental damage until the ISB has worn off.
Systemic analgesia
Despite the excellent immediate postoperative analgesia provided by a regional anaesthetic technique, one fifth of patients report their postoperative pain after shoulder surgery as ‘the worst pain imaginable’ once the block has worn off.17 It is therefore vital to ensure that patients begin a multimodal oral analgesic regimen before the block has worn off. Paracetamol and a non-steroidal anti-inflammatory agent (unless contraindicated) should be provided. Strong opioids are often required in the first 48 h after block resolution, and the majority of patients cope well with titrating immediate release oral morphine at home.
Perineural catheters
Major shoulder procedures such as arthroplasty are associated with moderate to severe postoperative pain which outlasts the typical 12–24 h of analgesia provided by single injection ISB. Postoperative continuous infusion of local anaesthetic into the interscalene groove via a perineural catheter reduces both rest and dynamic shoulder pain and opioid consumption compared with single injection ISB and is associated with higher patient satisfaction.18 Catheter insertion may be performed either in-plane or out-of-plane according to operator preference. Although overall complication rates are low, the consequences of interscalene catheter malposition or dislodgement may be significant.19 For this reason, the catheter tip position should be verified with ultrasound and a test bolus of local anaesthetic with or without adrenaline administered while the patient is still in a monitored environment. If the catheter and tip is difficult to visualise, small ‘pulses’ of fluid can be detected using Doppler-mode ultrasound. Injection of a tiny volume of air or manual ‘wiggling’ of the catheter can also help confirm position. Secure fixation is essential and a number of methods including skin glue, specialist anchor dressings, and tunneling have all been described to prevent dislodgement. Skin glue is additionally helpful in preventing leakage of local anaesthetic from the skin puncture site. Interscalene catheter placement is an advanced technique requiring skilled operators for block placement and the infrastructure to monitor and troubleshoot the infusion after surgery. Pharmacological adjuncts such as dexamethasone can prolong analgesia following single injection ISB to 20–24 h, and for many anaesthetists this offers an acceptable compromise between extended duration and ease of technique.
Subacromial/intra-articular analgesia
This can be performed by the surgeon at the end of the procedure, either as a single injection of 20–40 ml local anaesthetic or accompanied by placement of a catheter. Such techniques are of marginal, if any, clinical benefit however and carry a risk of iatrogenic chondrolysis.
Selective peripheral nerve blocks for analgesia
Blockade of the suprascapular nerve provides an alternative to ISB for postoperative analgesia in patients who receive general anaesthesia for surgery. The early postoperative analgesia provided by suprascapular nerve blockade is inferior to that of ISB but superior to intra-articular infiltration.20 The suprascapular nerve may be blocked posteriorly in the supraspinatus fossa or anteriorly as it exits the upper trunk of the plexus and moves laterally deep to the omohyoid muscle (Fig. 3).
Combining selective suprascapular nerve with axillary nerve block21 provides superior analgesia to suprascapular nerve block alone.22 As all nerves innervating the shoulder are not blocked, this technique is unsuitable for awake surgery but is reserved as an analgesic alternative to ISB. The axillary nerve may be blocked by imaging the posterior surface of the humerus just distal to the humeral head. The posterior circumflex humeral artery and axillary nerve may be seen at this point, and local anaesthetic is injected deep to the deltoid muscle. A volume of 5–7 ml may be sufficient to travel proximally through the quadrilateral space to block the axillary articular supply to the shoulder.23 The axillary nerve may alternatively be targeted by an infraclavicular approach also aiming to block the subscapular, musculocutaneous, and lateral pectoral nerves and thus achieve fairly complete shoulder analgesia with a low incidence of phrenic nerve palsy. At present evidence is limited to case reports,24, 25 and further larger-scale studies are warranted to establish effectiveness compared with ISB.
Up to 40% of patients may fail to demonstrate either sensory or motor block after combined suprascapular and axillary nerve block, and in our experience this limits its role compared with the very reliable and reproducible ISB.
Declaration of Interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
David Hewson BSc (Hons) PGCert FHEA FRCA is a consultant anaesthetist and honorary assistant professor in Nottingham with clinical and research interests in regional anaesthesia, sedation practice, and patient experience. He is a current NIHR Investigator undertaking research into sedation with propofol.
Matt Oldman FRCA PGCert EDRA is a consultant anaesthetist in Plymouth who has clinical interests in orthopaedic and regional anaesthesia. He is a former secretary of Regional Anaesthesia UK and a current examiner for the European Diploma in regional anaesthesia. He teaches ultrasound-guided regional anaesthesia in the UK and internationally.
Nigel Bedforth BMedSci FRCA a consultant anaesthetist and honorary associate professor in Nottingham whose clinical interests include regional and orthopaedic anaesthesia. His research interests include training in regional anaesthesia and patient experiences during regional anaesthesia. He is a current NIHR Principal Investigator.
Matrix codes: 1D02, 2G01, 3A09
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2018.12.004.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
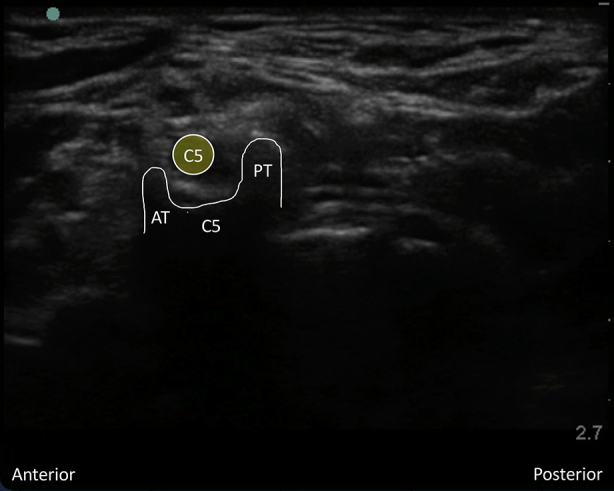
Supplementary Fig 1 Characteristic ultrasound appearance of respective cervical transverse processes. (Figs S1a and S1 b) C7 transverse process acoustic shadow with posterior tubercle and absent anterior tubercle. Note the C5, C6, and C7 nerve roots superficial to the transverse process. (Figs S1c and S1d) Colour Doppler applied to the C7 transverse process demonstrating the proximity of the vertebral artery. (Figs S1e and S1f) C6 transverse process acoustic shadow with less prominent posterior tubercle (PT) and more prominent anterior tubercle (AT); also known as Chassaignac's tubercle. Note the C5 and C6 nerve roots superficial to the transverse process. (Figs S1g and S1h) C5 transverse process acoustic shadow with prominent posterior tubercle (PT) and less prominent anterior tubercle (AT). Note the C5 nerve root superficial to the transverse process.
Fig. S1A.
Video 1Interscalene brachial plexus block prescan. Prescan for interscalene brachial plexus block. Scan the plexus from inferior to superior, observing the formation of the trunks, the superficial supraclavicular nerves coalescing, and the nerves passing through the middle scalene muscle. If reading the pdf online, click on the image to view the video
Supraclavicular trunk and divisions prescan. Prescan for the supraclavicular trunk and divisions. Scan from superior to inferior starting superior to the C5 nerve root. The supraclavicular nerve is seen passing superficially and posteriorly before dividing into terminal branches within the superficial cervical fascia. If reading the pdf online, click on the image to view the video.
Suprascapular nerve prescan. Prescan to observe the suprascapular nerve departing posteriorly from the upper trunk. Scan inferiorly from the upper trunk to observe the suprascapular nerve departing posteriorly underneath the omohyoid muscle. If reading the pdf online, click on the image to view the video.
Interscalene brachial plexus block placement 1. Short axis (view of the nerves), in-plane (needle with respect to the transducer) needle insertion technique. Local is placed deep to C6 root. If reading the pdf online, click on the image to view the video.
Interscalene brachial plexus block placement 2. Local anaesthetic is placed around C5 nerve root. If reading the pdf online, click on the image to view the video.
Interscalene brachial plexus block placement 3. Local anaesthetic is placed around the supraclavicular nerve branches within the superficial cervical fascia. If reading the pdf online, click on the image to view the video.
Fig. S1B.
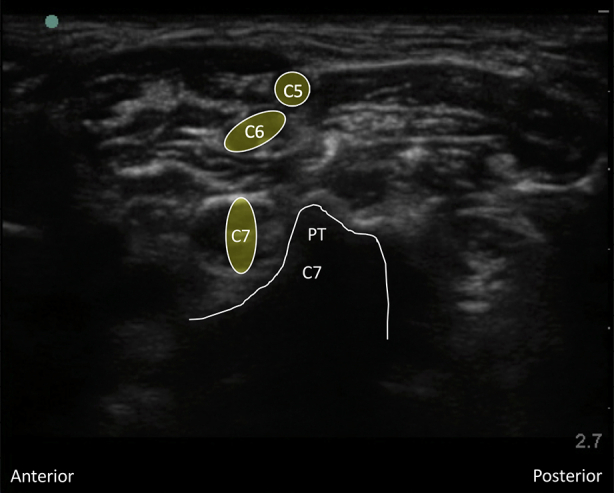
Fig. S1C.
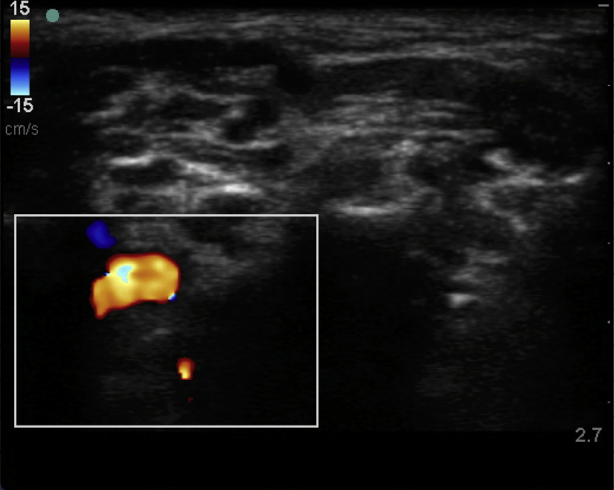
Fig. S1D.
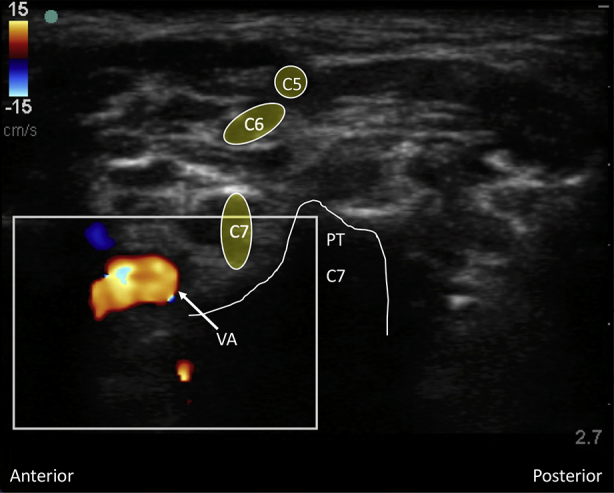
Fig. S1E.
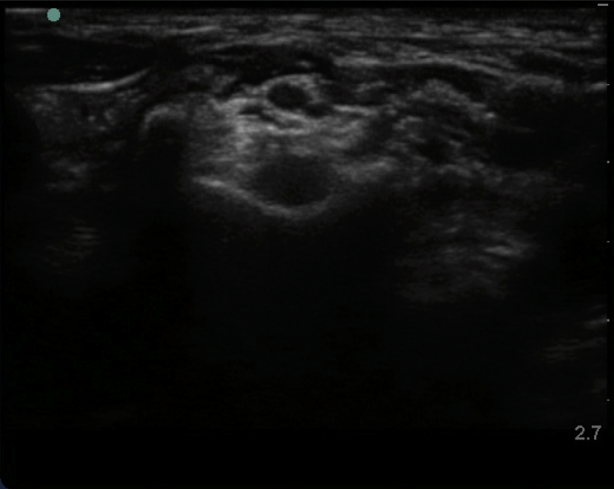
Fig. S1F.
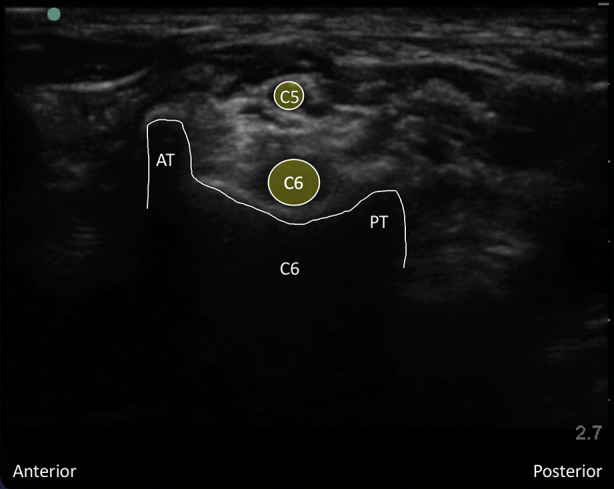
Fig. S1G.
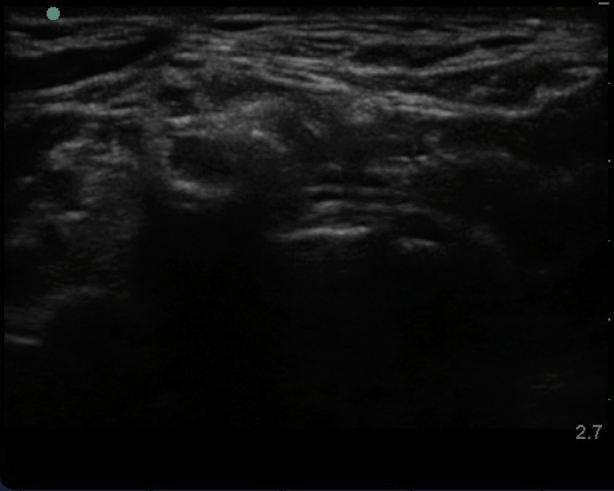
Fig. S1H.
References
- 1.Linsell L., Dawson J., Zondervan K. Prevalence and incidence of adults consulting for shoulder conditions in UK primary care; patterns of diagnosis and referral. Rheumatology. 2006;45:215–221. doi: 10.1093/rheumatology/kei139. [DOI] [PubMed] [Google Scholar]
- 2.Hadzic A., Williams B.A., Karaca P.E. For outpatient rotator cuff surgery, nerve block anesthesia provides superior same-day recovery over general anesthesia. Anesthesiology. 2005;102:1001–1007. doi: 10.1097/00000542-200505000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Hewson D.W., Bedforth N.M., Hardman J.G. Peripheral nerve injury arising in anaesthesia practice. Anaesthesia. 2018;73:51–60. doi: 10.1111/anae.14140. [DOI] [PubMed] [Google Scholar]
- 4.McNaught A., Shastri U., Carmichael N. Ultrasound reduces the minimum effective local anaesthetic volume compared with peripheral nerve stimulation for interscalene block. Br J Anaesth. 2011;106:124–130. doi: 10.1093/bja/aeq306. [DOI] [PubMed] [Google Scholar]
- 5.Maybin J., Townsley P., Bedforth N., Allan A. Ultrasound guided supraclavicular nerve blockade: first technical description and the relevance for shoulder surgery under regional anaesthesia. Anaesthesia. 2011;66:1053–1055. doi: 10.1111/j.1365-2044.2011.06907.x. [DOI] [PubMed] [Google Scholar]
- 6.Urmey W.F., Talts K.H., Sharrock N.E. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg. 1991;72:498–503. doi: 10.1213/00000539-199104000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Riazi S., Carmichael N., Awad I., Holtby R.M., McCartney C.J.L. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth. 2008;101:549–556. doi: 10.1093/bja/aen229. [DOI] [PubMed] [Google Scholar]
- 8.El-Boghdadly K., Alberto Goffi M., Chan V. Point of care diaphragmatic ultrasonography made easy. Can J Anesth. 2017;64:327–328. doi: 10.1007/s12630-016-0766-z. [DOI] [PubMed] [Google Scholar]
- 9.Hogan Q.H. Phrenic nerve function after interscalene block revisited. now, the long view. Anesthesiol. 2013;119:250–252. doi: 10.1097/ALN.0b013e31829c2f3a. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman M., Elkwood A., Rose M. Surgical treatment of permanent diaphragm paralysis after interscalene nerve block for shoulder surgery. Anesthesiol. 2013;119:484–487. doi: 10.1097/ALN.0b013e31829c2f22. [DOI] [PubMed] [Google Scholar]
- 11.Borgeat A., Ekatodramis G., Kalberer F., Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiol. 2001;95:875–880. doi: 10.1097/00000542-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Benumof J. Permanent loss of cervical spinal cord function associated with interscalene block performed under general anesthesia. Anesthesiol. 2000;93:1541–1544. doi: 10.1097/00000542-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Song S., Roh W. Hypotensive bradycardic events during shoulder arthroscopic surgery under interscalene brachial plexus blocks. Korean J Anesthesiol. 2012;62:209–219. doi: 10.4097/kjae.2012.62.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryu T., Kil B.T., Kim J.H. Comparison between ultrasound-guided supraclavicular and interscalene brachial plexus blocks in patients undergoing arthroscopic shoulder surgery: a prospective, randomized, parallel study. Medicine (Baltimore) 2015;94:e1726. doi: 10.1097/MD.0000000000001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo C.W., Ma J.X., Ma X.L. Supraclavicular block versus interscalene brachial plexus block for shoulder surgery: a meta-analysis of clinical control trials. Int J Surg. 2017;45:85–91. doi: 10.1016/j.ijsu.2017.07.098. [DOI] [PubMed] [Google Scholar]
- 16.Pohl A., Cullen D. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17:463–469. doi: 10.1016/j.jclinane.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Wilson A.T., Nicholson E., Burton L., Wild C. Analgesia for day-case shoulder surgery. Br J Anaesth. 2004;92:414–415. doi: 10.1093/bja/aeh071. [DOI] [PubMed] [Google Scholar]
- 18.Vorobeichik L., Brull R., Bowry R., Laffey J.G., Abdallah F.W. Should continuous rather than single-injection interscalene block be routinely offered for major shoulder surgery? A meta-analysis of the analgesic and side-effects profiles. Br J Anaesth. 2018;120:679–692. doi: 10.1016/j.bja.2017.11.104. [DOI] [PubMed] [Google Scholar]
- 19.Yanovski B., Gaitini L., Volodarski D., Ben-David B. Catastrophic complication of an interscalene catheter for continuous peripheral nerve block analgesia. Anaesthesia. 2012;67:1166–1169. doi: 10.1111/j.1365-2044.2012.07222.x. [DOI] [PubMed] [Google Scholar]
- 20.Singelyn F.J., Lhotel L., Fabre B. Pain relief after arthroscopic shoulder surgery: a comparison of intraarticular analgesia, suprascapular nerve block, and interscalene brachial plexus block. Anesth Analg. 2004:589–592. doi: 10.1213/01.ANE.0000125112.83117.49. [DOI] [PubMed] [Google Scholar]
- 21.Price D. The shoulder block: a new alternative to interscalene brachial plexus blockade for the control of postoperative shoulder pain. Anaesth Intensive Care. 2007;35:575. doi: 10.1177/0310057X0703500418. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.J., Kim D.-Y., Hwang J.-T. Effect of ultrasonographically guided axillary nerve block combined with suprascapular nerve block in arthroscopic rotator cuff repair: a randomized controlled trial. Arthrosc J Arthrosc Relat Surg. 2014;30:906–914. doi: 10.1016/j.arthro.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Price D. How I do it: ultrasound-guided combined suprascapular and axillary nerve block. Am Soc Reg Anesth Pain Med News. 2013;13:22–25. [Google Scholar]
- 24.Casanova M.G., Choi S., McHardy P.G. Ultrasound-guided posterior cord and selective suprascapular block for shoulder surgery. Br J Anaesth. 2016;117:835. doi: 10.1093/bja/aew373. [DOI] [PubMed] [Google Scholar]
- 25.Vagh F., Baker E., Arndt C., Billstrand M.M. Anterior approach to the suprascapular nerve. Reg Anesth Pain Med. 2017;42:680. doi: 10.1097/AAP.0000000000000627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1Interscalene brachial plexus block prescan. Prescan for interscalene brachial plexus block. Scan the plexus from inferior to superior, observing the formation of the trunks, the superficial supraclavicular nerves coalescing, and the nerves passing through the middle scalene muscle. If reading the pdf online, click on the image to view the video
Supraclavicular trunk and divisions prescan. Prescan for the supraclavicular trunk and divisions. Scan from superior to inferior starting superior to the C5 nerve root. The supraclavicular nerve is seen passing superficially and posteriorly before dividing into terminal branches within the superficial cervical fascia. If reading the pdf online, click on the image to view the video.
Suprascapular nerve prescan. Prescan to observe the suprascapular nerve departing posteriorly from the upper trunk. Scan inferiorly from the upper trunk to observe the suprascapular nerve departing posteriorly underneath the omohyoid muscle. If reading the pdf online, click on the image to view the video.
Interscalene brachial plexus block placement 1. Short axis (view of the nerves), in-plane (needle with respect to the transducer) needle insertion technique. Local is placed deep to C6 root. If reading the pdf online, click on the image to view the video.
Interscalene brachial plexus block placement 2. Local anaesthetic is placed around C5 nerve root. If reading the pdf online, click on the image to view the video.
Interscalene brachial plexus block placement 3. Local anaesthetic is placed around the supraclavicular nerve branches within the superficial cervical fascia. If reading the pdf online, click on the image to view the video.