Abstract
Background
It is unknown if there are cardiac abnormalities in participants recovered from COVID-19 without cardiac symptoms and those who have normal biomarkers and normal ECGs.
Purpose
To evaluate cardiac involvement in participants recovered from COVID-19 without clinical evidence of cardiac involvement using cardiac MRI
Materials and methods
In this prospective observational cohort study, 40 participants recovered from COVID-19 with moderate(n=24) or severe(n=16) pneumonia and no cardiovascular medical history, without cardiac symptoms, with normal ECG, normal serological cardiac enzyme levels, and discharged > 90 days between May and September 2020. Demographic characteristics, serum cardiac enzymes, and cardiac MRI were obtained. Cardiac function, native T1, ECV and Two-dimensional (2D) strain were quantitatively evaluated and compared with controls (n = 25).The Comparison among the 3 groups were performed using one-way analysis of variance (ANOVA) with Bonferroni corrected post-hoc comparisons(for normal distribution) or Kruskal-Wallis tests with post-hoc pairwise comparisons(for non-normal distribution).
Results
Forty participants (54±12 years; 24 men) enrolled with a mean time between admission and CMR of 158 ±18 days and discharge and CMR examination of 124 ±17 days. There was no LV and RV size or functional differences among participants recovered from COVID-19 and healthy controls. Only one (3%) participants had positive LGE located at the mid inferior wall. Global ECV values were elevated in both participants recovered from COVID-19 with moderate or severe pneumonia, compared to the healthy controls [median ECV (IQR)], [29.7% (28.0%-32.9%), versus 31.4% (29.3%-34.0%), versus 25.0% (23.7%-26.0%); both p<.001]. The 2D-global LV longitudinal stains (GLS) were reduced in both groups of participants [COVID-19 moderate group,-12.5%(-10.7%--15.5%), COVID-19 severe group, -12.5%(-8.7%--15.4%) compared to healthy control group -15.4%(-14.6%-17.6%), p=.002 and p=.001, respectively].
Conclusion
CMR myocardial tissue and strain imaging parameters suggest that a proportion of participants recovered from COVID-19 had subclinical myocardial abnormalities detectable months after recovery.
Summary
MRI-derived extracellular volume fraction and 2D global longitudinal strain could serve as markers of cardiac involvement in participants recovered from COVID-19 without cardiac symptoms or clinical findings of myocardial injury.
Key Results
■ In a prospective, single-center study, cardiac MRI revealed extracellular volume fraction (ECV) was elevated in 24 of 40 participants recovered from moderate or severe COVID-19 and without cardiac symptoms or structural cardiac abnormalities compared to healthy controls [29.7% vs 25.0%, respectively, p<.001)].
■ 28 of 40 participants recovered from COVID-19 had subclinical changes of myocardial dysfunction with lower left ventricular 2D-global longitudinal strain (GLS) compared to healthy controls (-12.5% vs. -15.4%, respectively, p=.002) regardless of the severity of pneumonia.
Introduction
The global pandemic of coronavirus disease 2019 (COVID-19) continues to cause considerable morbidity and mortality worldwide (1). As of November 23 , 2020, there has been over 58 million confirmed cases globally with over 1.3 million deaths. Initially recognized as a respiratory illness, there has been a growing body of evidence of cardiovascular complications of this disease (2-7). Multiple data sets now confirm the increased risk for morbid and mortal complications due to COVID-19 in individuals with preexisting cardiovascular diseases or risk factors (8, 9). Among hospitalized patients,7%-15% patients with COVID-19 had increased cardiac troponins indicating myocardial injury, which was associated with worse outcomes (10, 11).
The exact mechanism of cardiac injury due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been difficult to confirm (7, 12), although some autopsy studies have showed direct myocyte involvement or secondary injury from the profound inflammatory response invoked by SARS-CoV-2 (7, 13, 14).
Cardiac MRI provides tissue characterization in addition to anatomical and functional assessment of the heart and vascular system, which has now become a “one-stop shop” for diagnostic and prognostic imaging to investigate myocardial injury (15). To date, there has been limited evaluation in participants recovered from COVID-19 who reported cardiac symptoms or had abnormal serological markers of cardiac injury (4, 5). These studies demonstrated myocardial edema, fibrosis, and impaired RV function (4) in patients recovered from COVID-19 with more than 50% having ongoing myocardial inflammation (5).
There remains limited understanding of the cardiovascular sequelae in participants recovered from COVID-19 without cardiac symptoms, with normal cardiac markers and normal ECG. The purpose of our study is to evaluate cardiac involvement in participants recovered from COVID-19 without clinical evidence of cardiac involvement using cardiac MRI.
Materials and Methods
Study Participants
This single-center, prospective, observational study was performed at No. 2 People's Hospital of Fuyang City, Anhui, China. Consecutive participants recovered from COVID-19 who were seen in follow-up clinics between May and September 2020 and met the following inclusion criteria were invited to participate: (1) Participants with confirmed SARS-CoV-2 infection by reverse transcription polymerase chain reaction (RT-PCR) swab test; (2) Hospitalized participants who were considered recovered and met the guideline discharge criteria (16) (a. normal temperature lasting longer than 3 days; b. resolved respiratory symptoms; c. substantially improved acute exudative lesions on chest CT images; d. two consecutive negative RT-PCR test results separated by at least 24 hours) and were isolated for 14 days; (3) Participants discharged from the hospital >90 days (4) Participants without any cardiac symptoms at any time prior to enrollment, including chest pain, chest pressure, palpitation, or syncope who also had no shortness of breath since discharge from the hospital; (5) Participants with normal serological markers of cardiac injury: creatine kinase (CK), creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH), cardiac troponin I (TnTI), and brain natriuretic peptide (BNP); (6) Participants with normal ECG. Exclusion criteria for participants were as follows: (1) A history of cardiovascular disease including coronary artery disease, myocardial infarction, diabetes, hypertension, stroke, vascular disease, or myocarditis; (2) Absolute contraindications for a contrast-enhanced MRI study such as anaphylaxis to contrast agent, brain aneurysm clips, or shrapnel injury; (3) cardiac MRI quality not sufficient for analysis. Hematocrit (Hct) and serum creatinine of all participants were determined within 24 hours prior to cardiac MRI. The baseline disease severity was determined according to the National Health Commission of the People's Republic of China’s Diagnosis and Treatment Protocol of Novel Coronavirus (Version 7) in Appendix E1. Briefly, a moderate case is defined as a confirmed case with fever, respiratory symptoms, and radiographic evidence of pneumonia, while a severe case is defined as a moderate case with dyspnea or respiratory failure(16).This prospective study was approved by our local ethic institutional review board (PJ2020-02-10) and all participants gave written consent.
Healthy controls of similar age and sex distributions was selected from a database (August 2017 to October 2019) of healthy individuals who previously underwent the MRI exams with the same protocol in our hospital without history of cardiovascular diseases or systemic inflammation or other co-morbidities. Healthy candidates were included as controls after they demonstrated normal electrocardiographic and echocardiographic results and normal CMR findings.
Cardiac MRI Imaging
Cardiac MRI were performed on a clinical 3T scanner (Skyra, Siemens Healthineers, Germany). For morphologic and functional analysis, steady-state free precession (SSFP) with breath-hold were performed, comprising a stack of contiguous parallel short-axis slices covering the entire left ventricle (LV) and right ventricle (RV) from base to apex and three LV long-axis slice (2-, 3-, and 4-chamber views) image was used for cardiac cine imaging with following parameters: field of view (FOV), 340-380 mm; repetition time (TR)/echo time (TE), 3.4/1.4 ms; matrix size, 202×182 mm; voxel size, 1.6×1.6×8.0 mm; bandwidth, 962 Hz/Px; flip angle (FA), 47°; slice thickness, 8 mm; and inter slice gap, 2 mm.
LGE images were performed approximately 10-15 minutes after intravenous administration of gadolinium-DTPA (Omniscan, GE Health Care, Ireland) at a dose of 0.15 mmol/kg in short-axis stack using a phase-sensitive inversion-recovery (PSIR) gradient echo sequence. Inversion times were adjusted to null the signal from normal myocardium (FOV, 340-380 mm; matrix size, 256×224 mm; voxel size, 1.3×1.3×8.0 mm; bandwidth, 781 Hz/Px; TR, 5.2 ms; TE, 1.24 ms; FA, 55°).
Native and post-contrast T1 mapping was acquired at the basal, mid and apical level of LV short-axis using a motion corrected Modified Look-Locker inversion-recovery (MOLLI) sequence with protocol 5(3)3 and 4(1)3(1)2, respectively during a breath hold before and 15-20 min after intravenous contrast (Omniscan, GE Health Care, Ireland) injection. Scanning parameters were as follows: FOV, 340-380 mm; matrix size, 192×172 mm; voxel size, 1.3×1.3×8.0 mm; bandwidth, 1085 Hz/Px; TR/TE, 3.8/1.2 ms; and FA, 35°; slice thickness,8mm. Inversion time was individually adjusted for complete nulling of the myocardium.
Cardiac MRI Imaging Analysis
MRI data were analyzed using commercially available postprocessing software (CVI42, v.5.1, Circle Cardiovascular Imaging, Calgary, Canada). Two radiologists (H.T.W with 20 years of MRI diagnostic experience and X.H.L with 15 years of MRI diagnostic experience) evaluated all cardiac MRI independently blinded to all identifying information. LV and right ventricular (RV) functional parameters were automatically processed including the trabeculations in the ventricular volume with manual adjustments.
LGE images were reviewed by the same two observers independently. The location (16 segments of AHA), and pattern (epicardial, mid-wall, or transmural) of LGE lesions were assessed by automatically delineating endo-and epicardial contours of myocardium in LGE images with manual adjustments, and LGE lesion was defined as SI>5SD above the mean SI of the remote reference myocardium(4). A senior observer (Y.Q.Y, with 30 years' experience in MRI) were designated to adjudicate any discrepancies between the two observers.
Global T1 values were derived with automated contouring of the LV myocardium (including regions of LGE lesion) on the T1 map with manual adjustments as needed to exclude cavity blood pool and epicardial fat. The global T1 values were the average of the three short axis slices. Native TI and post-contrast Tl of myocardium and blood pool were used to derive Global ECV as described (17). Hematocrit level was determined for each individual from a venous blood sample drawn less than 24 hours prior to the cardiac MRI examination.
Cardiac MRI feature tracking (FT) was measured using voxel-tracking postprocessing software (CVI42, v.5.1, Circle Cardiovascular Imaging, Calgary, Canada). Two-dimensional (2D) global peak LV strain from feature tracking were assessed as previously described (18, 19).
Using extracellular volume fraction and myocardial mass, the composition of the myocardium volume can be further divided into cell and matrix components. The cell volume represents intact myocardial cellular components, providing a method to measure myocyte volume (20). The myocardial matrix volume= left ventricular mass (LVM)/1.05 g/ml ×ECV. The myocardial cell volume= LVM/1.05 g/ml×[1 − ECV].
Inter-and Intra-observer Reproducibility
Intra-and inter-observer reliability of 2D strain and T1 values was assessed in 15 participants randomly selected from 65 subjects using Bland-Altman plots. Intra-observer reliability was derived from the repeated measurement by one radiologist (X.H.L) after at least a one-month interval blinded to the previous results. Inter-observer reliability was independently assessed by another radiologists (T.T.W) blinded to the first radiologist's measurements.
Statistical analysis
All statistical analysis was performed using SPSS (version 22.0, IBM statistics, Armonk, NY) and GraphPad Prism(Version7.04, GraphPad Software Inc.). Categorical variables were expressed as counts (percentage), and continuous variable as mean ± standard deviation (SD) or median (interquartile range). Normality of distribution was tested using Shapiro-Wilk test. Comparison between two groups were performed by unpaired Student's t-test (for normal distribution) or Mann-Whitney U test (for non-normal distribution) with continuous variables, or Chi-squared test with categorical variable. The Comparison among the 3 groups were performed using one-way analysis of variance (ANOVA) with Bonferroni corrected post-hoc comparisons (for normal distribution) or Kruskal-Wallis tests with post-hoc pairwise comparisons (for non-normal distribution). To assess the qualitative data, comparisons of the three groups were performed using Pearson’s chi-square test or Fisher’s exact test. Two-tailed p < 0.05 was considered to be statistically significant. Because 27 cardiac MRI parameters were compared within the three groups, we used the Bonferroni correction with a significance level of P = 0.002 (0.05/27) to adjust for multiplicity.
Results
Participant characteristics
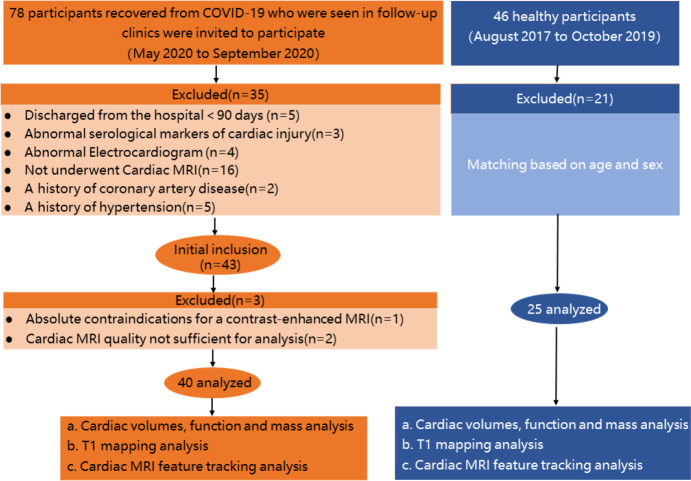
78 participants were screened from the COVID-19 clinic and 35 were excluded due to discharge < 90 days (n = 5), abnormal serological markers of cardiac injury (n = 3), abnormal ECG findings (n = 4), not underwent Cardiac MRI (n=16), a history of coronary artery disease(n=2) ,a history of hypertension(n=5). (Fig.1). 43 participants were enrolled in the study and 1 patient was excluded due to contrast allergy and 2 were excluded due to image quality. The final analysis cohort consisted of 40 participants (54±12 years; 24 men).Twenty-five healthy controls matched for age (50±15years, p=.23) and sex (16 men, p=.75). Twenty-four COVID-19 participants (60%) were diagnosed as moderate pneumonia and 16 (40%) as severe.
Figure 1:
Flowchart of participant enrollment. COVID-19 = coronavirus disease 2019
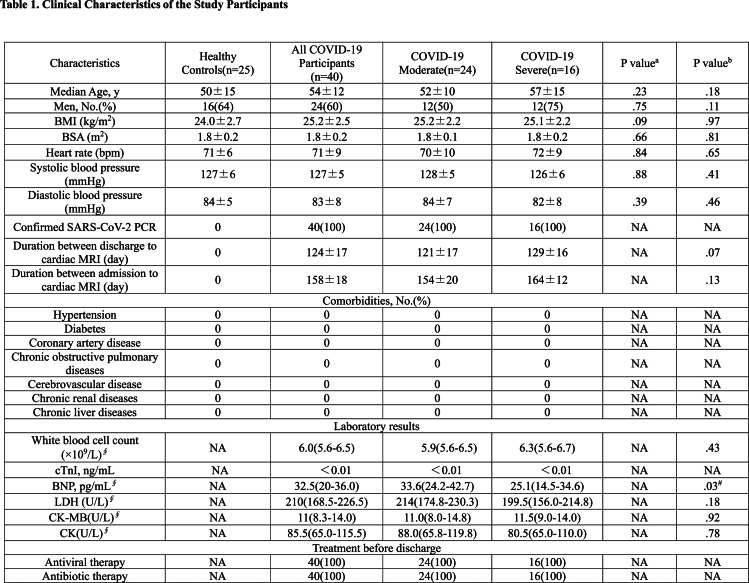
Clinical characteristics and laboratory testing results of participants recovered from COVID-19 are reported in Table 1. During hospitalization, all participants were administered antiviral and antibiotic therapy including lopinavir / ritonavir and umifenovir, and moxifloxacin and cefoperazone sulbactam according to the treatment protocol(16). The mean duration from admission and discharge to cardiac MRI examination were 158±18 days and 124 ±17 days, respectively. The laboratory testing results within 24 hours of cardiac MRI were in the normal range (Table1).
Table 1.
Clinical Characteristics of the Study Participants
Cardiac MRI parameters
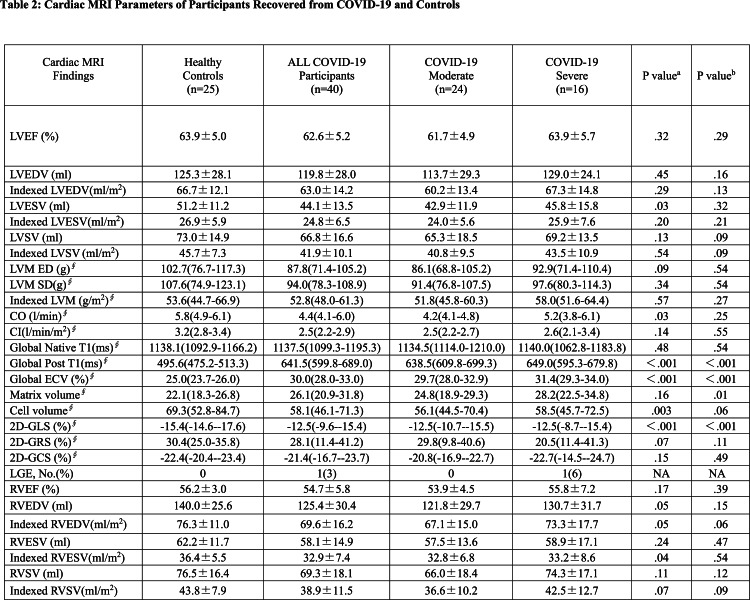
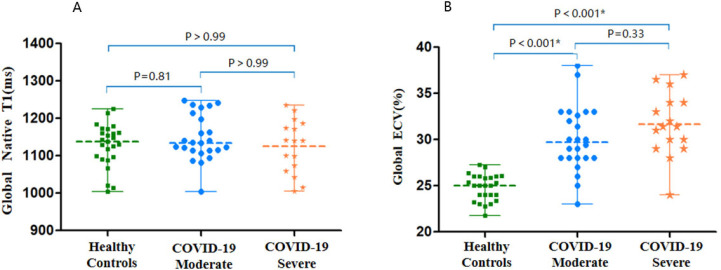
Cardiac morphological and functional parameters are summarized in Table 2. There was no differences of LV or RV size and function among participants with COVID-19 and healthy controls. Only one participant with COVID-19 had positive LGE located at the mid inferior wall (Fig 2). Global ECV values was elevated in participants recovered from COVID-19 in both moderate and severe disease groups, compared with the healthy controls [median ECV (IQR) COVID-19 moderate group,29.7% (28.0%-32.9%), COVID-19 severe group ,31.4% (29.3%-34.0%), healthy control group, 25.0% (23.7%-26.0%); p<.001 for both moderate and severe disease groups compared to control] (Fig 3) (Table 3). 24 of 40 participants (60%) were above the top normal ECV cutoff of 29%.
Table 2:
Cardiac MRI Parameters of Participants Recovered from COVID-19 and Controls
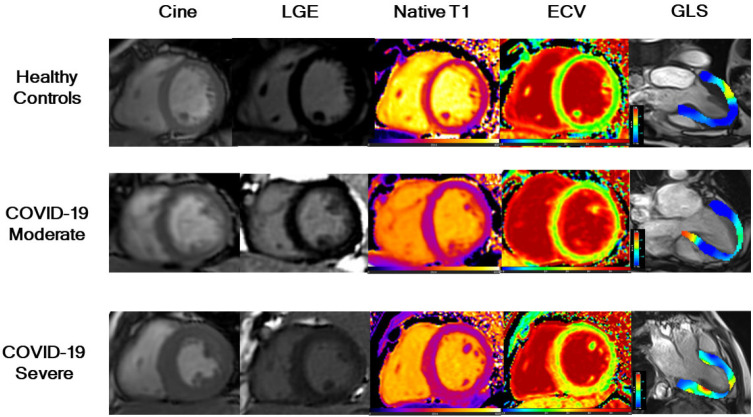
Figure 2:
Examples of cardiac MRI in participants recovered from COVID-19 and healthy controls. Short-axis SSFP cine, corresponding phase sensitive inversion recovery (PSIR) late gadolinium enhancement (LGE), native T1 maps, extracellular volume (ECV) maps, and global longitudinal strain (GLS) of three groups (Healthy Control, participants with moderate COVID-19, and participants with severe COVID-19). A 41-year-old healthy man (control) (first row) with negative LGE, normal global native T1 (1161ms), global ECV (27%), and GLS (-17.2%). A 66-year-old woman (second row) with moderate COVID-19 and negative LGE, normal global native T1 (1139 ms), elevated global ECV (31%) and reduced GLS (-12.6%). A 44-year-old man (third row) with severe COVID-19 and focal LGE in the left ventricular septal segment. Native T1 (1216 ms) was increased, ECV values were elevated (34%) and LGS was lower (-9.1%). A moderate case is defined as a confirmed case with fever, respiratory symptoms, and radiographic evidence of pneumonia, while a severe case is defined as a moderate case with dyspnea or respiratory failure.
Figure 3:
Native T1 and ECV scatterplots by group. There were no differences in, A, global native T1. There was, B, global ECV difference in participants with COVID-19 compared with the controls. Scatter dot plot with midlines indicate medians and whiskers indicate interquartile ranges.
Table 3:
Subgroups Comparisons with Cardiac MRI Parameters of Participants Recovered from COVID-19 Compared with Controls
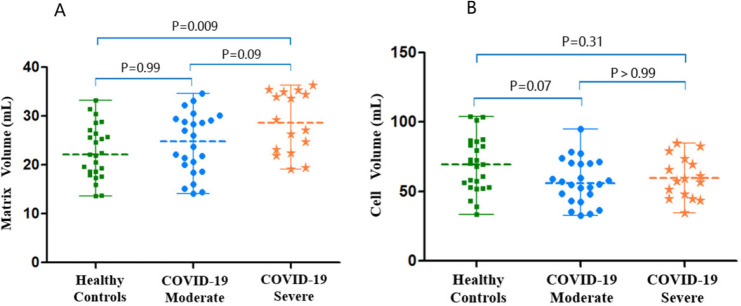
The myocardial matrix volume showed no difference in participants recovered from COVID-19 who had severe pneumonia, compared with the healthy controls (28.2(22.5-34.8) versus 22.1(18.3-26.8), p=.009 (p<.002 is considered to indicate statistical significance). There were no differences between the moderate disease group and the healthy controls or between the two groups of participants recovered from COVID-19 (p=.09, p=.99, respectively). The myocardial cell volume showed no differences among these three groups (p=.06) (Fig 4).
Figure 4:
LV matrix volume and cell volume scatterplots by group. A, The left ventricular matrix volumes and, B, cell volumes of all study participants (healthy controls, participants with moderate COVID-19, and participants with severe COVID-19). Scatter dot plot with midlines indicate medians and whiskers indicate interquartile ranges. A moderate case is defined as a confirmed case with fever, respiratory symptoms, and radiographic evidence of pneumonia, while a severe case is defined as a moderate case with dyspnea or respiratory failure.
The 2D-global LV longitudinal stains (GLS) demonstrated reduction in participants recovered from COVID-19 in both moderate and severe disease groups, compared with the healthy controls [COVID-19 moderate group,-12.5%(-10.7%--15.5%), COVID-19 severe group,-12.5%(-8.7%--15.4%), healthy control group,-15.4%(-14.6%--17.6%), p=.002 and p=.001, respectively](Table 3). 28 of 40 participants (70%) were below the normal GLS cutoff (-15%). 2D global radial strain (GRS) and 2D global circumferential strain (GCS) showed no differences among these groups (p=.11 and p=.49, respectively) (Fig5).
Figure 5:
Cardiac MRI Feature-tracking LV strain scatterplots by group. A, Two-dimensional global longitudinal strain, B, global circumferential strain, C, global radial strain in the left ventricle (LV) of all study participants (healthy controls, participants with moderate COVID-19, and participants with severe COVID-19). Scatter dot plot, midlines indicate medians, and whiskers indicate interquartile ranges. A moderate case is defined as a confirmed case with fever, respiratory symptoms, and radiographic evidence of pneumonia, while a severe case is defined as a moderate case with dyspnea or respiratory failure.
Intra-and Inter-observer Reliability
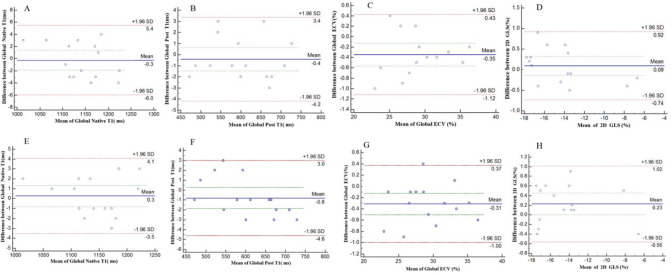
Global native T1 had an intra-observer reliability of 0.3 ms ± 1.9 ms and an interobserver reliability of -0.3ms ± 2.9 ms. Global post T1 had an intra-observer reliability of -0.8ms ± 1.9 ms and an inter-observer reliability of-0.4 ms ± 1.9 ms. Global ECV had an intra-observer reliability of -0.31%±0.35% and an inter-observer reliability of-0.35%±0.39%. 2D GLS had an intra-observer reliability of 0.23%±0.40% and an inter-observer reliability of 0.09%±0.42%. The Bland-Altman plots are presented in Figure 6.
Figure 6:
Bland-Altman analysis for A-D, interobserver reproducibility of global native T1, global post T1, global extracellular volume (ECV) and 2D global longitudinal strain (GLS). E-H, Intra-observer reproducibility of global native T1, global post T1, global extracellular volume (ECV) and 2D global longitudinal strain (GLS). The blue line indicates the mean value; the dashed red line indicates 95% confidence interval. A moderate case is defined as a confirmed case with fever, respiratory symptoms, and radiographic evidence of pneumonia, while a severe case is defined as a moderate case with dyspnea or respiratory failure.
Discussion
We found extracellular volume fraction (ECV) elevation>29% in 24 of 40 participants (60%) recovered from moderate or severe COVID-19 discharged from the hospital for more than 90 days, showing no cardiac symptoms or clinical findings of myocardial injury, and without functional or structural cardiac impairment (LV ejection fraction was within normal range 62.6%±5.2%). We also found 28 of these 40 participants (70%) had subclinical changes of myocardial function demonstrated by a reduction in LV 2D-global longitudinal strain (GLS) [ -13%(-10%--15%] compared with healthy controls regardless of pneumonia severity. Only one of the forty participants (3%) had positive late gadolinium enhancement (LGE) positive, located at the mid inferior wall. In addition, we found that the both cell volumes and matrix volume were not different in the participants recovered from COVID-19 and control groups (p=.06 and p=.01, respectively).
Our understanding of COVID-19 involvement in the myocardium continue to evolve. The pathological evidence for acute myocarditis was reported in a patient with COVID-19 who presented with typical manifestations of fulminant myocarditis (21). Myocardial inflammation was confirmed, and coronavirus particles were detected in macrophages but not in myocardial or endothelial cells (7). Another autopsy study reported infiltration of myocardial tissue by mononuclear inflammatory cells in a postmortem of a patient with COVID-19(22).
A few studies have reported cardiac MRI findings on patients recovered from COVID-19.Huang(4) described 26 patients in a single-center retrospective cohort study demonstrating abnormal cardiac MRI findings in patients recovered from COVID-19 without evidence of cardiac involvement during their initial treatments, but presented one to three months after discharge with chest discomfort or palpitations, and other non-specific cardiac symptoms. This study found 14 (54%) of participants with evidence of myocardial edema using T2 weighted imaging. In the acute phase of myocardial inflammatory injury, LGE represents myocardial edema and necrosis, and in the chronic phase, LGE represents the formation of myocardial fibrosis (6). Huang et al showed in symptomatic patients, 31% of patients and 4% segments had evidence of small focal subepicardial and patchy mid-wall LGE (4). The median time between onset of cardiac symptoms and cardiac MRI was 47 days (4). These findings support previous reports of COVID-19 related myocarditis (23-25), and demonstrated that cardiac MRI abnormalities of active inflammation/edema may extend into the convalescent phase of COVID-19 in individuals with cardiac symptoms. The first prospective report on a cohort of unselected 100 recently recovered patients with COVID-19 also found evidence for myocardial inflammation in 60% patients, positive LGE findings in 41% patients with 38% demonstrating an ischemic pattern of myocardial LGE (5). Overall, some form of cardiac abnormalities were present in 78% of patients at a median of 71 days (IQR of 64-92 days) post diagnosis (5). In our study, only one of the forty participants (3%) was LGE positive. Nonetheless, our study demonstrated a high prevalence of subclinical cardiac abnormalities in participants recovered from COVID-19 in a convalescent phase later than both of these studies.
Abnormal native T1 and ECV can be found in diffuse myocardial fibrosis (26, 27). ECV, which quantifies the relative expansion of the extracellular matrix as a result of diffuse reactive fibrosis in multiple cardiac pathologies, can be used as a noninvasive alternative to myocardial biopsies and histochemical analysis (17). Our findings showed in both moderate and severe pneumonia groups, there was increased global ECV compared with healthy controls, which suggests that diffuse interstitial fibrosis may be present in 60% participants recovered from COVID-19 who had ECV elevation higher than the healthy cutoff previously reported by Gottbrecht et al (28). However, we saw no difference on native T1 in COVID-19 recovered participants and controls (P=.48), which is not consistent with the findings of increased T1 values from Puntmann et a l(5)or Huang et al (4). This difference may reflect different patient populations dictated by the inclusion and exclusion criteria. We have a more homogenous population with participants hospitalized for pneumonia without cardiac symptoms or clinical findings, as compared to Puntamnn’s mixture of hospitalized and ambulatory patients with a number of cardiovascular risk factors, or Huang’s patients with cardiac symptoms. Our patient cohort with increased ECV but no difference in native T1 is consistent with previous observations in non-ischemic cardiomyopathy (29) and in the MESA cohort (30). The significance of these results is unknown, which highlights the need for long-term follow-up.
Combining extracellular volume fraction and myocardial volume, it is possible to examine the changes of the two components of myocardium volume, which are the myocardial cell volume and the matrix volume. The cell volume represents intact myocardial cellular component, providing a way to measure the myocyte volume. In diseases with simultaneous cellular hypertrophy and extracellular matrix expansion, the ECV fraction may not change because it depicts the ratio between cell and matrix volumes(20).Although there were no statistical significant difference among the groups when a P value of 0.002 was used, separating into cell and matrix volumes allow us to gain insight into how each component of the myocardium change in the COVID-19 disease.
Feature-tracking technology is a post-processing method applied to routinely acquired cine cardiac MRI to study myocardial strain. Global longitudinal strain (GLS) is more sensitive than LV ejection fraction (EF) as a measure of systolic function and may identify subclinical LV dysfunction in cardiomyopathies (31, 32). Our study demonstrated that subclinical LV dysfunction was prevalent in participants recovered from COVID-19 and the impact of this finding on long-term outcome remains unknown.
Our study has limitations. First, the sample size is small, limited by the reduction of advanced cardiac MRI utilization and resources in the region during the early phase of the epidemic. Second, we did not perform T2 imaging. In our study, the median time between admission and cardiac MRI examination was 159 days with IQR (145-170days). At the time of study design, according to the pathogenesis and acute versus chronic phase of myocarditis and the enrollment criteria of this study, we anticipated that edema would no longer be present during this time period (33, 34). Therefore, T2 weighted imaging or T2 mapping were not performed in this study.
In conclusion, we demonstrated subclinical functional and myocardial tissue characteristic abnormalities in individuals recovered from moderate or severe COVID-19 without cardiac symptoms or clinical findings of myocardial injury three months after recovery from SARS-CoV-2 infection. Given the pressing burden of the ongoing COVID-19 pandemic, long-term cardiovascular consequences of COVID-19 need to be investigated. Cardiac MRI can be a sensitive imaging tool for identifying cardiac involvement in COVID-19. The clinical significance of our results is unknown, and this work highlights the need for longitudinal follow-up to understand the importance and progression of subclinical myocardial findings in participants with COVID-19.
Acknowledgments
Acknowledgements
We thank all colleagues who contributed to the present study. We are especially grateful to our front-line medical staff for their professionalism, dedication, and courage in the face of the COVID-19 outbreak.
Supported in part by the Anhui Natural Science Foundation (202004a0702003, 201904a07020060). Fuyang Natural Science Foundation (FK20202815-3). National Natural Science Foundation of China (82071897,81970446).
Disclosures of Conflicts of Interest: X.L. disclosed no relevant relationships. H.W. disclosed no relevant relationships. R.Z. disclosed no relevant relationships. T.W. disclosed no relevant relationships. Y.Z. disclosed no relevant relationships. Y.Q. disclosed no relevant relationships. B.L. disclosed no relevant relationships. Y.Y. disclosed no relevant relationships. Y.H. disclosed no relevant relationships.
Abbreviations:
- 2D
- 2 dimensional
- COVID-19
- coronavirus disease 2019
- ECV
- extracellular volume fraction
- GLS
- global longitudinal strain
- IQR
- interquartile range
- LGE
- late gadolinium enhancement
- LV
- left ventricle
- RV
- right ventricle
- MRI
- magnetic resonance imaging
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
References
- 1.Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart 2020;106(15):1132. doi: 10.1136/heartjnl-2020-317056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol 2020. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. The Lancet 2020;395(10235):1517-1520. doi: 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac involvement in recovered COVID-19 patients identified by magnetic resonance imaging. JACC: Cardiovascular Imaging 2020. doi: 10.1016/j.jcmg.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salerno M, Kwong R. CMR in the era of COVID-19: Evaluation of Myocarditis in the Subacute Phase. JACC: Cardiovascular Imaging 2020. 10.1016/j.jcmg.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei ZY, Qian HY, Huang J, Geng YJ. Pathogenesis and Management of Myocardial Injury in Coronavirus Disease 2019. Eur J Heart Fail 2020. doi: 10.1002/ejhf.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020;323(11):1061-1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus I, Research T. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382(8):727-733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhakal BP, Sweitzer NK, Indik JH, Acharya D, William P. SARS-CoV-2 Infection and Cardiovascular Disease: COVID-19 Heart. Heart Lung Circ 2020. doi: 10.1016/j.hlc.2020.05.101 [DOI] [PMC free article] [PubMed]
- 13.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovascular Research 2020;116(6):1097-1100. doi: 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harikrishnan S, Mohanan PP, Chopra VK, Ambuj R, Sanjay G, Bansal M, Chakraborty RN, Chandra S, Chattarjee SS, Chopra HK, Mathew C, Deb PK, Goyal A, Goswami KC, Gupta R, Guha S, Gupta V, Hasija PK, Wardhan H, Jabir A, Jayagopal PB, Kahali D, Katyal VK, Kerkar PG, Khanna NN, Majumder B, Mandal M, Meena CB, Naik N, Narain VK, Pathak LA, Ray S, Roy D, Routray SN, Sarma D, Shanmugasundaram S, Singh BP, Tyagi SK, Venugopal K, Wander GS, Yadav R, Das MK. Cardiological society of India position statement on COVID-19 and heart failure. Indian Heart J 2020;72(2):75-81. doi: 10.1016/j.ihj.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy J-P, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. Journal of the American College of Cardiology 2009;53(17):1475. doi: 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Commission of the People's Republic of China’s Diagnosis and Treatment Protocol of Novel Coronavirus (Version 7) . Translated by the Chinese Society of Cardiology available at website:http://kjfy.meetingchina.org/msite/news/show/cn/3337.html
- 17.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc Imaging 2016;9(1):67-81. doi: 10.1016/j.jcmg.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 18.Guo Q, Wu L-M, Wang Z, Shen J-Y, Su X, Wang C-Q, Gong X-R, Yan Q-R, He Q, Zhang W, Xu J-R, Jiang M, Pu J. Early Detection of Silent Myocardial Impairment in Drug-Naive Patients With New-Onset Systemic Lupus Erythematosus. Arthritis & Rheumatology 2018;70(12):2014-2024. doi: 10.1002/art.40671 [DOI] [PubMed] [Google Scholar]
- 19.Neisius U, Myerson L, Fahmy AS, Nakamori S, El-Rewaidy H, Joshi G, Duan C, Manning WJ, Nezafat R. Cardiovascular magnetic resonance feature tracking strain analysis for discrimination between hypertensive heart disease and hypertrophic cardiomyopathy. PLOS ONE 2019;14(8):e0221061. doi: 10.1371/journal.pone.0221061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamori S, Dohi K, Ishida M, Goto Y, Imanaka-Yoshida K, Omori T, Goto I, Kumagai N, Fujimoto N, Ichikawa Y, Kitagawa K, Yamada N, Sakuma H, Ito M. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC: Cardiovascular Imaging 2018;11(1):48-59. 10.1016/j.jcmg.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 21.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. European Journal of Heart Failure 2020;22(5):911-915. doi: 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 2020;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravinay P, Issa N, Girard D, Camou F, Cochet H. CMR and serology to diagnose COVID-19 infection with primary cardiac involvement. European Heart Journal - Cardiovascular Imaging 2020. doi: 10.1093/ehjci/jeaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiology 2020;5(7):819-824. doi: 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, Schnuriger A, Lorrot M, Guedj R, Ducou le Pointe H. Cardiac MRI of Children with Multisystem Inflammatory Syndrome (MIS-C) Associated with COVID-19: Case Series. Radiology 2020:202288. doi: 10.1148/radiol.2020202288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Meester de Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, Pasquet A, Vancraeynest D, Pouleur A-C, Vanoverschelde J-LJ, Gerber BL. Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. Journal of Cardiovascular Magnetic Resonance 2015;17(1):48. doi: 10.1186/s12968-015-0150-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Florian A, Ludwig A, Rosch S, Yildiz H, Sechtem U, Yilmaz A. Myocardial fibrosis imaging based on T1-mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Imaging 2014;15(9):1004-1012. doi: 10.1093/ehjci/jeu050 [DOI] [PubMed] [Google Scholar]
- 28.Gottbrecht M, Kramer CM, Salerno M. Native T1 and Extracellular Volume Measurements by Cardiac MRI in Healthy Adults: A Meta-Analysis. Radiology 2018;290(2):317-326. doi: 10.1148/radiol.2018180226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.aus dem Siepen F, Buss SJ, Messroghli D, Andre F, Lossnitzer D, Seitz S, Keller M, Schnabel PA, Giannitsis E, Korosoglou G, Katus HA, Steen H. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur Heart J Cardiovasc Imaging 2015;16(2):210-216. doi: 10.1093/ehjci/jeu183 [DOI] [PubMed] [Google Scholar]
- 30.Ambale-Venkatesh B, Liu CY, Liu YC, Donekal S, Ohyama Y, Sharma RK, Wu CO, Post WS, Hundley GW, Bluemke DA, Lima JAC. Association of myocardial fibrosis and cardiovascular events: the multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging 2019;20(2):168-176. doi: 10.1093/ehjci/jey140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? European Heart Journal 2015;37(15):1196-1207. doi: 10.1093/eurheartj/ehv529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamperidis V, Marsan NA, Delgado V, Bax JJ. Left ventricular systolic function assessment in secondary mitral regurgitation: left ventricular ejection fraction vs. speckle tracking global longitudinal strain. European Heart Journal 2015;37(10):811-816. doi: 10.1093/eurheartj/ehv680 [DOI] [PubMed] [Google Scholar]
- 33.Bohnen S, Radunski UK, Lund GK, Ojeda F, Looft Y, Senel M, Radziwolek L, Avanesov M, Tahir E, Stehning C, Schnackenburg B, Adam G, Blankenberg S, Muellerleile K. Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis. European Heart Journal - Cardiovascular Imaging 2017;18(7):744-751. doi: 10.1093/ehjci/jex007 [DOI] [PubMed] [Google Scholar]
- 34.von Knobelsdorff-Brenkenhoff F, Schuler J, Doganguzel S, Dieringer MA, Rudolph A, Greiser A, Kellman P, Schulz-Menger J. Detection and Monitoring of Acute Myocarditis Applying Quantitative Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging 2017;10(2). doi: 10.1161/CIRCIMAGING.116.005242 [DOI] [PubMed] [Google Scholar]