Key points.
-
•
There is an increasing number of women with congenital heart disease surviving to childbearing age, with more complex conditions.
-
•
Women with adult congenital heart disease may tolerate the physiological changes of pregnancy poorly, and can deteriorate at any point, including postpartum.
-
•
Such women should be counselled of the risks of pregnancy with their condition, and be managed by a specialist multidisciplinary team.
-
•
Early epidural and a ‘low cardiac output’ approach allows many women to achieve a normal delivery, but others may require Caesarean section to prevent deterioration in labour.
-
•
Caution is advised with the commonly used uterotonic drugs.
Cardiac disease has remained the leading cause of maternal mortality since the 2000–2002 triennium. Between 2009 and 2014 the deaths of 189 women who died from heart disease associated with, or aggravated by, pregnancy were reported by MBRRACE-UK (Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK). Of these deaths, only a small number (11, 7%) were in women with adult congenital heart disease (ACHD), a finding that the review programme felt may reflect good prepregnancy counselling and antenatal and perinatal care.1 Nevertheless, these women still present a challenge to manage to anaesthetists, obstetricians, and cardiologists, and there are increasing numbers of patients with more complex disease surviving to adulthood. We review the management of women with ACHD throughout pregnancy.
Background
Congenital heart disease (CHD) affects up to nine in every 1000 babies born in the UK, and ranges from simple complexity lesions e.g. atrial septal defects (17% of all CHD, most common) to moderate e.g. tetralogy of Fallot (11%), to severe lesions such as transposition of the great arteries (5%).2
Due to the advances in modern imaging, surgical techniques, and postoperative care, the survival of patients with CHD has improved dramatically, and today at least 85% of all patients are expected to reach adulthood, such that there are now more adults than children (<18 yr) with CHD. The percentage of ACHD patients with severe disease has also increased from 9% in 2000 to 14% in 2010.3
Regarding the obstetric population, the prevalence is 0.8% of all pregnancies,4 but this may increase with more women with CHD surviving to childbearing age, with more complex disease being more challenging to manage.
Physiological changes of pregnancy
Pregnancy induces changes in the cardiovascular system to meet the increased metabolic demands of the mother and fetus5, 6: (i) total blood volume increases by 45% on average; (ii) cardiac output (CO) increases by 30–50%, peaking in the second trimester; (iii) heart rate rises progressively, peaking in the third trimester at 20–25% above baseline (Fig. 1); and (iv) hormonal-induced vasodilatation causes systemic blood pressure to fall in early gestation.
Fig 1.
Cardiovascular changes throughout pregnancy. Reproduced with permission.6
These changes may be poorly tolerated by women with ACHD, particularly those with impaired ventricular function, obstructive lesions, and pulmonary hypertension, with the peak effects at 24–32 weeks of gestation. Women with mild or asymptomatic disease prepregnancy can develop symptoms such as breathlessness, orthopnoea, and fatigue. Those with more severe disease may deteriorate significantly.
The risk of decompensation increases with the physiological events of labour: (i) blood is autotransfused into the systemic circulation during uterine contraction; and (ii) CO increases during labour, rising to 50% above baseline during expulsive efforts, and reaches a peak increase of 80% early postpartum due to autotransfusion associated with uterine involution and resorption of dependent oedema.
These events can rapidly precipitate critical decompensation in women with ACHD.
Risks of pregnancy
Maternal risk
ACHD carries an increased risk of morbidity and mortality. There are several scoring systems available, such as the World Health Organisation (WHO) classification of maternal cardiovascular risk, the CARPREG (CARdiac disease and PREGnancy) risk score and the ZAHARA (Zwangerschap bij Aangeboren HARtAfwijkingen [Dutch; English translation: Pregnancy with Congenital Heart Defects]) predictors of maternal cardiovascular events.7, 8 The European Society of Cardiology guidance forms the basis for the management of cardiovascular disease in pregnancy, including ACHD, and recommends using a modified version of the WHO classification (Table 1). The predicted mortality risk of the most severe forms (e.g. Eisenmenger’s) is as high as 50%.9
Table 1.
European Society of Cardiology modified version of the WHO classification of pregnancy risk with cardiac disease, including congenital diseases. Reproduced with permission9
| WHO risk class | I | II | III | IV |
|---|---|---|---|---|
| Risks of pregnancy | No detectable increased risk of maternal mortality and no/mild increase in morbidity. | Small increased risk of maternal mortality or moderate increase in morbidity | Significantly increased risk of maternal mortality or severe morbidity. Expert counselling required. If pregnancy is decided upon, intensive specialist cardiac and obstetric monitoring needed throughout pregnancy, childbirth, and the puerperium. |
Extremely high risk of maternal mortality or severe morbidity; pregnancy contraindicated. If pregnancy occurs termination should be discussed. If pregnancy continues, care as for class III. |
| Conditions/lesions |
Uncomplicated, small or mild
|
If otherwise well and uncomplicated:
Repaired coarctation Mild left ventricular impairment Hypertrophic cardiomyopathy Native or tissue valvular heart disease not considered WHO I or IV Marfan syndrome without aortic dilatation Aorta <45 mm in aortic disease associated with bicuspid aortic valve |
Mechanical valve Systemic right ventricle Fontan circulation Cyanotic heart disease (unrepaired) Other complex congenital heart disease |
Pulmonary arterial hypertension of any cause Severe systemic ventricular dysfunction Severe mitral stenosis, severe symptomatic aortic stenosis Marfan syndrome with aorta dilated >45 mm Aortic dilatation >50 mm in aortic disease associated with bicuspid aortic valve Native severe coarctation |
The incidence of maternal obstetric complications is increased in women with certain forms of ACHD, particularly hypertension-related disorders such as pre-eclampsia.
Fetal risk
Neonatal complications, such as premature birth, small for gestational age, and respiratory distress syndrome occur in 20–28% of pregnancies complicated by heart disease. Fetal or neonatal death occurs in 1–4%.8 Children of women with ACHD are at a higher risk of having CHD themselves. Problems diagnosed antenatally may be a factor in obstetricians’ decisions to deliver by emergency Caesarean section (CS) if there is evidence of fetal hypoxia in labour. Anaesthetists should also be aware of the additional psychological burden such complications can present when dealing with such a situation.
Antenatal management
Prepregnancy
Women with ACHD should seek advice from a congenital cardiologist with expertise in transition to adulthood and pregnancy before attempting conception.1 In a retrospective 10-yr study of women with a Fontan circulation across nine UK congenital cardiac centres 82% of women received pre-pregnancy counselling.10 Many women are followed-up from childhood, and receive pregnancy and contraception advice from adolescence.
However, women may be lost to follow-up, or have undiagnosed ACHD discovered in pregnancy. The latter is more likely in women from low resource countries, and such a diagnosis should be considered in the deteriorating parturient from such backgrounds.
At the prepregnancy review, cardiologists use a combination of scoring systems and investigations together with clinical judgement and experience to give the woman a prediction of the risks of pregnancy. Those with the highest risk are advised against pregnancy, and termination is offered if it occurs. Other paths are discussed including adoption and surrogacy. In our experience, the desire of women and their partners to have or complete a family often seems to overcome even the direst of predictions, with few couples being put off conceiving.
Multidisciplinary clinic
It is essential that all women with ACHD are referred to centres experienced in their management as early as possible in their pregnancy, to avoid the potentially disastrous situation of a complex problem only being discovered when labour is imminent. Women with the most minor and corrected conditions (e.g. a ligated patent ductus arteriosus with no ongoing sequelae) may be able to be managed as normal in their local hospital following multidisciplinary review in the specialist centre.
Otherwise women are reviewed at least every trimester by the multidisciplinary team (MDT) including an ACHD cardiologist, an obstetrician, and an obstetric anaesthetist.
Maternal and fetal well-being are assessed and treatments reviewed. Serial echocardiography examinations and clinical assessment monitor the effects of the pregnancy. Other investigations, such as magnetic resonance imaging for aortopathies, can also be arranged.
The MDT provides the opportunity for the woman to plan her delivery with appropriate guidance. For the majority of women with ACHD, labour and a vaginal delivery is possible and should be the goal, with CS more commonly performed for obstetric indications.7 Delivery is usually recommended in the tertiary cardiac centre, unless the cardiac condition is minor or fully corrected with no ongoing sequelae.
Assuming the maternal and fetal conditions permit it, spontaneous labour is generally preferable, but induction of labour at term can allow for a more planned delivery, which is advantageous if the woman lives far from the cardiac centre, or intervention is expected to be required. Those planning to labour are advised of the benefits of early epidural analgesia to reduce pain, sympathetic drive, and associated anxiety, and to facilitate a ‘low CO’ approach (see below) to delivery which can mitigate the need for CS in many cases.
For some women with specific cardiac conditions, it is felt that CS is the safest mode of delivery. This is often in patients with aortopathy, severe left ventricular outflow tract obstruction, or severely impaired cardiac function.
The deteriorating patient antenatally
It is crucial to identify women whose condition worsens during pregnancy to prevent poor outcomes, and the frequency of clinical review is tailored to the risk of decompensation to facilitate early detection of an impending problem. If women present to other services, e.g. primary care and emergency departments, they should be urgently discussed with the cardiology and obstetric teams, and admitted for assessment and treatment.
Medical optimization (e.g. diuretics or β-blockers) may be required. Specific interventions may be indicated, such as balloon valvuloplasty in severe pulmonary or aortic stenosis. One should never underestimate the power of hospital bed rest in a struggling parturient. This reduces cardiac demand, and in patients around the cusp of fetal viability can achieve even a few extra days of gestation, making significant differences to fetal outcomes.11 Such women should have daily assessment of maternal and fetal status, and a written plan in place for delivery, which is adhered to after a multidisciplinary discussion. Venous thromboembolism prophylaxis should be provided to women admitted.
Surgery is only indicated if medical management has failed or life-threatening conditions (valve thrombosis, aortic dissection) are present. Although cardiopulmonary bypass in pregnancy has similar maternal outcomes to that in non-pregnant women, fetal outcomes are poor. Normothermic bypass with pulsatile flow, higher mean arterial pressures, and maintenance of strict potassium balance are thought to reduce stillbirth rate, however fetal mortality is still quoted at 14–33%.12
High risk lesions
Pulmonary hypertension
Pulmonary hypertension (PH) is defined as a mean pulmonary artery pressure of >25 mm Hg and encompasses a range of heterogeneous conditions. Regardless of the cause, PH in pregnancy carries an extremely poor prognosis; mortality remains at 20–30%, even with optimal management.
PH is best managed in an expert centre. Patients should continue with vasodilator therapy and may require fluid and salt restriction if they develop right heart failure. Labour and vaginal delivery is generally avoided; CO in PH is dependent on preload, which may be critically reduced by Valsalva manoeuvres associated with expulsive efforts. Furthermore, labour induced pain, hypoxia, hypercarbia, and acidosis can increase pulmonary vascular resistance (PVR). Therefore, the Pulmonary Vascular Research Institute13 recommends CS as the optimal mode of delivery, under titrated regional anaesthesia, i.e. epidural or combined spinal-epidural anaesthesia as described below. General anaesthesia (GA) should be avoided as all induction and inhalational agents, except etomidate, reduce right ventricular contractility, and PVR is increased in response to laryngoscopy and by positive pressure ventilation.14
Severe systemic ventricular impairment
Impairment of the systemic ventricle can arise from acquired or congenital conditions. Congenital causes include residual dysfunction following surgical correction of conditions such as tetralogy of Fallot, or when the systemic ventricle is a morphological right ventricle e.g. following an atrial switch procedure for transposition of the great arteries.
Even the mildest forms of impairment can be exacerbated by the haemodynamic changes of pregnancy. Those with severe impairment are at the highest risk and labour should be avoided where possible, with delivery by CS.
Left ventricular outflow tract obstruction
In the pregnant population, left ventricular outflow tract obstruction is most commonly due to aortic stenosis from a bicuspid aortic valve. Women may only develop symptoms once pregnant, therefore asymptomatic women should undergo testing to evaluate tolerance and physiological responses to exercise and thus the need for intervention prepregnancy. Women with a bicuspid aortic valve and associated aortopathy are at increased risk of aortic dissection and rupture, and should have imaging of their aorta before and during pregnancy.
Severe symptomatic left ventricular outflow tract obstruction carries the risks of heart failure and arrhythmia and should be regarded as a contraindication to pregnancy. Women should be counselled appropriately, and referred for surgery if necessary. Valve choice should be sensitive to the woman’s wishes to have children. If such women choose to continue with pregnancy, CS delivery is recommended.
Aortopathy
There are several familial conditions that predispose to the risk of aortic dissection, aneurysm formation and rupture, including Marfan syndrome, Loeys–Dietz syndrome, Ehlers–Danlos type IV, and Turner syndrome, in addition to bicuspid aortic valve with aortic dilatation, and previous complex coarctation repairs. The risks are greatly increased in pregnancy due to the effects of cardiovascular and hormonal changes to the aortic media. Most deaths from aortic dissection in women of reproductive age are related to pregnancy.15
Such deaths feature prominently in MBRRACE reports, with delayed or missed diagnosis a recurring theme. Chest or severe back pain in pregnancy should be thoroughly investigated and the possibility of dissection actively considered. Cardiac tamponade, from extension into the aortic root, should be considered as a cause of cardiac arrest in the parturient.
Women at risk should have their aorta imaged before attempting pregnancy, and counselled of the risks of acute aortic syndromes and of their children inheriting familial conditions. Aortic dilatation of 50 mm, or 45 mm with Marfan syndrome, should be regarded as a contraindication to pregnancy, and may require surgery prepregnancy. Those electing to continue with the pregnancy should have interval imaging to assess for progressive dilatation of the aorta.16
Mode of delivery will depend on the condition and extent of dilatation, but in our centre CS is often considered safest.
Mechanical valves
Mechanical valves are implanted for congenital and acquired causes. It is vital that women receive adequate anticoagulation to minimize the risk of valve thrombosis, particularly given the prothrombotic state of pregnancy. All regimens carry an increased risk of miscarriage and of haemorrhagic complications. Warfarin with strict international normalized ratio monitoring has been shown to minimize the risk of valve thrombosis; however, it can have teratogenic effects. The rates of embryopathy may be dose-dependent,17 and the risks are further reduced if substituted for low molecular weight heparin (LMWH), which is not teratogenic, in Weeks 6–12. Women may elect to continue with LMWH, particularly if taking a larger dose of warfarin, but this requires intense monitoring of anti-factor Xa levels. Unresolved questions concern optimal anti-Xa levels and monitoring schedules. We monitor peak and trough levels in our institution, but valve thrombosis and haemorrhagic complications remain significant concerns.18
The European Society of Cardiology9 makes the following recommendations regarding peripartum anticoagulation management: (i) replace warfarin with either LMWH or unfractionated heparin (UFH) from 36 weeks gestation; and (ii) LMWH should be replaced by i.v. UFH at least 36 hours before planned delivery. UFH should be continued until 4–6 hours before planned delivery and restarted 4–6 hours after delivery if there are no bleeding complications.
A plan should be made by the MDT on how to manage the woman if she should unexpectedly present in labour whilst anticoagulated. Women do not require full reversal of anticoagulation for labour or CS but the urgency of delivery, degree of maternal haemorrhage and type of valve are factors in such decisions. We advise following the Association of Anaesthetists of Great Britain and Ireland guidance regarding timings of anticoagulation and regional anaesthesia; however, if insufficient time has elapsed prior to surgery and GA is felt to be prohibitively high risk, the use of point of care testing (e.g. thromboelastography or thromboelastometry) may be useful in assessing the risks and benefits of regional anaesthesia.
Cyanotic disease
Most cyanotic disease is corrected in childhood. Adults with cyanosis have either not had the appropriate corrective surgery—more likely in women from less developed countries—or have complex lesions not amenable to biventricular repair.
Maternal complications (heart failure, pulmonary or systemic thrombosis, supraventricular arrhythmias, infective endocarditis) occur in 30% of cyanotic pregnant patients. Cyanosis is also associated with an increased risk of postpartum haemorrhage.7 The degree of hypoxaemia is an important predictor of fetal outcome; with arterial oxygen saturations of 85%, the chance of a live birth is approximately 12%, and cyanotic patients should be counselled regarding such figures.9
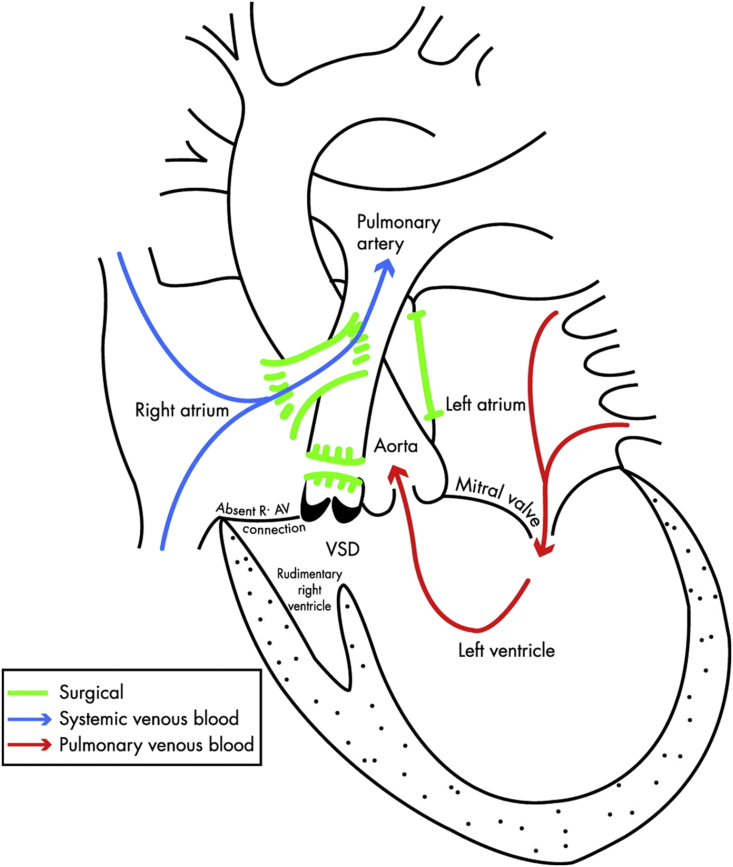
Most univentricular conditions will have been palliated by the Fontan procedure (Fig. 2).6 Even a woman with an optimal Fontan circuit is still at considerable risk of decompensation during pregnancy and should be closely monitored. If women have cyanosis, poor ventricular function, AV regurgitation or protein-losing enteropathy they should be counselled against pregnancy.
Fig 2.
Schematic drawing of a completed Fontan circuit for triscupid atresia (i.e. an absent right atrioventricular connection) with a ventricular septal defect. Systemic venous blood passes directly to the pulmonary arteries via surgical connections; pulmonary blood flow is hence dependent on adequate venous return and low pulmonary vascular resistance. Reproduced with permission.6
Vaginal delivery in these women is possible, but requires confidence from all members of the MDT, including the patient, that she has the physiological reserve to tolerate the haemodynamic stresses of labour, and meticulous planning to facilitate the management of any complications, including emergency CS. In the Fontan series discussed above, there were 53 live births, of which 12 were spontaneous, 10 had an assisted delivery, and 31 (58.5%) were delivered by CS (12 elective, 19 emergency).10
Delivery
General considerations
Access
Venous and arterial access can be difficult in women who have had multiple previous operations and/or prolonged hospitalisations. This can include conventional routes of central venous access, and expert help may be required.
Monitoring
The level of monitoring will depend on the mode of delivery and complexity of disease. For a planned vaginal delivery, pulse oximetry, and non-invasive blood pressure are routine, with the use of continuous ECG monitoring depending on concerns regarding cardiac rhythm. Invasive arterial blood pressure monitoring is preferable in the presence of significant ventricular dysfunction or for women requiring operative delivery. Central venous pressure monitoring and pulmonary artery flotation catheters are rarely used in our institution except in cases of severe heart failure. Midwives caring for such women should be trained in the use and interpretation of invasive and ECG monitoring.
Accurate recording of fluid balance is vital in those at risk of decompensation. Urine output should be recorded hourly. Blood loss post-delivery can be difficult to measure but every effort should be made to determine this accurately by weighing swabs and other materials.
Haemorrhage
A moderate degree of blood loss following delivery may be beneficial in preventing ventricular decompensation due to auto-transfusion from the placental bed; however, significant haemorrhage in women with limited ability to compensate can be catastrophic. Fluid resuscitation can be difficult to judge, and if done aggressively may precipitate cardiac failure. Avoidance of large volumes of crystalloid is advisable, and expert assistance from cardiology and cardiac anaesthesia, and the use of echocardiography, may be crucial.
Uterotonic agents
Haemorrhage may be very poorly tolerated, especially by women dependent on preload (e.g. Fontan circulation); however, the drugs normally used to treat uterine atony can have deleterious cardiovascular effects. Non-pharmacological methods should be used where possible such as bimanual uterine massage, and, at operative delivery, brace sutures and intrauterine balloon tamponade.
Oxytocin causes systemic vasodilation and pulmonary vasoconstriction. Its use as an i.v. bolus can precipitate cardiovascular collapse in susceptible patients with as little as 1 IU. An infusion at standard rates (10 IU h−1) does not usually cause haemodynamic instability, and can be titrated. I.M. administration gives lower peak concentrations.
Ergometrine and carboprost both cause pulmonary and systemic vasoconstriction which may overload impaired ventricles, and are best avoided. Ergometrine also causes coronary vasoconstriction, which may impair ventricular function further. PR misoprostol has no cardiovascular effects and is safe to use.19
Intravascular air
Death can occur if even a small amount of air crosses a shunt and reaches the coronary or cerebral circulation. Epidurals should be sited using loss of resistance to saline, and care should be taken with i.v. and epidural infusion lines, using filters if possible. Venous air embolism can occur during CS and should be considered as a potential cause of deterioration in a patient with a known shunt.20
Antibiotics
Women with ACHD are at increased risk of bacterial endocarditis. There is little clear evidence or guidance for the role of peripartum antibiotics in ACHD—NICE advises against routinely offering prophylaxis for obstetric procedures or childbirth, but also that this guidance does not override the responsibility to discuss all options with the patient and to use clinical judgement. MBRRACE recommends discussing prophylaxis with women prior to childbirth.
Labour
Most women with ACHD are able to labour and delivery vaginally. The risks of decompensation are mitigated by the early initiation of epidural analgesia, to reduce cardiac afterload and pain-related sympathetic drive. Low dose regimens should be used, with cautious administration of any boluses. It is vital to ensure adequate blockade and pain relief is achieved, with a low threshold for replacing a failing epidural.
A working epidural is the cornerstone of the ‘low CO’ approach to delivery. It reduces the urge to push, and once the cervix is fully dilated, allows time for the fetal head to passively descend in the pelvis. Once the head is below the ischial spines, expulsive efforts can commence, which are limited to minimize the effects of Valsalva manoeuvres and risks of autotransfusion, with delivery by forceps or ventouse if necessary.
An epidural also allows conversion to surgical anaesthesia for operative delivery if necessary. This should also be done cautiously and decisions concerning urgency of delivery should be made in this context.
Elective CS
Regional anaesthesia is our preference for most women with ACHD requiring elective CS. This is done with a combined spinal–epidural, with a low-dose spinal, and gradual supplementation via the epidural to minimize haemodynamic instability. GA is also possible, and should be tailored towards haemodynamic stability, and opioids should not be withheld for fear of causing neonatal respiratory depression. The usual precautions to prevent aspiration should be taken.
If decompensation is a reasonable possibility and potentially amenable to cardiac surgery, CS is performed under GA in cardiac theatres, with the facility and personnel for emergency cardiopulmonary bypass and surgery.
Care after delivery
Close monitoring postpartum is required in women with ACHD. In the immediate period, if the woman does not require critical care support, then the optimal location for this is the delivery suite, allowing one to one midwifery care, and close obstetric, neonatal, and anaesthetic support. In our unit we monitor such women for a minimum of 24 hours, with cardiology input, and postoperative deliveries are monitored in a high dependency environment on the delivery suite. Women requiring organ support or at higher risk of deterioration are managed in critical care with frequent obstetric and midwifery review, although this requires separating mother and baby.
The haemodynamic changes persist for several weeks postpartum, and 55% of deaths from heart disease in 2009–2014 occurred in the 6 weeks following delivery.1 The duration of inpatient observation varies, but women who required admission antenatally for decompensation may need a prolonged period of observation until their physiology approaches the prepregnant state.
Conclusion
The number of women with all types and complexities of ACHD surviving to childbearing age is increasing, resulting in greater clinical challenges to anaesthetic, obstetric, and cardiology teams. Multidisciplinary care by specialized teams has resulted in low numbers of pregnancy related deaths in this group in the recent MBRRACE report, yet the physiological challenges of pregnancy and labour remain a threat to many women, some of whom can decompensate rapidly. Effective epidural analgesia and an active obstetric approach can mitigate the need for CS in many women with ACHD.
Declaration of interest
None.
MCQs
The associated MCQs (to support CME/CPD activity) can be accessed at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Luke Bishop MB ChB FRCA is an advanced trainee in obstetric anaesthesia.
Alison Lansbury MB ChB FRCA is a consultant anaesthetist who runs the combined obstetric-anaesthetic-cardiology clinic at Leeds General Infirmary with KE.
Kate English MB ChB MRCP PhD is a consultant cardiologist with a specialist interest in ACHD and ACHD in pregnancy.
Matrix codes: 1A01, 2B01, 2B02, 2B03, 2B06, 3A09, 3B00
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bjae.2017.11.001.
Supplementary material
The following is the supplementary data related to this article:
References
- 1.Knight M., Nair M., Tuffnell D. National Perinatal Epidemiology Unit, University of Oxford; Oxford: 2016. Saving Lives, Improving mothers' care – surveillance of maternal deaths in the UK 2012–14 and lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009–14. [Google Scholar]
- 2.Kelleher A.A. Adult congenital heart disease (grown-up congenital heart disease) Cont Educ Anaesth Crit Care Pain. 2012;12:28–32. [Google Scholar]
- 3.Ntiloudi D., Giannakoulas G., Parcharidou D. Adult congenital heart disease: a paradigm of epidemiological change. Int J Cardiol. 2016;218:269–274. doi: 10.1016/j.ijcard.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Steer P. Royal College of Obstetricians and Gynaecologists; London: 2011. Cardiac disease and pregnancy. Good practice guide No. 13. [Google Scholar]
- 5.Sanghavi M., Rutherford J.D. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 6.Thorne S.A. Pregnancy in heart disease. Heart. 2004;90:450–456. doi: 10.1136/hrt.2003.027888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siu S.C., Sermer M., Colman J.M. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–521. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 8.Drenthen W., Boersma E., Balci A. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31:2124–2132. doi: 10.1093/eurheartj/ehq200. [DOI] [PubMed] [Google Scholar]
- 9.The Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) ESC guidelines on the management of cardiovascular diseases during pregnancy. Eur Heart J. 2011;32:3147–3319. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 10.Cauldwell M., Steer P.J., Bonner S. Retrospective UK multicentre study of the pregnancy outcomes of women with a Fontan repair. Heart. 2017 doi: 10.1136/heartjnl-2017-311763. [DOI] [PubMed] [Google Scholar]
- 11.Soe A., David A.L.M., Roberts A.D., Costeloe K. Royal College of Obstetricians and Gynaecologists; London: 2014. Perinatal management of pregnant women at the threshold of infant viability – the obstetric perspective (Scientific impact paper No. 41) [Google Scholar]
- 12.John A.S., Gurley F., Schaff H.V. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg. 2011;91:1191–1196. doi: 10.1016/j.athoracsur.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Hemnes A., Kiely D.G., Cockrill B.A. Statement on pregnancy in pulmonary hypertension from the Pulmonary Vascular Research Institute. Pulm Circ. 2015;5:435–465. doi: 10.1086/682230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilkington S.A., Taboada D., Martinez G. Pulmonary hypertension and its management in patients undergoing non-cardiac surgery. Anaesthesia. 2015;70:56–70. doi: 10.1111/anae.12831. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee A., Begaj I., Thorne S. Aortic dissection in pregnancy in England: an incidence study using linked national databases. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiratzka L.F., Bakris G.L., Beckman J.A. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. Anesth Analg. 2010;111:279–315. doi: 10.1213/ANE.0b013e3181dd869b. [DOI] [PubMed] [Google Scholar]
- 17.Vitale N., De Feo M., De Santo L.S. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol. 1999;33:1637–1641. doi: 10.1016/s0735-1097(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 18.Vause S., Clarke B., Tower C.L., Hay C., Knight M. (on behalf of UKOSS). Pregnancy outcomes in women with mechanical prosthetic heart valves: a prospective descriptive population based study using the United Kingdom Obstetric Surveillance System (UKOSS) data collection system. Br J Obstet Gynaecol. 2017;124:1411–1419. doi: 10.1111/1471-0528.14478. [DOI] [PubMed] [Google Scholar]
- 19.Allen R., O’Brien B.M. Uses of misoprostol in obstetrics and gynecology. Rev Obstet Gynecol. 2009;2:159–168. [PMC free article] [PubMed] [Google Scholar]
- 20.Mirski M.A., Lele A.V., Fitzsimmons L. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106:164–177. doi: 10.1097/00000542-200701000-00026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.