Abstract
Background
The combination of a microtubule inhibitor (eribulin) with a nucleoside analog (gemcitabine) may synergistically induce tumor cell death, particularly in triple negative breast cancer (TNBC) characterized by high cell proliferation, aggressive behavior, and chemo-resistance.
Patients and methods
This is an open-label, multicenter phase II study evaluating the combination of eribulin (0.88 mg/m2) plus gemcitabine (1000 mg/m2) on days 1 and 8 of a 21-day cycle as either first- or second-line treatment of locally advanced or metastatic TNBC. The primary endpoint was the objective response for evaluable patients. A prospective, molecular correlative study was carried out to assess the role of germinal BRCA pathogenic variants and single nucleotide polymorphisms (SNPs) in predicting efficacy and toxicity of the combination regimen.
Results
From July 2013 to September 2016, 83 evaluable patients were enrolled. They received a median number of six cycles of treatment. An overall response rate (ORR) of 37.3% (31 patients) was observed, with a complete response rate of 2.4% and a partial response rate of 34.9%; the clinical benefit rate was 48.8%. With a median follow-up of 28.8 months, the median response duration was 6.6 months, the median progression-free survival (PFS) was 5.1 months, and the median overall survival (OS) was 14.5 months. The most common grade 3-4 adverse events were aminotransferase elevation (in 25% of the patients) and neutropenia (in 23.8%). Women with BRCA1/2 pathogenic variants were associated with worse ORR, PFS, and OS than BRCA1/2 wild-type carriers. CYP3A4 and FGD4 SNPs were associated with increased risk of liver toxicity. Three different SNPs in CDA∗2, RRM1, and CYP2C8 genes were significantly associated with poorer OS.
Conclusions
The combination of eribulin and gemcitabine showed promising activity and a moderate toxicity profile in metastatic TNBC. BRCA status and pharmacogenetics tests may help identify patients with high probability of response with negligible toxicity.
EudraCT number
2012-003505-10.
Key words: breast cancer, TNBC, eribulin, gemcitabine, metastatic, pharmacogenetics
Highlights
-
•
Eribulin plus gemcitabine showed a remarkable best ORR of 37.3% and a clinical benefit rate of 48.8%.
-
•
The most common grade 3/4 toxicities were liver toxicity and neutropenia without febrile neutropenia.
-
•
The study regimen partially lost its efficacy in patients harboring BRCA1/2 pathogenic variants.
-
•
SNPs in CYP3A4 and FGD4 genes were associated with increased risk of liver toxicity.
-
•
Three different SNPs in CDA∗2, RRM1, and CYP2C8 genes were significantly associated with poorer OS.
Introduction
Triple negative breast cancer (TNBC) is a heterogeneous disease characterized by high cell proliferation, chemo-resistance, and unfavorable prognosis.1 In the early setting, neoadjuvant and adjuvant chemotherapy containing anthracyclines and taxanes are the standard of care.2,3
None the less, almost 40% of TNBC patients relapse after local surgery and neo/adjuvant therapies,4 whereas about 6%-10% of patients with newly diagnosed TNBC present with stage IV disease.5 Patients who develop a metastatic disease have a very poor prognosis with a median survival of approximately 1 year.6
The systematic use of pre- and post-operative chemotherapy administered in patients with TNBC prompts malignant cells to be exposed early to anti-cancer drugs and may render tumors resistant to them by the time the disease recurs. Therefore, using more active chemotherapy drugs in the early setting may reduce the number of therapeutic options for metastatic disease, making novel and ground-breaking approaches necessary for the treatment of advanced TNBC patients.7,8 Gemcitabine is a nucleoside (pyrimidine) analogue that replaces one of the building blocks of cytidine, a nucleic acid, during DNA replication, causing the stall of the replication fork.9 Gemcitabine is an active drug against chemotherapy-naive and chemotherapy-pretreated breast cancer (BC) with response rates, as a single agent, of 12%-33% and favorable toxicity profile.9 Gemcitabine has also shown activity in metastatic TNBC in various combination regimens, particularly in combination with taxanes.9 Eribulin mesylate (E7389, Eisai Research Institute, Andover, MA) is a synthetic analogue of the macrolide halichondrin B, which is a large polyether macrolide found in a rare Japanese sponge, Halichondria okadai.10,11 Like halichondrin B, eribulin inhibits tubulin polymerization by binding the β-tubulin subunit. This activity explains its ability to overcome taxane resistance conferred by β-tubulin mutations.12 Three phase II trials and one phase III trial have demonstrated the efficacy of eribulin in patients with metastatic breast cancer13, 14, 15, 16; furthermore, the pooled analysis, conducted by Pivot et al., has shown that the drug is particularly active in metastatic TNBC.17
A single phase I study has evaluated the combination of eribulin and gemcitabine in 21 pretreated patients with advanced solid tumors.18 Eribulin and gemcitabine were given on days 1 and 8 of a 21-day cycle. Best responses were as follows: partial response (PR) in two patients (ovarian cancer and head and neck cancer), stable disease (SD) in eight patients {minor response: 4 [non-small-cell lung cancer (NSCLC) 2, endometrial cancer 1, head and neck cancer 1]}, progressive disease (PD) in seven patients; four patients were not evaluable. According to this trial, the investigators recommended the following dosing regimen for further investigation: eribulin mesylate 1.0 mg/m2 (equivalent to 0.88 mg/m2 eribulin) plus gemcitabine 1000 mg/m2, on days 1 and 8 every 21 days.18
Based on these considerations, the objective of this multicenter, single-arm, phase II trial was to assess the activity and safety of eribulin in combination with gemcitabine in patients with locally advanced BC or TNBC (ERIGE trial; EudraCT number: 2012-003505-10). The impact of genetic and pharmacogenetic profiles of enrolled patients on clinical outcomes and toxicity was also evaluated.
Patients and methods
Patient selection
Patients with locally advanced and/or metastatic TNBC were enrolled. Eligibility criteria included previous neoadjuvant and/or adjuvant chemotherapy containing an anthracycline and a taxane (unless one or both were clinically contraindicated), ≥1 measurable tumor lesion according to RECIST 1.1,19 Eastern Cooperative Oncology Group (ECOG) status ≤2, and adequate organ function. The study protocol and three protocol amendments, which included the permission to enroll patients previously treated with no more than one line of therapy for metastatic disease and/or with treated/stable brain metastases, were approved by the independent local ethics committee of each participating hospital. The study was conducted according to the Declaration of Helsinki and ICH-GCP guidelines. All patients gave informed consent before any study-related procedure.
Study treatment
Patients received eribulin (0.88 mg/m2) as an intravenous (i.v.) infusion over 2-5 min on days 1 and 8 of every 21-day cycle and gemcitabine (1000 mg/m2) as an i.v. infusion over 30 min after eribulin administration on days 1 and 8 of every 21-day cycle. The treatment was administered for three cycles (9 weeks) and in case of objective response or stabilized disease at that time, the same therapy was given for three additional courses (9 weeks). After a minimum of six courses (18 weeks) of chemotherapy, patients could continue the treatment until disease progression, unacceptable toxicity, or any other reason for discontinuation.
Molecular analyses
BRCA1/2 genetic testing
Genomic DNA was isolated from peripheral blood lymphocytes. DNA library preparation and sequencing were carried out using the Illumina MiSeq platform as specified by the manufacturer's instructions.20 All sequence variants were named according to the nomenclature used by the Human Genome Variation Society (HGVS) guidelines,21 and classification of BRCA1/2 variants was also provided using the ClinVar variation report and interpretation.22
Pharmacogenetics
We investigated a panel of 10 single nucleotide polymorphisms (SNPs) in seven genes involved in either eribulin or gemcitabine metabolism pathway: RRM1 (ribonucleotide reductase catalytic subunit M1), CDA1 (cytidine deaminase 1), CDA2, ABCB1 (ATP binding cassette subfamily B member 1), CYP3A4 (cytochrome P450 family 3 subfamily A member 4), CYP2C8 (cytochrome P450 family 2 subfamily C member 8), and FGD4 (FYVE, RhoGEF, and PH domain containing 4). Genotyping was carried out using pre-designed TaqMan® probes according to the manufacturer's instructions.23 FcγRIIa and FcγRIIIa polymorphisms were evaluated in study patients as negative controls.24 Genotyping was carried out by researchers blinded to clinical outcome and toxicity data. SNP genotypes were assessed for Hardy–Weinberg equilibrium.25,26
Statistical analysis
The primary endpoint of this multicenter, single-arm, phase II trial was the objective response, defined as the best response identified by RECIST 1.1 criteria,19 and recorded from the start of the trial until disease progression or death occurred, or the patient discontinued study intervention. Overall response rate (ORR) was defined as the proportion of patients who achieved a complete or partial response as their best overall response. The Simon's two-stage optimal design27 was planned to test the null hypothesis that the ORR would be 20% or less against a one-sided alternative (i.e. before the investigators could proceed to stage 2 of the study, at least 9 of 37 patients had to have a response). We calculated that a sample size of 83 patients would have yielded a one-sided type I error rate of 5% and power of 90% if the true response rate is 35%. The exact method was used to calculate the two-sided 90% confidence intervals for the response rate. Secondary endpoints were the response duration, progression-free survival (PFS), overall survival (OS), clinical benefit rate (disease response plus stable disease lasting ≥6 months), and safety. Adverse events were recorded at each visit and classified according to the National Cancer Institute-Common Terminology Criteria (NCI-CTCAE) version 4.0.28 The response duration was defined as the time from documentation of tumor response to disease progression. PFS was defined as the time between the date of enrolment to progression or death due to any cause or last contact the patient was known to be progression-free or alive. OS was measured from the start of treatment to the date of death or the last follow-up at which the patient was known to be alive. Survival curves were estimated according to the Kaplan–Meier method.29 All efficacy and toxicity endpoints were updated in August 2018.
The association of genetic factors with binary endpoints was tested with the chi-square test for heterogeneity,26 or the Fisher's exact test30 when appropriate. The association of genetic factors with survival endpoints was tested with the log-rank test.31 All reported P values are two-sided; P values ≤ 0.05 were considered statistically significant. SAS System version 9.2 was used in all analyses.
Results
Patient characteristics
From July 2013 to September 2016, 89 patients were registered in the trial at 22 centers in Italy. Five out of these were not eligible because of severe violations of inclusion/exclusion criteria, and one patient was registered two times in the web-based registration database by mistake. Therefore, the modified intention-to-treat efficacy population, as predefined in the study protocol, was composed of 83 eligible and evaluable patients. One out of the five non-eligible patients received study drugs and, therefore, was included in the safety analysis population of 84 patients.
Baseline characteristics are summarized in Table 1.
Table 1.
Clinical characteristics of the patients at baseline
| Parameter | Evaluable population (total, n = 83) n (%) |
|---|---|
| Age, years | |
| Median | 56 |
| Range | 23-81 |
| BRCA1/2 status | |
| Pathogenic variant | 15 (18) |
| Wild type | 53 (64) |
| Untested | 15 (18) |
| ECOG performance status | |
| 0 | 74 (89) |
| 1 | 9 (11) |
| Prior neoadjuvant chemotherapy | |
| Anthracycline | 18 (22) |
| Taxane | 18 (22) |
| Platinum salts | 2 (2) |
| Prior adjuvant chemotherapy | |
| Anthracycline | 48 (58) |
| Taxane | 48 (58) |
| Platinum salts | 4 (5) |
| Prior lines of chemotherapy for metastatic disease | |
| 0 | 66 (80) |
| 1a | 17 (20) |
| Sites of metastatic disease | |
| 1 site | 11 (13) |
| ≥2 | 66 (80) |
| Bone and visceral | 21 (25) |
| Visceral only | 57 (69) |
| Brain | 7 (8) |
ECOG, Eastern Cooperative Oncology Group; n, number.
Capecitabine, seven patients; carboplatin, six patients; vinorelbine/other, four patients.
Median age at baseline was 56 years; the majority of the patients (80%) were pretreated with anthracyclines and/or taxanes in the (neo)adjuvant setting. Sixty-six and 17 patients were in first- and second-line treatment, respectively. Median interval from the end of (neo)adjuvant treatment to the time of initiation of first-line therapy was 14 months [interquartile range (IQR) 6.9 to 24.9].
Sixty-eight out of the 83 (82%) eligible patients enrolled in the trial were successfully genotyped. Genotyping was not carried out for the remaining patients due to the following reasons: patient's informed refusal (n = 8), lack of informed proposal (n = 5), and consent withdrawal (n = 2). There was no significant difference in pretreatment characteristics between genotyped patients and the whole study population (data not shown). Germline pathogenic BRCA mutations (9 BRCA1 and 6 BRCA2) were detected in 15 (22%) out of 68 tested patients (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2020.100019). Frameshift mutations were detected in eight women: five in BRCA1 and three in BRCA2. Four nonsense, two splice, and one missense mutations were also reported. The same pathogenic variant BRCA1 c.5030_5034del was detected in two women. All these mutations result in loss of function of the protein product and are considered to be clinically significant.
Treatment administration
Eighty-four patients received at least one dose of the study treatment. The median number of cycles administered was six (range 1-27). With an estimated median follow-up of 28.8 months (IQR 16.8-38.9), all patients discontinued therapy. Reasons for treatment discontinuation included progression of disease in 62 patients, patient or investigator decision for 18 patients, and adverse events in four patients.
Fifty-two (62%) and 49 (58%) patients required a dose reduction for eribulin and gemcitabine, respectively. Therapy delays occurred for 60 (71%) patients in 142 (22%) out of 645 cycles.
Efficacy
Based on the predefined interim analysis conducted at the completion of the first stage, enrollment was allowed to continue, as per protocol. A total of 83 eligible and evaluable patients (37 in the first stage, 46 in the second stage) were included in the efficacy analysis. Three out of 83 patients interrupted study treatment before first tumor evaluation (two for toxicity and one for withdrawal of consent) and were considered non-responders. The response rate for all patients was 37.3% [90% confidence interval (CI), 28.5 to 46.9], with 2.4% of patients having a complete response and 34.9% having a PR (Table 2).
Table 2.
Efficacy results by BRCA status
| Variable | BRCA pathogenic variants (n = 15) |
BRCA wild type (n = 53) |
BRCA untested (n = 15) |
Pa | All patients (N = 83) |
|---|---|---|---|---|---|
| Response n (%)b | |||||
| Overall | 4 (26.7) | 22 (41.5) | 5 (33.3) | 0.375 | 31 (37.3) |
| Complete | 0 | 1 (1.9) | 1 (6.7) | 2 (2.4) | |
| Partial | 4 (26.7) | 21 (39.6) | 4 (26.7) | 29 (34.9) | |
| Nonec | 11 (73.3) | 31 (58.5) | 10 (66.6) | 52 (62.7) | |
| Clinical benefit rated | 4 (26.7) | 30 (57.7) | 6 (40) | 0.043 | 40 (48.8) |
| Response duratione months | |||||
| Median | 2.4 | 6.6 | 5.1 | 0.008 | 6.1 |
| 95% CI | 2.0-6.1 | 4.1-8.4 | 2.0-7.1 | 4.1-7.2 | |
| Progression-free survival months | |||||
| Median | 2.6 | 6.4 | 5.1 | <0.001 | 5.1 |
| 95% CI | 2.0-4.5 | 4.4-9.3 | 2.4-6.9 | 4.1-6.9 | |
| Overall survival months | |||||
| Median | 9.6 | 17.9 | 13.0 | 0.020 | 14.5 |
| 95% CI | 2.6-11.3 | 11.1-21.5 | 4.6-20.1 | 10.1-19.8 | |
CI, confidence interval; n/N, number.
Fisher's exact test or log-rank test for the comparison between BRCA-mutated and wild-type patients.
First stage results of 37 patients: overall response: 16/37 (43.2%), complete response: 0, partial response: 16/37; no response: 21/37 (56.8%).
No response was defined as stable, progressive, or non-assessed disease.
One BRCA wild-type patient was not evaluable for clinical benefit rate because they were not assessed for short (<6 months) versus long (≥6 months) stable disease duration.
Response duration was defined as the time from documentation of tumor response to disease progression.
The clinical benefit rate was 48.8% (90% CI 39.2 to 58.4). For the 31 patients who had a response at the time of data analysis, the estimated median response duration was 6.1 months (95% CI 4.1 to 7.2). The estimated median PFS among all patients was 5.1 months (95% CI 4.1 to 6.9). The median OS for this study was 14.5 months (95% CI 10.1 to 19.8) (Table 2).
Safety
The majority of the adverse events observed in the 84 patients who received at least one dose of study drugs were grade 1 or 2. The most common nonhematologic adverse events occurring in more than 10% of patients were fatigue (in 66.7% of patients), elevation of aspartate aminotransferase (AST)/alanine aminotransferase (ALT) (in 58.3%), nausea (in 36.9%), alopecia (in 23.8%), diarrhea (in 19.0%), constipation (in 17.9%), peripheral neuropathy (in 14.3%), rash (in 14.3%), vomiting (in 11.9%), and oral mucositis (in 10.7%) (Table 3).
Table 3.
Adverse eventsa
| Event | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|---|---|---|---|---|---|
| No. of patients with event (%) | |||||
| Hematologic event | |||||
| Neutropenia | 8 (9.5) | 22 (26.2) | 14 (16.7) | 6 (7.1) | 50 (59.5) |
| Anemia | 23 (27.4) | 12 (14.3) | 1 (1.2) | 0 | 36 (42.9) |
| Thrombocytopenia | 18 (21.4) | 6 (7.1) | 2 (2.4) | 0 | 26 (30.9) |
| Nonhematologic event | |||||
| Fatigue | 25 (29.8) | 26 (30.9) | 5 (5.9) | 0 | 56 (66.6) |
| Elevation of AST/ALT | 14 (16.7) | 14 (16.7) | 21 (25.0) | 0 | 49 (58.3) |
| Nausea | 20 (23.8) | 10 (11.9) | 1 (1.2) | 0 | 31 (36.9) |
| Alopecia | 10 (11.9) | 7 (8.3) | 2 (2.4) | 1 (1.2) | 20 (23.8) |
| Diarrhea | 12 (14.3) | 4 (4.8) | 0 | 0 | 16 (19.0) |
| Constipation | 12 (14.3) | 2 (2.4) | 1 (1.2) | 0 | 15 (17.9) |
| Peripheral neuropathy | 8 (9.5) | 3 (3.6) | 1 (1.2) | 0 | 12 (14.3) |
| Rash | 8 (9.5) | 2 (2.4) | 2 (2.4) | 0 | 12 (14.3) |
| Vomiting | 8 (9.5) | 1 (1.2) | 1 (1.2) | 0 | 10 (11.9) |
| Oral mucositis | 6 (7.1) | 3 (3.6) | 0 | 0 | 9 (10.7) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; no., number.
The safety analysis set included the 84 patients who received at least one dose of study drugs.
Grade 3 and 4 hematologic adverse events included neutropenia (in 23.8% of patients), thrombocytopenia (in 2.4%), and anemia (in 1.2%); two patients (2.4%) had grade 3/4 febrile neutropenia.
An adverse event leading to discontinuation of therapy occurred in four patients (4.7%). No patient died during treatment due to an adverse event.
Efficacy and safety by BRCA status and pharmacogenetics
Among the 68 genotyped patients, the response rate was 41.5% (90% CI 30.0-53.7) and 26.7% (90% CI 9.7-51.1) for BRCA wild-type and BRCA-mutated subgroups, respectively (P = 0.375) (Table 2). Furthermore, the clinical benefit rate was 57.7% (90% CI 45.4-69.3) in wild-type patients and only 26.7% (90% CI 9.7-51.1) in BRCA-mutated ones (P = 0.043). Similarly, subjects with BRCA pathogenic variants were associated with significantly worse response duration, PFS, and OS than non-carriers (Table 2; Figure 1). No correlation was observed between toxicity and BRCA status (data not shown).
Figure 1.
Progression-free survival (A) and overall survival (B) according to the BRCA1/2 status.
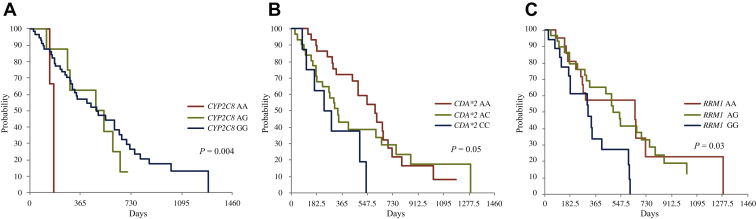
Genotyped patients were also evaluated using a panel of 10 SNPs in seven genes involved in either eribulin or gemcitabine metabolism pathway. All SNP genotypes were in Hardy–Weinberg equilibrium; none of them were associated with treatment response (data not shown). Conversely, the CYP3A4∗1B (392A>G) SNP, which is involved in the hepatic metabolism of both eribulin and gemcitabine, was associated with grade 3/4 AST and ALT elevations {23% in AA genotype [variant frequency (VF): 94%] versus 75% in AG/GG genotypes [VF: 6%]; P = 0.05}. The same association was also reported for the FGD4 (2044236G>A) SNP, which is involved in the regulation of actin cytoskeleton assembly and cell shape: the rates of grade 3/4 hypertransaminasemia were 20% in AA/AG genotypes (VF: 85.5%) versus 60% in GG genotype (VF: 14.5%; P = 0.04). Moreover, after adjustment for BRCA status, three different SNPs were significantly and independently associated with poorer over survival (Figure 2): CDA∗2 (79A>C) and RRM1 (2455A>G), which are involved in the metabolism of nucleoside analogues, and the CYP2C8 (416G>A) SNP involved in the hepatic metabolism of both eribulin and gemcitabine.
Figure 2.
Overall survival according to CYP2C8 (416G>A) (A), CDA∗2 (79A>C) (B), and RRM1 (2455A>G) (C) single nucleotide polymorphisms.
Discussion
The ERIGE trial is a multicenter, Italian study enrolling a homogenous population of patients with advanced TNBC, testing a novel treatment regimen that combines eribulin with gemcitabine.
The combination therapy showed a remarkable best ORR of 37.3% and a clinical benefit rate of 48.8%. Adverse events of the trial were consistent with the known safety profiles of the two drugs. The administration of eribulin and gemcitabine revealed a manageable safety profile and the most common grade 3/4 toxicities were liver toxicity and neutropenia without febrile neutropenia. At a median follow-up of 28.8 months, the median PFS was 5.1 months and the median OS was 14.5 months.
For patients with metastatic breast cancers, both combination and sequential, single-agent chemotherapy are reasonable options.2 Current guidelines recommend sequential monotherapy as the preferred choice. Combination chemotherapy should be reserved for patients with rapid clinical progression, life-threatening visceral metastases, or the need for rapid symptom and/or disease control. In patients pretreated (in the adjuvant and/or metastatic setting) with an anthracycline and a taxane, single-agent capecitabine, carboplatin, vinorelbine, or eribulin may be offered.3
The combination of eribulin with gemcitabine used in the ERIGE trial seems to increase the activity of eribulin monotherapy, which accounts for an ORR of 12%, according to the results of the EMBRACE trial in heavily pretreated patients with metastatic breast cancer.16 Furthermore, the activity of eribulin plus gemcitabine observed in our trial was almost higher compared with the first-line monotherapies tested in the triple negative breast cancer trial (TNT),32 i.e. carboplatinum and taxotere, showing an ORR of 31% and 34%, respectively.32 Some studies were conducted in patients with metastatic TNBC evaluating other combination regimens, such as carboplatin/gemcitabine,33 iniparib plus carboplatin/gemcitabine,34 gemcitabine/paclitaxel,35 ixabepilone/capecitabine,36 and cisplatin/gemcitabine.37 In summary, those combination treatments revealed response rates of 23%-36%, median PFS times of 4.1-6.0 months, and median OS times of 11-12 months. With an ORR of 37.3%, PFS of 5.1 months, and OS of 14.5 months, the combination of eribulin and gemcitabine compares well with those results. For interpretation purposes, it is however important to take into account that the ERIGE study did not perform independent central reviews of neither triple negative status nor survival endpoints.
Almost 40% of patients with metastatic TNBC, which express the programmed death-ligand 1 (PD-L1) on immune cells of the tumor microenvironment, are currently treated in the first-line setting with the anti-PD-L1 monoclonal antibody atezolizumab in combination with nab-paclitaxel.38 Furthermore, the 20% of patients with TNBC who harbored BRCA1/2 pathogenic variants and had previously received ≤2 lines of chemotherapy for metastatic disease obtained benefit from PARP inhibitors (PARPi), such as olaparib and talazoparib.39, 40, 41 Nevertheless, ~50% of TNBC do not present biomarkers predictive of response to targeted therapies and, in this population, there is no general agreement on the best first- or second-line treatment to provide for metastatic disease, especially if patients already received adjuvant regimens including anthracyclines and taxanes (+/− carboplatin).42 Considering the complementary mechanisms of action of eribulin and gemcitabine, the activity of their combination observed in the ERIGE trial, and the manageable toxicity, we propose the use of our regimen in patients with ‘biomarker-orphan’, metastatic TNBC.
Looking at the results of our pre-planned genetic and pharmacogenetic analyses, the study regimen partially lost its efficacy in patients harboring BRCA1/2 pathogenic variants, showing an ORR of 26.7% and a median PFS and OS of 2.6 and 9.6 months, respectively. Interestingly, in the PARP inhibitor OlympiAD and EMBRACA trials, pretreated patients with BRCA-mutated, HER2-negative metastatic breast cancer who were treated with treatment of physician's choice (TPC) as control arm (i.e. eribulin, gemcitabine, capecitabine, or vinorelbine) showed comparable response rates of 27%-29% and a median PFS of 4.2-5.6 months.39,40 Furthermore, the TNT trial demonstrated that docetaxel was less active than carboplatin in BRCA-mutated tumors.32 Taking into account all these data, we can hypothesize that therapeutic agents such as microtubule inhibitors and nucleotide analogues, which, unlike platinum salts and PARPi, do not exploit the vulnerability of impaired DNA damage repair mechanism in BRCA-mutant cancers, do not get the most therapeutic benefit in this group of patients. Our data confirm the current guideline statement that BRCA1/2 germline testing has proven clinical utility and therapeutic impact in metastatic breast cancer and should be carried out as early as possible.3
Pharmacogenomic studies are conducted to investigate biomarkers that can predict the toxicity or efficacy of chemotherapies. A relatively limited number of pharmacogenomic studies have been conducted to investigate these biomarkers in BC with inadequate accumulated evidence to warrant specific dosing or chemotherapy regimens. Apart from the guidelines regarding dihydropyrimidine dehydrogenase (DPYD) genetic testing before fluoropyrimidine use, there are no other guidelines related to other chemotherapeutic agents used in BC.42 To our knowledge, the ERIGE trial is the first trial prospectively investigating the role of SNPs involved in the metabolism of eribulin and gemcitabine in predicting toxicities and efficacy of those drugs. SNPs in CYP3A4 and FGD4 genes were associated with increased risk of liver toxicity. Moreover, three different SNPs in CDA∗2, RRM1, and CYP2C8 genes were significantly associated with poorer OS. For all that, additional data from large-scale, prospective randomized clinical trials are required.
Conclusion
The combination of eribulin and gemcitabine demonstrated promising antitumor activity as first- or second-line therapy for advanced TNBC. The treatment was well-tolerated with manageable toxicity. The pre-planned subgroup analysis indicated that this combination regimen was particularly efficacious in BRCA wild-type patients. Pharmacogenetic testing was encouraging, suggesting that SNP data analysis may represent for this disease setting a reliable and affordable approach in the precision medicine scenario.
Ethics statement
This study was approved by the local independent ethics committee (IEC). The patients provided informed written consent for participation in the trial publication of clinical data and images.
Acknowledgements
The authors thank the patients involved in the trial, their families, and the hospital staff whose support and collaboration made this study possible.
Funding
This work was supported by sponsorship from the Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC) and ESMO to BP with the aid of a grant from Roche. Support for study drug supply and trial management was provided by Eisai Co., Ltd (no grant number).
Disclosure
CZ reports grants, personal fees, and non-financial support from Roche; grants from Eisai; grants, personal fees, and non-financial support from Novartis; grants, personal fees, and non-financial support from AstraZeneca; grants, personal fees, and non-financial support from Pfizer; grants from PharmaMar; grants and personal fees from Amgen; grants and personal fees from Tesaro; personal fees from QuintilesIMS; grants from Pierre Fabre; grants from Istituto Gentili; grants from Takeda; grants from TEVA; grants from Medivation; grants from AbbVie; grants from Array BioPharma; grants from Morphotek; grants from Synthon; grants from Seattle Genetics; grants from Lilly, grants from Celgene, outside the submitted work. AF reports personal fees from Roche, personal fees from Novartis, personal fees from Pfizer, personal fees from Lilly, outside the submitted work. AS reports personal fees from Novartis, personal fees from Amgen, personal fees from Istituto Gentili, outside the submitted work. AR reports personal fees from Pfizer, personal fees from Novartis, personal fees from Eli Lilly, personal fees from Roche, outside the submitted work. FP reports personal fees from Novartis, personal fees from Pfizer, personal fees from Amgen, outside the submitted work. FM reports personal fees from Roche, personal fees from Novartis, personal fees from Pfizer, personal fees from Pierre Fabre, personal fees from Eli Lilly, personal fees from Daiichi Sankyo, personal fees from Astra Zeneca, outside the submitted work. AA reports grants and personal fees from BMS, grants from MSD, grants from Roche, grants from Astra Zeneca, grants from Eli Lilly, grants from Takeda, grants from Bayer, outside the submitted work. AM reports grants and other from Eisai Co., Ltd, during the conduct of the study; grants and personal fees from Roche; personal fees from MacroGenics; personal fees from Merck; grants and personal fees from Lilly; grants from Pfizer, outside the submitted work. All other authors have declared no conflicts of interest.
Disclaimer
Any views, opinions, findings, conclusions, or recommendations expressed in this material are those solely of the authors and do not necessarily reflect those of ESMO, Roche, or Eisai.
Supplementary data
References
- 1.Lehmann B.D., Bauer J.A., Chen X. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge A.H., Rumble R.B., Carey L.A. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2 negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32(29):3307–3329. doi: 10.1200/JCO.2014.56.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F., Paluch-Shimon S., Senkus E. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent R., Hanna W.M., Trudeau M., Rawlinson E., Sun P., Narod S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 5.Malmgren J.A., Mayer M., Atwood M.K., Kaplan H.G. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990–2010. Breast Cancer Res Treat. 2018;167(2):579–590. doi: 10.1007/s10549-017-4529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gobbini E., Ezzalfani M., Dieras V. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24. doi: 10.1016/j.ejca.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Angulo A.M., Morales-Vasquez F., Hortobagyi G.N. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 8.Cazzaniga M.E., Pinotti G., Montagna E. Metronomic chemotherapy for advanced breast cancer patients in the real world practice: final results of the VICTOR-6 study. Breast. 2019;48:7–16. doi: 10.1016/j.breast.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Glueck S., Montero A.J., Glück S. Gemcitabine and taxanes in metastatic breast cancer: a systematic review. Ther Clin Risk Manag. 2008;4(6):1157–1164. [PMC free article] [PubMed] [Google Scholar]
- 10.Hirata Y., Uemura D. Halichondrins – antitumor polyether macrolides from a marine sponge. Pure Appl Chem. 1986;58(5):701–710. [Google Scholar]
- 11.Uemura D., Takahashi K., Yamamoto T. Norhalichondrin A: an antitumor polyether macrolide from a marine sponge. J Am Chem Soc. 1985;107(16):4796–4798. [Google Scholar]
- 12.Alday P.H., Correia J.J. Macromolecular interaction of halichondrin B analogues eribulin (E7389) and ER-076349 with tubulin by analytical ultracentrifugation. Biochemistry. 2009;48(33):7927–7938. doi: 10.1021/bi900776u. [DOI] [PubMed] [Google Scholar]
- 13.Vahdat L.T., Pruitt B., Fabian C.J. Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2009;27(18):2954–2961. doi: 10.1200/JCO.2008.17.7618. [DOI] [PubMed] [Google Scholar]
- 14.Cortes J., Vahdat L., Blum J.L. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28(25):3922–3928. doi: 10.1200/JCO.2009.25.8467. [DOI] [PubMed] [Google Scholar]
- 15.Iwata H., Aogi K., Masuda N. Efficacy and safety of eribulin in Japanese patients (pts) with advanced breast cancer. J Clin Oncol. 2010;28(suppl 15):1081. [Google Scholar]
- 16.Cortes J., O'Shaughnessy J., Loesch D. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 17.Pivot X., Marmé F., Koenigsberg R., Guo M., Berrak E., Wolfer A. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann Oncol. 2016;27(8):1525–1531. doi: 10.1093/annonc/mdw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lheureux S., Oza A.M., Laurie S.A. A phase I combination dose-escalation study of eribulin mesylate and gemcitabine in patients with advanced solid tumours: a study of the Princess Margaret Consortium. Br J Cancer. 2015;113(11):1534–1540. doi: 10.1038/bjc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Illumina Methods guide 2020; version 6: catalog no. 770-2014-018 QB6152. 2020. https://emea.illumina.com/landing/methods-guide.html Available at:
- 21.Human Genome Variation Society HGVS website. http://www.hgvs.org Available at: Accessed April 6, 2020.
- 22.NCBI ClinVar. https://www.ncbi.nlm.nih.gov/clinvar/ Available at: Accessed April 6, 2020.
- 23.Technologies Corporation L. TaqMan® Universal PCR Master Mix User Guide (Pub. no. 4304449 Rev. E), 2014.
- 24.Negri F.V., Musolino A., Naldi N. Role of immunoglobulin G fragment C receptor polymorphism-mediated antibody-dependent cellular cytotoxicity in colorectal cancer treated with cetuximab therapy. Pharmacogenomics J. 2014;14(1):14–19. doi: 10.1038/tpj.2012.54. [DOI] [PubMed] [Google Scholar]
- 25.Musolino A., Naldi N., Bortesi B. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 26.Hardy G.H. Mendelian proportions in a mixed population. Science. 1908;28(706):49–50. doi: 10.1126/science.28.706.49. [DOI] [PubMed] [Google Scholar]
- 27.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 28.NCI CTCAE Files. https://evs.nci.nih.gov/ftp1/CTCAE/About.html Available at: Accessed April 6, 2020.
- 29.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 30.Freedman D. 2nd ed. Cambridge University Press; Cambridge: 2009. Statistical Models: Theory and Practice. [Google Scholar]
- 31.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 32.Tutt A., Tovey H., Cheang M.C.U. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24(5):628–637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maisano R., Zavettieri M., Azzarello D. Carboplatin and gemcitabine combination in metastatic triple-negative anthracycline-and taxane-pretreated breast cancer patients: a phase II study. J Chemother. 2011;23(1):40–43. doi: 10.1179/joc.2011.23.1.40. [DOI] [PubMed] [Google Scholar]
- 34.O'Shaughnessy J., Hellerstedt B., Schwartzberg L. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32(34):3840–3847. doi: 10.1200/JCO.2014.55.2984. [DOI] [PubMed] [Google Scholar]
- 35.Aogi K., Yoshida M., Sagara Y. The efficacy and safety of gemcitabine plus paclitaxel combination first-line therapy for Japanese patients with metastatic breast cancer including triple-negative phenotype. Cancer Chemother Pharmacol. 2011;67(5):1007–1015. doi: 10.1007/s00280-010-1390-1. [DOI] [PubMed] [Google Scholar]
- 36.Perez E.A., Patel T., Moreno-Aspitia A. Efficacy of ixabepilone in ER/PR/HER2-negative (triple-negative) breast cancer. Breast Cancer Res Treat. 2010;121(2):261–271. doi: 10.1007/s10549-010-0824-0. [DOI] [PubMed] [Google Scholar]
- 37.Koshy N., Quispe D., Shi R., Mansour R., Burton G.V. Cisplatin-gemcitabine therapy in metastatic breast cancer: improved outcome in triple negative breast cancer patients compared to non-triple negative patients. Breast. 2010;19(3):246–248. doi: 10.1016/j.breast.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Schmid P., Rugo H.S., Adams S. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 39.Robson M., Im S.-A., Senkus E.E. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 40.Litton J.K., Rugo H.S., Ettl J. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caparica R., Lambertini M., de Azambuja E. How I treat metastatic triple-negative breast cancer. ESMO Open. 2019;4(suppl 2):e000504. doi: 10.1136/esmoopen-2019-000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Mahayri Z.N., Patrinos G.P., Ali B.R. Toxicity and pharmacogenomic biomarkers in breast cancer chemotherapy. Front Pharmacol. 2020;11:445. doi: 10.3389/fphar.2020.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.