Key points.
-
•
EEG and somatosensory evoked potentials (SSEP) can provide objective measures of cerebral activity.
-
•
EEG is a sensitive measure of cerebral disturbance, although it is not specific to particular aetiologies.
-
•
Diagnostic uses of EEG in critical care include the identification and grading of encephalopathy, and the identification and guidance for management of patients with non-convulsive epileptic seizures.
-
•
Therapeutic indications of EEG include assessment of the depth of sedation in induced coma.
-
•
EEG and SSEP, in combination with neuroimaging and biochemical measurements, can provide valuable prognostic information after cardiac arrest or traumatic brain injury.
Learning objectives.
By reading this article, you should be able to:
-
•
Describe the basic principles of EEG, including waveforms seen in the healthy adult population.
-
•
Summarise the common causes of encephalopathy and their associated features on the electroencephalograph.
-
•
Discuss the neurophysiological features associated with favourable and unfavourable outcomes in critically ill patients.
EEG is an investigation often used in critical care, but the application of the EEG findings in clinical care by ICU physicians can prove challenging.
Common conditions in which EEG can inform diagnosis and neurological prognosis include encephalopathy, seizure disorders, hypoxic and traumatic brain injuries, cerebral structural abnormalities, and coma. EEG also has therapeutic indications, such as determining the depth of anaesthesia in induced coma and the cessation of non-convulsive status epilepticus by anticonvulsant or anaesthetic agents.
Patterns that are frequently encountered in critical care include slow (wave) activity, frontal intermittent rhythmic delta activity (FIRDA), triphasic waves, epileptiform patterns and periodic discharges (PDs), suppression, and burst suppression. Detailed definitions of these patterns can be found in the internationally agreed recently revised glossary of terms most commonly used by clinical electroencephalographers.1
This overview discusses the basic principles of EEG, recording and interpretation, followed by EEG patterns present in the aforementioned pathologies, as illustrated in Fig 1, Fig 2, Fig 3, Fig 4, Fig 5, Fig 6, Fig 7, Fig 8 (also see additional details in Supplementary data).
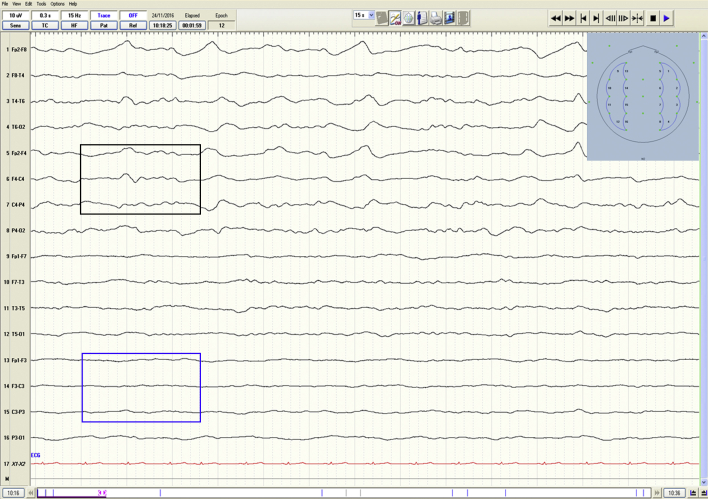
Fig 1.
Normal adult EEG demonstrating alpha rhythm (highlighted in box), with blocking on eye opening (vertical line).
Fig 2.
Frontal intermittent rhythmic delta activity (highlighted in box) appears as intermittent symmetrical rhythmic delta activity over frontal regions. It is non-specific and can arise secondary to structural or non-structural pathologies, but is most often associated with metabolic disturbances, for example uraemia or hyperglycaemia, commonly in combination with cerebrovascular insufficiency.6
Fig 3.
Triphasic waves (highlighted in box) occurring with a periodicity of 1–2 Hz in a 63 yr old male as a result of hepatic encephalopathy. Triphasic waves are symmetrical positive sharp transients (downward deflection) preceded and followed by relatively low amplitude negative waves (upward deflection), dominant over frontal cortex with an anterio-posterior lag.
Fig 4.
Lateralised periodic discharges over the left hemisphere (highlighted in box), consisting of sharp waves at a frequency of 1 Hz, in a 74 yr old female patient recorded 24 h after an episode of complex partial status epilepticus. LPDs are confined to one hemisphere and can suggest ictal activity; however, they are most often considered interictal.11
Fig 5.
Generalised periodic discharges (highlighted in box, but seen throughout the image) at a frequency of 2.5 Hz in a 70 yr old male, secondary to hypoxic ischaemic encephalopathy, after in-hospital cardiac arrest. Generalised periodic discharges (GPDs) are generalised, synchronous, periodic, or quasi-periodic complexes that occupy at least 50% of a recording, with a repetition rate of 0.5–1.5 Hz.
Fig 6.
Diffuse slow wave activity (highlighted in black box) and left hemisphere suppression (highlighted in blue box), in a 62 yr old male secondary to traumatic brain injury with associated subarachnoid and bilateral subdural haemorrhage. Where suppression is unilateral (or bilateral) and CT or MRI has not yet taken place, urgent neuroimaging should be considered to assess for evidence of subdural haemorrhage or cerebral infarction.
Fig 7.
Interictal discharges (highlighted in box) in a 31 yr old female with primary generalised epilepsy.
Fig 8.
Median nerve somatosensory evoked potentials (SSEPs) (left and right, respectively), demonstrating bilateral presence of cortical responses (N20, black arrowheads), after electrical stimulation at the wrist. Stimulus was applied at the start of each trace. Negative potentials are shown as an upward deflection.
Basic principles
The EEG records electrical activity generated by cerebral neurones, in the form of potential differences between electrodes positioned over the scalp. The EEG is digitised, amplified (as in a healthy adult it is typically 20–100 μV), filtered to remove or reduce noise (usually from 0.3 to 70 Hz), and displayed as waveforms of varying morphologies and frequencies (Table 1). Interpretation of these waveforms is usually by visual inspection, but can be performed by quantitative analysis of the frequency, amplitude, topographical distribution, cross correlation, and reactivity; these analyses enable an objective assessment of cerebral activity.2
Table 1.
EEG waveforms in the healthy adult population. (Not to scale, in general terms lower frequency activity is higher in amplitude and vice versa.).
| Wave form | Frequency (Hz) | Location | Morphology (1s) |
|---|---|---|---|
| Gamma | >30 | Somatosensory cortex | |
| Beta | 13–30 | Frontal | |
| Alpha | 8–13 | Occipital | |
| Theta | 4–8 | Diffuse | |
| Delta | <4 | Diffuse |
Standard recording typically uses 21 electrodes, applied according to the international 10–20 classification system, over the frontal (F), temporal (T), central (C), parietal (P), and occipital (O) regions. Even-numbered electrodes relate to the right hemisphere and odd ones to the left, whilst a post-fixed z refers to the midline. A standard recording is usually 20–30 min in duration.
Normal adult EEG
Alpha activity is usually evident over posterior cortical regions and is ‘reactive’ to arousal stimulation, which means it attenuates (or blocks) on eye opening or mental activity. Beta activity is present over anterior regions.
Effect of drugs on EEG patterns
Beta activity is initially increased by several sedative, anaesthetic, and anticonvulsant medications (e.g. barbiturates, propofol, benzodiazepines).3 However, in simplified terms, increasing depth of anaesthesia results in progressive increase of slower frequencies, although different agents can induce several effects.4 In the case of propofol, the beta activity decreases first with an increase in coherent alpha activity, which becomes more prominent over the anterior part of the head (‘anteriorisation’), before slower incoherent frequencies emerge, followed by burst suppression and finally suppression of all cortical activity.5
While epileptiform activity can be assessed in the presence of pharmacological sedation, temporary weaning of such agents should produce a more reliable EEG for interpretation of the ongoing background activity.
EEG in encephalopathy
Encephalopathy, or global cerebral dysfunction, is a common complication in the critically ill and its aetiology is wide ranging. A few examples of common causes include metabolic derangement, hepatic or renal failure, systemic infection, toxins, and traumatic and hypoxic ischaemic encephalopathies.
While the EEG is generally not specific to aetiology, it is a very sensitive measure of cerebral dysfunction and can give information regarding the severity of encephalopathy.6 The earliest changes of encephalopathy are usually ‘slowing’, or fall out of the alpha rhythm. With an advancing clinical picture, patterns visualised include slow (wave) activity, FIRDA, triphasic waves, PDs, intermittent suppression, and burst suppression.
Slow wave activity
Slow waves are theta and delta frequency activity and should be present for a significant proportion (i.e. approximately >50%) of a recording in an awake patient to be deemed pathological. Common neuropsychiatric drugs associated with diffuse slow wave activity include tricyclic antidepressants, lithium, and clozapine.3 Structural cerebral abnormalities—such as tumour; gliosis; encephalitis; and cerebrovascular insults caused by infarction, subdural haematoma, and subarachnoid haemorrhage—generally produce asymmetrical slow-wave activity.
Frontal intermittent rhythmic delta activity
FIRDA is fairly regular, approximately sinusoidal or sawtooth waves, occurring in intermittent bursts at 1.5-2.5 Hz synchronously over the frontal regions (occasionally unilaterally). FIRDA is most commonly associated with mild to moderate unspecified encephalopathy in responsive patients, often with metabolic derangement and cerebrovascular disease.
Triphasic waves
Triphasic waves arise as a result of toxic, metabolic, and hypoxic ischaemic encephalopathies, and prion disease (Creutzfeldt–Jakob disease).7 Triphasic waves can occur in overdose of several drugs including sodium valproate, baclofen, lithium, levodopa, barbiturates, and serotonergic drugs.3
Periodic discharges
PDs are repeating waveforms of relatively uniform morphology, duration, and quantifiable inter-discharge interval, occupying the majority of the recording.8 PDs are described by their topography and inter-discharge interval, usually 0.3 s to several seconds, which can provide clues to their aetiology.7 Topographic descriptions include lateralised periodic discharges (LPDs), bilateral independent periodic discharges (BIPDs), and generalised periodic discharges (GPDs).1 In response to an alerting event, stimulus-induced rhythmic ictal or periodic discharges (SIRPIDs) have been described in comatose patients. The significance of PDs should be interpreted within the clinical context in which they occur, as there is no formal standard of care, but a prophylactic anticonvulsant may be warranted because of their frequent association with seizures.1 For example, in a patient with fever, delirium, and an EEG revealing LPDs over the left hemisphere, there should be a strong suspicion of herpes simplex encephalitis (HSE). The same EEG from a patient with acute right-sided hemiparesis would suggest significant left hemispheric dysfunction and structural damage secondary to a stroke.
Lateralised periodic discharges
LPDs can be seen in acute destructive lesions such as cerebrovascular insults, space-occupying lesions, and encephalitides, including HSE.9 The literature suggests that up to 90% of patients with LPDs will experience seizures during the course of their illness.10
Bilateral independent periodic discharges
BIPDs refers to the presence of two independent, asynchronous, lateralised discharges in both hemispheres.11 Common pathologies include hypoxic ischaemic encephalopathy (HIE), severe hypoglycaemia, central nervous system (CNS) infections, and HSE.10 BIPDs are also frequently associated with seizures, and often a poor neurological prognosis.
Generalised periodic discharges
GPDs are commonly seen in toxic, metabolic, and hypoxic ischaemic encephalopathies, but can occur in NCS and NCSE. Discrimination between these conditions based on the GPD appearance alone is unreliable, and must be interpreted within the clinical context.12 Management should be focused on treatment of the underlying cause and prognosis guided by both response and reversibility of the underlying cause, but is generally poor.
Stimulus-induced rhythmic periodic or ictal discharges
In comatose patients, rhythmic periodic or ictal discharges can arise in the context of alerting stimuli. SIRPIDs may not correlate with any obvious clinical change, but on occasions clinical seizures do occur. Discrimination between ictal and non-ictal activity can prove difficult. Clinical significance of SIRPIDs is therefore uncertain and should be assessed in the clinical context, but are often associated with a poor prognosis.12
Suppression
Suppression implies a low amplitude recording, defined as voltage <10 μV throughout all channels.8 As previously noted, intermittent suppression can occur as a result of deeper states of anaesthesia, which are reversible. In pathological states, suppression is suggestive of gross cerebral disturbance and generally indicates a poor prognosis. Although not aetiology-specific, possibilities include cerebral intoxication, post-ictal suppression, brainstem haemorrhage, cerebral ischaemia, and oedema.
Electrocerebral inactivity (ECI) represents extreme suppression caused by the global loss of electrical activity, defined as <2 μV. Profound hypothermia, severe hypotension, and CNS depressant intoxication are potentially reversible causes of ECI, which must be excluded before ascribing this pattern to irreversible coma.11
Burst suppression
As the EEG becomes discontinuous, bursts of sharp and slow wave activity of variable duration alternate with variable periods of attenuation or suppression below 10 μV. When more than 50% of the record consists of attenuation or suppression, this pattern has recently been defined as burst suppression.8 An alternative definition, sometimes used clinically, describes burst suppression as a state of unconsciousness and profound brain inactivation in which the EEG shows periods of electrical activity alternating with periods of isoelectricity or electrical silence.5 Common causes of this pattern include sedative and general anaesthetic agents, status epilepticus, and hypoxic ischemic encephalopathy.13 Consideration should be given to a trial period of sedation hold, unless being used for therapeutic purposes, as this pattern can be seen accompanying myoclonic status epilepticus after a profound hypoxic ischaemic insult and carries a grave prognosis.
EEG in epileptiform disorders
Non-convulsive seizures and non-convulsive status epilepticus
Non-convulsive seizures and status epilepticus (NCS and NCSE, respectively) should be considered in patients with disordered consciousness, particularly those in coma with a history of epilepsy; after neurosurgery, stroke, or cerebral infection. Intensivists should also be vigilant for the possibility of ongoing subclinical seizures after convulsive status epilepticus appears to have terminated, and in the setting of hypoxic–ischaemic encephalopathy.
To confirm a diagnosis of NCS or NCSE, a repetitive pattern of focal or generalised epileptiform discharges at a frequency >2.5 Hz should be present for ≥10 s or ≥30 min, respectively.14 Where repetitive epileptiform discharges at a frequency <2.5 Hz or rhythmic activity at a frequency of >0.5 Hz are present, a diagnosis of NCS or NCSE may also be appropriate if spatio-temporal EEG evolution is evident or if a trial of rapidly acting intravenous anticonvulsant therapy results in both clinical and EEG improvement.14
There is considerable debate as to the best treatment of subclinical (or electrographic only) NCSE, as there is a risk–benefit equation which must be considered on an individual basis.
Continuous EEG
Continuous EEG (cEEG), with or without video, is a relatively recent innovation in the ICU, whereby cerebral activity alongside clinical behaviour is recorded over a period ranging from hours to weeks. It can be used to identify NCS and NCSE and hence is increasing in clinical application. In cases of refractory status epilepticus it is also used to guide depth of anaesthesia, with evidence of burst suppression acting as a surrogate marker for adequate depth of anaesthesia. It is also used to distinguish non-epileptic events that may closely mimic epileptic seizures, and to detect early, real-time changes in brain function such as might occur in cerebral ischaemia complicating subarachnoid haemorrhage.
The use of cEEG in critically ill patients has been endorsed in recommendations by both the American Clinical Neurophysiological Society and European Society of Intensive Care Medicine, and guidelines for its use have been recently proposed.15, 16 However, cEEG is still a field of active clinical research, and more evidence is required to establish its cost-effectiveness. cEEG is also resource-intensive and often requires real-time expert interpretation, which may not always be readily available.
Prognostication in coma
Coma is defined as ‘an eyes-closed state of unresponsiveness with severely impaired arousal and cognition’.17 In comatose patients the EEG can be used to assess for evidence of potentially reversible causes, such as intoxication with sedatives, NCS, and NCSE. The EEG can also aid neuroprognostication in coma, particularly after cardiorespiratory arrest and traumatic brain injury (TBI).18 Favourable outcomes are seen when continuous rhythmic EEG activities are present, which are reactive to alerting stimuli.19 Reactivity is a reproducible EEG change in response to external alerting, noxious stimuli, or both. This can manifest as a change in amplitude or frequency or both, although this has not yet been precisely quantified. Unfavourable outcomes are generally seen in patients with a non-reactive, discontinuous, or suppressed EEG, and with the presence of epileptiform discharges, GPDs, or both.20
Somatosensory evoked potentials
Somatosensory evoked potentials (SSEPs), which record electrical activity from myelinated peripheral and central (sensory) neurones, have been used in the ICU setting to aid neurological prognosis, particularly after HIE and TBI. Electrical stimulation is usually applied to the median nerve at the wrist, and responses are recorded at the brachial plexus, cervical spine, and ipsilateral and contralateral sensory cortices.
The principle component of interest for neurological prognosis is the N20 response, which by electrophysiological convention is denoted by an upward deflection. The nomenclature is derived as a result of the polarity of the potential, in this case negative (N), and the typical peak latency in milliseconds of approximately 20 ms from wrist to sensory cortex. It is generated in the primary sensory cortex and can be dichotomised into either present or absent.
In comparison with EEG waveforms, muscle activity, movement, and electromagnetic artefacts, the amplitudes of SSEP responses are typically low. In order to eliminate or attenuate such artefacts and the background ‘noise’ computer averaging of the individual responses is performed, whereby hundreds of responses in relation to a time-locked stimulus are summated and random background noise is subtracted out by phase cancellation.
The bilateral absence of N20 confers an unfavourable neurological prognosis, with specificity nearing 100% mortality in HIE. The presence of N20 renders prognosis indeterminate in HIE, but is more favourable in TBI. SSEPs can evolve during the first 24 h after cardiac arrest, and are therefore best performed after this time when the patient is under light sedation and normothermic.21
Conclusion
EEG is a real-time objective measure of cerebral activity available to clinicians in the ICU, particularly where clinical neurological examination is difficult or unreliable. EEG can indicate cortical, subcortical, or arousal disturbance and detect epileptiform activity, which can inform diagnosis and management. It is complimentary to neuroimaging and can be used in conjunction with other neurophysiological techniques such as SSEPs in neuroprognostication for patients in coma.
Declaration of interest
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Laura Sewell MRCP is a specialty trainee in clinical neurophysiology and neurology at North Bristol NHS Trust.
Ahmed Abbas MRCP FHEA is a specialty trainee in clinical neurophysiology at Queen Elizabeth Hospital, Birmingham.
Nick Kane MSc, MD (Hons), FRCS, FRCP (by election), is a consultant in clinical neurophysiology at Southmead Hospital, North Bristol NHS Trust.
Matrix codes: 1A01, 2C01, 3F00
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2018.11.002.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Figs1.
Figs2.
Figs3.
Figs4.
Figs5.
Figs6.
Figs7.
Figs8.
References
- 1.Kane N., Acharya J., Benickzy S. Glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Clin Neurophysiol Pract. 2017;2:170–185. doi: 10.1016/j.cnp.2017.07.002. Revision 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisch B.J. Spehlmann’s EEG primer. 2nd Edn. Elsevier; The Netherlands: 1991. Descriptors of EEG activity; pp. 164–165. [Google Scholar]
- 3.Blume W.T. Drug effects on EEG. J Clin Neurophysiol. 2000;23:306–311. doi: 10.1097/01.wnp.0000229137.94384.fa. [DOI] [PubMed] [Google Scholar]
- 4.Rampil I.J. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. doi: 10.1097/00000542-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Purdon P.L., Sampson A., Pavone K.J., Brown E.N. Clinical electroencephalography for anesthesiologists: Part I. Background and basic signatures. Anesthesiology. 2015;123:937–960. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan P.W. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004;21:307–318. [PubMed] [Google Scholar]
- 7.Brenner R.P., Schaul N. Periodic EEG patterns: classification, clinical correlation, and pathophysiology. J Clin Neurophysiol. 1990;7:249–267. [PubMed] [Google Scholar]
- 8.Hirsch L.J., LaRoche S.M., Gaspard N. American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 9.Sutter R., Kaplan P.W., Valença M., De Marchis G.M. EEG for diagnosis and prognosis of acute nonhypoxic encephalopathy: history and current evidence. J Clin Neurophysiol. 2015;32:456–464. doi: 10.1097/WNP.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.W. EEG in the ICU: what should one treat, what not? Epileptologie. 2012;29:210–217. [Google Scholar]
- 11.Husain A.M. Electroencephalographic assessment of coma. J Clin Neurophysiol. 2006;23:208–220. doi: 10.1097/01.wnp.0000220094.60482.b5. [DOI] [PubMed] [Google Scholar]
- 12.Gaspard N., Hirsch L.J. Pitfalls in ictal EEG interpretation: critical care and intracranial recordings. Neurology. 2013;80:S26–S42. doi: 10.1212/WNL.0b013e31827974f8. [DOI] [PubMed] [Google Scholar]
- 13.Amorim E., Rittenberger J.C., Baldwin M.E., Callaway C.W., Popescu A. Malignant EEG patterns in cardiac arrest patients treated with targeted temperature management who survive to hospital discharge. Resuscitation. 2015;90:127–132. doi: 10.1016/j.resuscitation.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beniczky S., Hirsch L.J., Kaplan P.W. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2014;54:28–29. doi: 10.1111/epi.12270. [DOI] [PubMed] [Google Scholar]
- 15.Herman S., Abend N.S., Bleck T.P. Consensus statement on continuous EEG in critically ill adults and children: Part I. Indications. J Clin Neurophysiol. 2015;32:87–95. doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claassen J., Taccone F.S., Horn P., Holtkamp M., Stocchetti N., Oddo M. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39:1337–1351. doi: 10.1007/s00134-013-2938-4. [DOI] [PubMed] [Google Scholar]
- 17.Sutter R., Kaplan P. Electroencephalographic patterns in coma: when things slow down. Epileptologie. 2012;29:201–209. [Google Scholar]
- 18.Sivaraju A., Gilmore E.J., Wira C.R. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med. 2015;41:1264–1272. doi: 10.1007/s00134-015-3834-x. [DOI] [PubMed] [Google Scholar]
- 19.Young G.B., Wang J.T., Connolly J.F. Prognostic determination in anoxic–ischaemic and traumatic encephalopathies. J Clin Neurophysiol. 2004;21:379–390. [PubMed] [Google Scholar]
- 20.Sandroni C., Cariou A., Cavallaro F. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European resuscitation council and the European society of intensive care medicine. Resuscitation. 2014;85:1779–1789. doi: 10.1016/j.resuscitation.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Kane N., Kaplan P.W., Nolan J.P. Neurophysiology contributes to outcome prediction after cardiac arrest. Clin Neurophysiol Pract. 2017;2:201–205. doi: 10.1016/j.cnp.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]