Learning objectives.
By reading this article, you should be able to:
-
•
Explain the pharmacology of mannitol and hypertonic saline.
-
•
Recall how mannitol and hypertonic saline can reduce raised intracranial pressure in the context of traumatic brain injury.
-
•
Differentiate between the different formulations of hypertonic saline and mannitol.
-
•
Discuss the evidence regarding the use of mannitol and hypertonic saline in traumatic brain injury.
Key points.
-
•
Osmotherapy can reduce intracranial pressure after traumatic brain injury.
-
•
Mannitol and hypertonic saline have distinct actions and adverse effects.
-
•
There is currently no evidence to support the overall superiority of one agent.
-
•
It is unknown whether osmotherapy improves neurological outcome or mortality in traumatic brain injury.
-
•
To ensure safety, any local guidelines should account for the different formulations available.
Severe traumatic brain injury (TBI) is classified in the acute phase as a post-resuscitation Glasgow coma score (GCS) of 3–8.1 It carries significant morbidity and has a mortality of 20–30%.1 Once primary brain injury has occurred, the overall goal of treatment is to prevent secondary damage. The care of patients with TBI is informed by the use of international evidence-based guidelines collated by the Brain Trauma Foundation (BTF). Central to these are targeted interventions to monitor and lower intracranial pressure (ICP), whilst maintaining cerebral perfusion.1
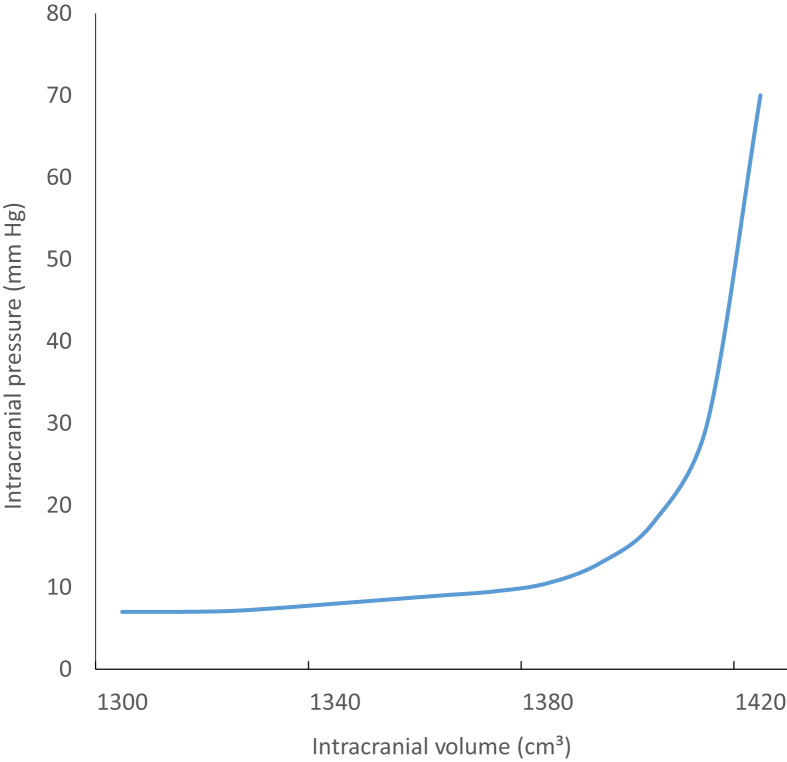
According to the Monro–Kellie hypothesis, the relationship between intracranial volume and pressure is linear, up to a point beyond which the ICP increases exponentially (Fig. 1).2 To begin with, an increased intracranial volume is compensated for by compression of compliant structures within the skull. The cerebrospinal fluid (CSF) is pushed out of the ventricular system into the spinal subarachnoid space and the blood volume is reduced as veins and arteries collapse. Cerebral perfusion is initially maintained by systemic hypertension. However, when these compensatory mechanisms are exhausted, the ICP increases exponentially and death occurs, either through cessation of cerebral blood flow (CBF) or brainstem herniation.2, 3
Fig 1.
The relationship between intracranial pressure and intracranial volume.
It has been almost 100 yr since the effects of osmotherapy to reduce CSF and brain volume were first recognised through the experimental use of i.v. hypertonic saline (HTS) in cats.3 Over time, many hypertonic solutions have been used in clinical practice, including bicarbonate 8.4%, sodium lactate, mannitol, and HTS (with or without dextrans).4 For decades, mannitol has been considered the gold standard. Its widespread use was demonstrated in a 1996 survey of UK neurosurgical centres, which showed it was used routinely in all units.5 More recently, however, there has been significant debate regarding the potential superiority of HTS.6 The aims of this article are to review the existing evidence on the use of osmotherapeutic agents, and provide pragmatic information regarding the clinical use of mannitol and HTS in TBI.
Pharmacology and physiology of mannitol and HTS
Mannitol (C6H14O6) is a naturally occurring six-carbon polyol isomer of sorbitol. It has a molecular weight of 182 Da and is readily filtered by the glomerulus. It does not undergo biotransformation, and is minimally reabsorbed and excreted by the kidney.
The term HTS describes a group of fluids containing sodium chloride in a greater than physiological concentration. The range of concentrations available for clinical use varies from 1.8% to 30% (Table 1). Some formulations also contain dextrans, with the aim of prolonged volume expansion.
Table 1.
Comparison of different concentrations of HTS available for clinical use, and mannitol as a 10 and 20% preparation.
| Solution | Sodium concentration (mmol L−1) | Osmolarity (mOsm L−1) | Equiosmolar dose ml (275 mOsm) | Dose (ml kg−1) for 80 kg person | Recommended dose in intracranial hypertension |
|---|---|---|---|---|---|
| NaCl 0.9% | 154 | 308 | 892 | 11.0 | N/A |
| Ringer's lactate | 130 | 275 | 1000 | 12.5 | N/A |
| Saline 1.7% | 291 | 582 | 472 | 5.9 | |
| Saline 3% | 513 | 1027 | 268 | 3.4 | 2 ml kg−1 |
| Saline 5% | 856 | 1711 | 161 | 2.0 | 1–2 ml kg−1=137.5–275 mOsm |
| Saline 7.2%/HES 6% (200/0.6) | 1232 | 2464 | 112 | 1.4 | |
| Saline 7.5% | 1283 | 2566 | 107 | 1.3 | 1–2 ml kg−1 |
| Saline 7.5%/dextran 70 6% | 1283 | 2568 | 107 | 1.3 | |
| Saline 10% | 1712 | 3424 | 80 | 1 | |
| Saline 23% | 4004 | 8008 | 34 | 0.43 | |
| Saline 30% | 5000 | 10,000 | 27.5 | 0.34 | |
| Mannitol 10% (1 g ml−1) | 549 | 502 | 6.3 | 0.25–2 g kg−1 over 30–60 min=40–160 ml (22–88 mOsm) | |
| Mannitol 20% (2 g ml−1) | 1098 | 251 | 3.1 | 20–80 ml (22–88 mOsm) |
After an i.v. bolus of mannitol or HTS, plasma osmolarity increases. The increase in plasma osmotic pressure draws water from the intracellular and interstitial compartments into the intravascular space. This causes an increase in blood volume, cardiac output, and pulmonary capillary wedge pressure.7, 8
In TBI, both mannitol and HTS act to reduce the ICP by promoting neuronal and endothelial cell dehydration. Neuronal dehydration reduces brain volume by limiting cerebral oedema, whereas endothelial dehydration reduces capillary wall thickness. A thinner capillary wall widens the vessel diameter, promoting blood flow and reducing the distance that oxygen must diffuse before it can reach the neuronal mitochondria. The movement of water into the capillary reduces blood viscosity, again facilitating flow and oxygen delivery to the brain. In response to better oxygenation, pial arterioles constrict, thus reducing cerebral blood volume and alleviating the increase in ICP.7, 8
However, this is where the similarities end. Approximately 45 min after a bolus of mannitol i.v., diuresis occurs, causing significant hypotension and a potential decrease in cerebral perfusion pressure (CPP).7, 8 As mannitol passes into the nephron, it increases the osmotic pressure in both the proximal convoluted tubule and descending loop of Henle. This prevents water from being reabsorbed back into the circulation and leads to a dilute tubular sodium concentration. Furthermore, the effects of mannitol on activating endogenous atrial natriuretic peptide and suppressing antidiuretic hormone release combine to result in the production of high volumes of dilute urine.
Conversely, HTS causes a prolonged increase in intravascular volume, which may benefit CPP especially in hypotensive trauma patients. Mannitol acts as a free-radical scavenger, which may limit damage to neuronal mitochondria.8 HTS is believed to restore endothelial Na+/glutamate pump activity controlling the toxic excitatory effects of excess glutamate release.7
Adverse effects and precautions when using osmotherapy
It is no surprise that mannitol and HTS can cause complications from cardiovascular changes, electrolyte disturbance and renal damage.7, 8 HTS may precipitate pulmonary oedema in patients with heart failure because of the sudden expansion of intravascular volume, whereas a rapid infusion of mannitol may result in acute arterial hypotension and cerebral hypoperfusion.1, 8
Mannitol promotes urinary excretion of magnesium, potassium, phosphate, and bicarbonate ions, and can cause hyperosmolar renal failure.8 In contrast, HTS can cause a profound hyperchloraemic acidosis and significant hypernatremia. As a result, serum sodium needs to be monitored closely. The highest recorded serum sodium in a patient that survived after HTS therapy for TBI was 169 mmol L−1. As a precaution, most studies set an upper serum sodium limit of 155 mmol L−1, a figure often used to guide clinical practice.9 HTS poses the theoretical risk of central pontine myelinolysis if administered to a patient with hyponatraemia. However, evidence from post-mortem studies of non-survivors and MRI scans of those recovering from TBI show no signs of this phenomenon.9
Because they are hyperosmolar, both mannitol and HTS can cause extravasation injuries. Mannitol can be given through a large peripheral vein, but it is recommended that HTS be infused through a central line, making its administration more complicated. However, an observational study in 2017 showed a low incidence of adverse incidents [one episode of thrombophlebitis and two episodes of infiltration (n=34)], when HTS 3% was infused through a peripheral cannula. The risks of administering low-concentration HTS (3–5%) may, therefore, be overstated.10 However, it is considered prudent to flush peripheral cannulae after an infusion to avoid blockage.
There are concerns over the ability of mannitol to cross the blood–brain barrier, accumulate in the brain, and worsen local oedema. This phenomenon has been demonstrated in rats, but its relevance in humans remains unknown.11 HTS has a slightly increased reflection coefficient (1.0 for HTS compared with 0.9 for mannitol), and is therefore less likely to cause this local cerebral oedema. However, prolonged administration of either agent can result in reversal of the water transfer gradient between the brain parenchyma and blood; this highlights the importance of a gradual reduction in dosage after a prolonged infusion of either agent.4, 7, 8
Evidence base: systematic reviews and meta-analyses
Many studies have attempted to address the question of whether HTS is superior to mannitol in the treatment of raised ICP, and the topic has been debated for more than 20 yr. Subsequent attempts to draw this research together have resulted in multiple systematic reviews, including two Cochrane systematic reviews and five other meta-analyses.12, 13, 14, 15, 16, 17, 18 Furthermore, in its aim to produce clear guidance, the BTF has reviewed and assessed the quality of the evidence base. To date, no clear conclusions have been drawn.
The combined analysis of the data is fraught with difficulty. The original studies (Table 2) considered patients with a wide range of underlying pathologies, and used different hypertonic solutions of varying osmolar concentrations and volumes. Furthermore, the treatment protocols, ICP definitions, and thresholds for administration were diverse, and clinical measures of efficacy, such as mortality and long-term neurological outcome, were rarely considered. As a result, the meta-analyses drew data from vastly heterogeneous studies (Table 3).
Table 2.
Summary of papers used in five meta-analyses and BTF guidelines on the use of osmotherapy in TBI. GOS, Glasgow outcome scale; HSD, hypertonic saline/dextran; PET, positron emission tomography; SAH, subarachnoid haemorrhage; SBP, arterial systolic blood pressure.
| Study design | Patient number | Pathology | Protocol | Outcome measure | |
|---|---|---|---|---|---|
| Schwartz et al. (1984)24 | RCT crossover | 54 | TBI GCS <8 | 5 ml kg−1 Mannitol 20% (5.49 mOsm kg−1) bolus or phenobarbitone | ICP reduction mortality |
| Fisher et al. (1992)27 | RCT crossover | 18 | Paediatric TBI | HTS 3% vs NaCl 0.9% or NaCl 0.9% | Mean ICP reduction |
| Schwartz et al. (1998)28 | RCT | 9 | Stroke ICP >25 mm Hg | Mannitol 20% (220 mOsm bolus) or HTS 7.5%+HES 6% (257 mOsm bolus) | Decrease (>10%) below ICP baseline |
| Shackford et al. (1998)23 | RCT pre-hospital†,‡ | 34 | TBI GCS <8 | HTS 1.6% or Ringer's lactate to maintain SBP >100 mm Hg | ICP reduction, GOS 6 months |
| Qureshi et al. (1999)9 | Retrospective cohort | 82 | TBI GCS <8 | Saline acetate 2–3% or NaCl 0.9% used as a continuous infusion of 75–150 ml h−1 | Mortality |
| Afifi et al. (2003)29 | RCT | 40 | Tumour | Mannitol 20% or HTS 3% both 5.49 mOsm kg−1 doses | ICP control <20 mm Hg |
| Vialet et al. (2003)19 | RCT∗ | 20 | TBI GCS <8 | Mannitol 20% (2 ml kg−1=2.2 mOsm kg−1) or HTS 7.5% (2 m kg−1=5.13 mOsm kg−1) | ICP control <22 mm Hg mortality, GOS |
| Cooper et al. (2004)20 | RCT pre-hospital | 229 | Blunt TBI GCS <9 and SBP <100 mm Hg | 250 ml HTS 7.5% or 250 ml Ringer's lactate bolus | Mortality and GOS |
| Battison et al. (2005)30 | RCT | 9 | TBI and SAH GCS <8 and ICP >20 mm Hg | Mannitol 20% (200 ml=249 mOsm) and NaCl 7.5%/dextran 70 6% (100 ml=250 mOsm); each patient received two boluses of each; the first agent was randomised | ICP reduction and duration below 20 mm Hg |
| Harutjunyan et al. (2005)31 | RCT | 40 | TBI GCS <8 | HTS 7.2%+HES 6% or mannitol infusion 15% until ICP <15 mm Hg | Mean effective dose; CPP |
| Bentson et al. (2006)32 | RCT | 22 | SAH, ICP 10–20 mm Hg | 2 ml kg−1 HTS 7.2%+HES 6% or 2 ml kg−1 NaCl 0.9% | Mean change in ICP, CPP |
| Francony et al. (2008)33 | RCT∗ | 20 | TBI (17), ICH (2), stroke (1) | 231 ml Mannitol 20% or 100 ml HTS 7.45% (both 255 mOsm boluses) | Mean ICP reduction |
| Ichai et al. (2009)34 | RCT† | 34 | TBI | Mannitol 20% (1.74 mOsm kg−1 bolus) or sodium lactate (1.65 mOsm kg−1 bolus) | ICP reduction, GOS at 12 months |
| Oddo et al. (2009)35 | Retrospective non-randomised crossover | 12 | TBI mean GCS 3 on admission | Mannitol 25% (0.75 g kg−1=4.12 mOsm kg−1) or 250 ml HTS 7.5%=641 mOsm bolus (range: 9.1–6.4 mOsm kg−1 for 70–100 kg person) | Brain tissue oxygenation, CPP |
| Bulger et al. (2010)21 | RCT pre-hospital | 1073 | Blunt TBI SBP >100 mm Hg | 250 ml Bolus of either HTS 7.5%/dextran 70 6% or HTS 7.5% or NaCl 0.9% | 28 day mortality; 6 month GOS |
| Bordreaux et al. (2011)36 | RCT∗ | 11 | TBI GCS <8 | NaHCO 8.4% (100 ml=200 mOsm) vs HTS 5% (85 ml=171 mOsm) infused over 30 min | Mean ICP reduction and duration |
| Cottenceau et al. (2011)37 | RCT∗ | 47 | TBI GCS <8 | Mannitol 20% (4.39 mOsm kg−1) vs HTS 7.5% (5.13 mOsm kg−1) bolus | Mean time ICP >20 mm Hg and GOS at 6 months |
| Morrison et al. (2011)38 | Feasibility study for pre-hospital RCT∗ | 106 | Blunt TBI GCS <9 | 250 ml HSD vs 250 ml NaCl 0.9% with 4 h of injury | Feasibility to do study (ICP, mortality CPP) |
| Sakellardis et al. (2011)39 | RCT∗ | 29 | TBI GCS <9 | Mannitol 20% (2 ml kg−1=2.2 mOsm kg−1) vs HTS 15% (0.42 ml kg−1=2.2 mOsm kg−1) infused alternatively | Mean ICP reduction and duration |
| Scafani et al. (2012)40 | Cohort study† | 8 | TBI GCS <7 | 1 g kg−1 Mannitol 20% (n=6) or 0.686 ml kg−1 HTS 23.4% (n=2); both doses are 5.49 mOsm kg−1 | CBF measured by PET 1 h post-osmotherapy |
| Mangat et al. (2015)22 | Retrospective cohort using BTF database | 50 1:1 matched | TBI GCS <8 | HTS 3% or HTS 23.4% vs mannitol bolus 20% | 14 day mortality; cumulative ICP burden |
Study not blinded.
Not disclosed if blinded.
Comparison groups not adequately matched.
Table 3.
Comparison of original evidence used in published meta-analyses and BTF guidelines. 3rd Edn, 2007 BTF guidelines, third edition; 4th Edn, 2017 BTF guidelines, fourth edition.
| Meta-analysis authors (Original research) | Kamel (2011)14 | Mortazavi (2012)15 | Rickard (2014)16 | Li (2015)17 | Berger-pelleiter (2016)18 | BTF1 |
|---|---|---|---|---|---|---|
| Schwartz et al. (1984)24 | ∗3rd Edn | |||||
| Fisher et al. (1992)27 | ∗ | |||||
| Shackford et al. (1998)23 | ∗,¶ | ∗3rd Edn | ||||
| Schwartz et al. (1998)28 | ∗ | ∗ | ||||
| Qureshi et al. (1999)9 | ∗3rd Edn | |||||
| Afifi et al. (2003)29 | ∗ | |||||
| Vialet et al. (2003)19 | ∗ | † | ∗,‡ | |||
| Cooper et al. (2004)20 | ∗,‡ | |||||
| Battison et al. (2005)30 | ∗ | ∗ | ∗ | ∗ | ||
| Harutjunyan et al. (2005)31 | ∗ | ∗ | ∗ | |||
| Bentson et al. (2006)32 | ∗ | |||||
| Francony et al. (2008)33 | ∗ | ∗ | ∗ | ∗ | ∗,¶ | |
| Ichai et al. (2009)34 | ∗ | ∗ | ∗ | ∗,¶ | ∗4th Edn | |
| Oddo et al. (2009)35 | ∗ | |||||
| Bulger et al. (2010)21 | ∗,‡,¶ | |||||
| Bordreaux et al. (2011)36 | ∗,¶ | |||||
| Cottenceau et al. (2011)37 | ∗ | ∗ | ∗,¶ | ∗4th Edn | ||
| Morrison et al. (2011)38 | ∗,‡ | |||||
| Sakellardis et al. (2011)39 | ∗ | ∗ | ∗ | |||
| Scafani et al. (2012)40 | ∗ | |||||
| Mangat et al. (2014)41 | ∗4th Edn |
Study used as part of meta-analysis.
Data not used for primary outcome analysis (osmolality data used only for secondary outcome analysis).
Mortality data extracted.
ICP data extracted.
The meta-analysis of Kamel and colleagues in 2011 reviewed RCTs comparing equimolar doses of HTS and mannitol for the treatment of increased ICP resulting from any underlying pathology.14 It drew data from five trials (Table 3) to include a total of 112 patients. The primary outcome was the proportion of successfully treated episodes of increased ICP (as defined by the individual studies). The results showed a pooled relative risk of 1.16 [95% confidence interval (CI): 1.00–1.33] in favour of HTS. However, this translates into a mean difference in ICP reduction of only 2.0 mm Hg (95% CI: 1.6–5.7), which is a small pressure difference of uncertain clinical relevance.
In 2012, Mortazavi and colleagues performed a systematic review of the use of HTS for treating increased ICP of any cause in patients with quantitative ICP monitoring in situ.15 As part of the review, the authors performed a meta-analysis of eight RCTs, pooling data to compare the overall rates of treatment failure in each group. The analysis concluded that there was a statistically increased incidence of treatment failure in patients receiving mannitol compared to those receiving HTS (odds ratio: 0.36; CI: 0.19–0.68; P=0.002), supporting the superiority of HTS over mannitol. However, the methods used were poorly described and broad inclusion criteria drew data from significantly heterogeneous studies. The overall quality of this study is called into further question by the inclusion of evidence rejected by Kamel and colleagues as being weak.14
In 2014, Rickard and colleagues undertook a meta-analysis designed specifically to compare the use of mannitol and HTS in haemodynamically stable adults with TBI.16 They included six studies that involved 171 patients and 599 episodes of raised ICP. The results showed a weighted mean difference in ICP reduction of 1.39 mm Hg (95% CI: 0.74–3.53) when using HTS solutions compared to mannitol. The authors reported a trend towards better ICP control with HTS, but concluded that both mannitol and HTS are equally effective at reducing increased ICP, with no statistically significant difference in their overall performance. Of note, this analysis excluded the data of Vialet and colleagues because of inconsistencies between the abstract and full publication.19 This study was included in the analyses of other researchers.15, 17, 18
In 2015, Li and colleagues performed a meta-analysis using data from six RCTs and one non-randomised crossover trial that used mannitol and HTS for the treatment of increased ICP in adults after TBI.17 There was a significant overlap with the studies used in the meta-analysis by Rickard and colleagues.16 A total of 169 patients were included. The primary outcome was the pooled mean change in ICP from baseline to the end of the hyperosmolar infusion. The secondary outcomes were mean ICP at 30, 60, and 120 min after the start of therapy. This analysis showed HTS to be a superior agent for lowering ICP compared to mannitol with the pooled mean difference in ICP=–1.69 (95% CI: –2.95 to –0.44; P.0.008).
The most recent published meta-analysis of Berger-Pelleiter and colleagues included only RCTs that used HTS in adults with severe TBI.18 It is the largest review to date and includes 11 studies and 1820 patients. However, only four of these studies included mortality data and six were used to extract ICP data. In contrast to the other reviews, they found that HTS did not improve ICP control (weighted mean difference: –1.25 mm Hg, 95% CI: –4.18 to 1.68=78%) or reduce mortality (risk ratio: 0.96; 95% CI: 0.83–1.11; I=0%) when compared to other solutions. This result can be explained by the inclusion of two large trials totalling 1302 patients, in which a single 250 ml bolus dose of either HTS 7.5% or Ringer's lactate was administered as a pre-hospital therapy before CT scan and knowledge of the true extent of the brain injury.20, 21 The methodology of these RCTs is very different from the in-hospital protocols used in other studies, where patients had known severe TBI and established intractable raised ICP. As the prehospital studies did not show any difference in survival or long-term neurological outcome between groups, it is no surprise that inclusion of this huge patient number produced a meta-analysis with a similar outcome. Adverse events were considered as secondary outcomes in three of the meta-analyses. No significant difference in safety was found between groups.
Two Cochrane reviews have been published concerning the use of mannitol in the control of brain volume. The first reviewed the use of mannitol or HTS for brain relaxation in patients undergoing craniotomy for elective tumour resection.12 It included six RCTs with 527 participants, and concluded that HTS significantly reduces the risk of tense brain during craniotomy (relative risk: 0.60; 95% CI: 0.44–0.83). Although not directly comparable to reviews of TBI, it does support the findings of Kamel and colleagues and Mortazavi and colleagues that HTS lowers ICP to a greater degree than mannitol.14, 15
The second systematic review aimed to quantify the effects of different mannitol regimes compared with other ICP-lowering agents in the context of acute TBI. Unfortunately, the review had very limited search criteria and identified only four eligible RCTs with highly diverse methodology. Each study had a different control agent, (phenobarbitone, HTS, HTS/hydroxyethyl starch (HES), Ringer's lactate, or NaCl 0.9%). The dose, timing, and duration of mannitol therapy varied, and the severity of TBI was mixed. Furthermore, the authors ignored their own exclusion criteria by including a study with an element of crossover. It is difficult to see how the results from this review can be used to draw a reliable and coherent conclusion, and the authors themselves concede that there is insufficient evidence to answer their questions.13
Current guidelines
Clinicians throughout the world refer to the BTF guidelines to inform and design local TBI management protocols. The guidelines are based on expert review of evidence, with systematic assessment of its quality followed by consensus recommendation. The process is both thorough and robust. A systematic review is undertaken and an analytical framework is applied to the raw search so that relevant studies can be identified. Selected studies are then assessed for bias as a marker of internal validity, and their overall quality is rated as either Class 1, Class 2, or Class 3 (Table 4). The most relevant studies are then amalgamated into a ‘body of evidence’. Aggregate quality, consistency, and precision of results, and whether the evidence provided is direct (has an effect on survival or outcome) or indirect (has an effect on a surrogate marker, such as ICP reduction) are then used to inform a judgement regarding the overall strength of this body and the subsequent level of recommendation that can be issued (Table 4).
Table 4.
Definitions used by the BTF to describe the quality of a study used in the production of their guidelines and definition of ‘levels of recommendation’ which can be made, regarding the overall strength of a BTF guidance.
| An individual study can be classed based on quality of its design and integrity of data. |
| Class 1: derived from good-quality RCTs with solid methodology and large patient numbers |
| Class 2: derived from cohort studies where comparison of two or more groups is clearly distinguished, or from flawed RCTs |
| Class 3: derived from case series, databases/registries, case reports, and expert opinion, flawed RCTs, cohort, or case-control studies |
| A body of evidence is used to formulate a guideline. The quality and strength of the body of evidence defines the level of recommendation given to each guideline. |
| Level I: a high-quality body of evidence. Two or more Class 1 studies |
| Level II A: a moderate-quality body of evidence. Class 1 studies with inconsistent results or class 2 studies |
| Level II B: a low-quality body of evidence consisting of Class 2 studies, with direct evidence but of overall low quality |
| Level III: a low-quality body of evidence consisting of Class 3 studies, or Class 2 studies providing only indirect evidence |
| Insufficient: no evidence identified or quality too poor to make a recommendation |
The production of guidelines and the strength of any recommendation made are limited by the quality of research underpinning any one topic. These challanges regarding the quality of evidence applied here, as described above. Indeed, a large number of studies included in other meta-analyses (Table 3) were rejected by the BTF as having insufficient quality.
In the most recent 2017 edition of the BTF guidelines, the group undertook a systematic review aimed at assessing the comparative effectiveness of different hyperosmolar agents.1 In the 10 yr after the publication of the third edition, only three new studies (see Table 3, Table 4) were identified (one Class 2 retrospective cohort study and two Class 3 RCTs) as relevant and of acceptable quality. The study by Mangat and colleagues is interesting, as it used data from the BTF's own TBI-trac New York State database, data not used in previous meta-analyses.22 However, it was a single, non-randomised retrospective study with a relatively small sample size (n=75) and limited matching to address confounding factors. Three Class 3 studies from the third edition were retained; however, each addressed different subtopics comparing HTS with normal saline, HTS with lactated Ringer's, and mannitol with barbiturates.9, 23, 24 These studies are, therefore, not considered to constitute a ‘body of evidence’.
Ultimately, the BTF had to concede that there is insufficient evidence to recommend the superiority of any one agent over another. They also removed the recommendation for the use of mannitol in patients with signs of trans-tentorial herniation or progressive neurological deterioration. The BTF states that ‘Osmotherapy may lower ICP’ adding ‘The committee is universal in its belief that hyperosmolar agents are useful in the care of patients with severe TBI’.1
In the UK, neither the National Institute for Health and Care Excellence in their 2014 guideline, ‘Head Injury, the Early Management’, nor the Trauma Audit and Research Network makes any reference to the use of osmotherapy in the management of TBI.25
Conclusion
Where does this leave the frontline clinician caring for a patient with signs of imminent brainstem herniation or persistently increased ICP? Given the belief that osmotherapy is useful, and the compelling evidence that both mannitol and HTS are effective at lowering raised ICP, it would seem pragmatic and reasonable for a bolus of either mannitol or HTS to be administered. Studies report few adverse events associated with their use, suggesting that the risks of administration are low compared to any potential benefit. This is especially true when the alternative option, to do nothing, may well result in patient death. However, the question still remains whether any ICP reduction achieved through osmotherapy actually conveys a survival benefit or an improved neurological outcome.
Ultimately, the choice between ‘sugar and salt’ to assist with the control of raised ICP after TBI falls to personal (or locally agreed) preference. A 2011 online survey of neurointensivists reported a fairly close split with 55% of clinicians favouring the use of HTS over mannitol.26 At present, there is no conclusive evidence regarding the superiority of either agent. However, when using mannitol or HTS, clinicians should be aware of potential pitfalls of the chosen therapy, and be familiar with the dose and formulation of the agent used (Table 1). Furthermore, when designing pragmatic clinical guidance for the use of mannitol or HTS, it would be prudent for the following to be taken into account:
-
(i)
Assessment and ongoing review of the patient's volume status, electrolytes and comorbidities.
-
(ii)
Documented response to the initial bolus.
-
(iii)
The use of locally agreed guidelines for the overall management of TBI with a view to other therapies being used (e.g. evacuation of CSF or space-occupying lesion).
-
(iv)
Local policies should aim to standardise formulations to limit prescribing error and potential harm in the event of accidental or erroneous administration.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Nikki Freeman MSc BSc MRCP FRCA FFICM is a specialty registrar in anaesthesia and intensive care medicine at Derriford Hospital, Plymouth.
Jess Welbourne FRCA FFICM is a consultant in intensive care medicine and neuroanaesthesia at Plymouth Hospitals NHS Trust, where she is the medical lead for neurointensive care. She is the local neurointensive care link for the Neurocritical Care Society of Great Britain and Ireland.
Matrix codes: 1A02, 2F01, 3F00
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bjae.2018.05.005.
Supplementary data
The following is the supplementary data related to this article:
References
- 1.Carney N., Trotten A.M., O’Reilley C. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80:6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg N. The saga of the Munro-Kellie doctrine. In: Ishii S., Nagai H., Brock M., editors. Intracranial pressure V. Springer; Berlin/Heidelberg: 1983. pp. 68–76. [Google Scholar]
- 3.Weed L., McKibben P. Experimental alteration of brain bulk. Am J Physiol. 1919;48:531–558. [Google Scholar]
- 4.Ropper A.H. Hyperosmolar therapy for raised intracranial pressure. N Engl J Med. 2012;367:746–752. doi: 10.1056/NEJMct1206321. [DOI] [PubMed] [Google Scholar]
- 5.Matta B., Menon D. Severe head injury in the United Kingdom and Ireland: a survey of practice and implications for management. Crit Care Med. 1996;24:1743–1748. doi: 10.1097/00003246-199610000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Marko N.F. Hypertonic saline, not mannitol, should be considered gold-standard medical therapy for intracranial hypertension. Crit Care. 2012;16:113. doi: 10.1186/cc11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Järvelä K., Koskinen M., Koobi T. Effects of hypertonic saline (7.5%) on extracellular fluid volumes in healthy volunteers. Anaesthesia. 2003;58:878–881. doi: 10.1046/j.1365-2044.2003.03332.x. [DOI] [PubMed] [Google Scholar]
- 8.Fandino W. Understanding the physiological changes induced by mannitol: from theory to the clinical practice in neuroanaesthesia. J Neuroanaesthesiol Crit Care. 2017;4:138–146. [Google Scholar]
- 9.Qureshi A.I., Suarez J.I., Castro A., Bhardwaj A. Use of hypertonic saline/acetate infusion in treatment of cerebral edema in patients with head trauma: experience at a single center. J Trauma. 1999;47:659–665. doi: 10.1097/00005373-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Perez C.A., Figueroa S.A. Complication rates of 3% hypertonic saline given through peripheral intravenous access. J Neurosci Nurs. 2017;49:191–195. doi: 10.1097/JNN.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 11.Zeng H.K., Wang Q.S., Deng Y.Y. A comparative study on the efficacy of 10% hypertonic saline and equal volume of 20% mannitol in the treatment of experimentally induced cerebral oedema in adult rats. BMC Neurosci. 2010;11:153. doi: 10.1186/1471-2202-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakar H., Singh G.P., Anand V., Kalaivani M. Mannitol versus hypertonic saline for brain relaxation in patients undergoing craniotomy. Cochrane Database Syst Rev. 2014;7 doi: 10.1002/14651858.CD010026.pub2. CD010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakai A., McCabe A., Roberts I., Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD001049.pub5. CD001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamel H., Navi B., Nakagawa K., Hemphill J.C., 3rd, Ko N.U. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med. 2011;39:554–559. doi: 10.1097/CCM.0b013e318206b9be. [DOI] [PubMed] [Google Scholar]
- 15.Mortazavi M.M., Romeo A.K., Deep A. Hypertonic saline for treating raised intracranial pressure: literature review with meta-analysis. J Neurosurg. 2012;116:210–221. doi: 10.3171/2011.7.JNS102142. [DOI] [PubMed] [Google Scholar]
- 16.Rickard A.C., Smith J.E., Newell P., Bailey A., Kehoe A., Mann C. Salt or sugar for your injured brain? A meta-analysis of randomised controlled trials of mannitol versus hypertonic sodium solutions to manage raised intracranial pressure in traumatic brain injury. Emerg Med J. 2014;31:679–683. doi: 10.1136/emermed-2013-202679. [DOI] [PubMed] [Google Scholar]
- 17.Li M., Chen T., Chen S.D., Cai J., Hu Y.H. Comparison of equimolar doses of mannitol and hypertonic saline for the treatment of elevated intracranial pressure after traumatic brain injury: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e736. [Google Scholar]
- 18.Berger-Pelleiter E., Lauzier F., Shields J.F., Turgeon A.F. Hypertonic saline in severe traumatic brain injury a systematic review and meta-analysis of randomized control trials. CJEM. 2016;18:112–120. doi: 10.1017/cem.2016.12. [DOI] [PubMed] [Google Scholar]
- 19.Vialet R., Albanèse J., Thomachot L. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 2003;31:1683–1687. doi: 10.1097/01.CCM.0000063268.91710.DF. [DOI] [PubMed] [Google Scholar]
- 20.Cooper D.J., Myles P.S., McDermott F.T. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291:1350–1357. doi: 10.1001/jama.291.11.1350. [DOI] [PubMed] [Google Scholar]
- 21.Bulger E.M., May S., Brasel K.J. Out-of-hospital resuscitation following severe traumatic brain injury a randomized controlled trial. JAMA. 2010;304:1455–1464. doi: 10.1001/jama.2010.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangat H.S., Chiu Y.L., Gerber L.M., Alimi M., Ghajar J., Härtl R. Hypertonic saline reduces cumulative and daily intracranial pressure burdens after traumatic brain injury. J Neurosurg. 2015;122:202–210. doi: 10.3171/2014.10.JNS132545. [DOI] [PubMed] [Google Scholar]
- 23.Shackford S.R., Bourguignon P.R., Wald S.L., Rogers F.B., Osler T.M., Clark D.E. Hypertonic saline resuscitation of patients with head injury: a prospective, randomized controlled trial. J Trauma. 1998;44:50–58. doi: 10.1097/00005373-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz M.L., Tator C.H., Rowed D.W., Reid S.R., Meguro K., Andrews D.F. The University of Toronto head injury treatment study: a prospective, randomized comparison of phenobarbital and mannitol. Can J Neurol Sci. 1984;11:434–440. doi: 10.1017/s0317167100045960. [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health Care and Excellence. Head injury and early management 2014. Available from: https://www.nice.org.uk/guidance/cg176.
- 26.Hays A.N., Lazaridis C., Neyens R., Nicholas J., Gay S., Chalela J.A. Osmotherapy: use among neurointensivists. Neurocrit Care. 2011;14:222–228. doi: 10.1007/s12028-010-9477-4. [DOI] [PubMed] [Google Scholar]; Refs 27–41 are available in an online-only appendix accompanying this article.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.